Determination of Low Muscle Mass by Muscle Surface Index of the First Lumbar Vertebra Using Low-Dose Computed Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. LDCT Image Acquisition and LDCT-Based Determination of Low Skeletal Muscle Mass
2.3. Development of Cut-Off Values of L1MI
2.4. The Use of the Sex Specific L1MI Cutoff Values in COPD Patients
2.5. Statistical Analyses
3. Results
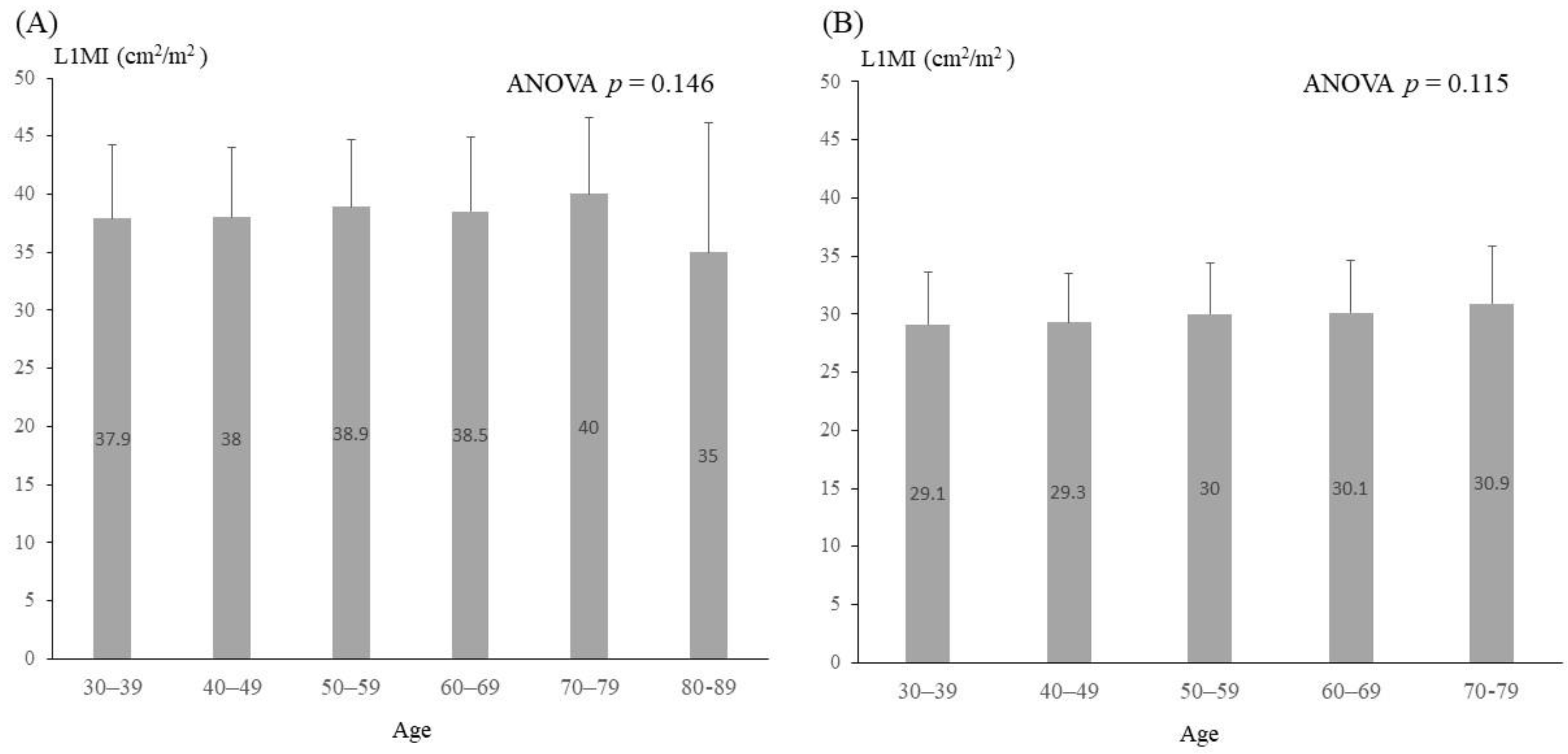
3.1. Characteristics of Subjects and Determination of Sex-Specific L1MI Cutoffs
3.2. Comparison between the Reference and Older Groups
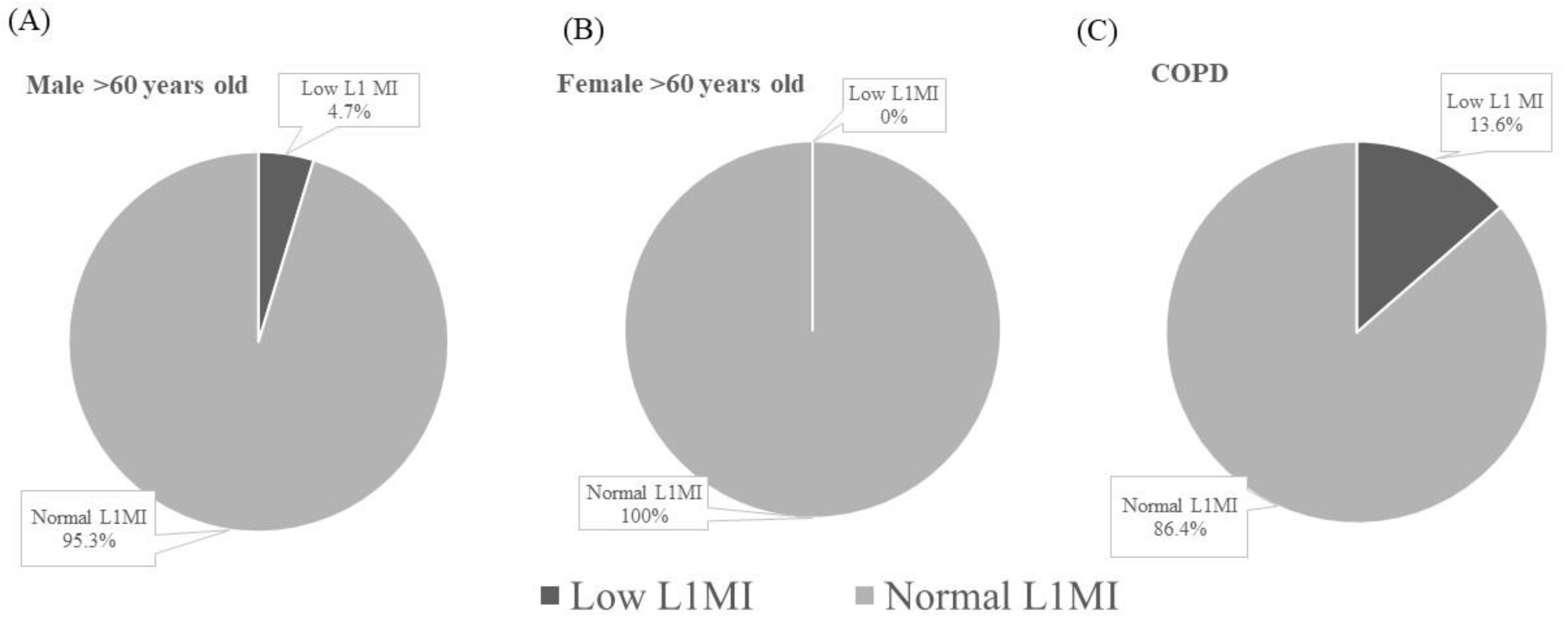
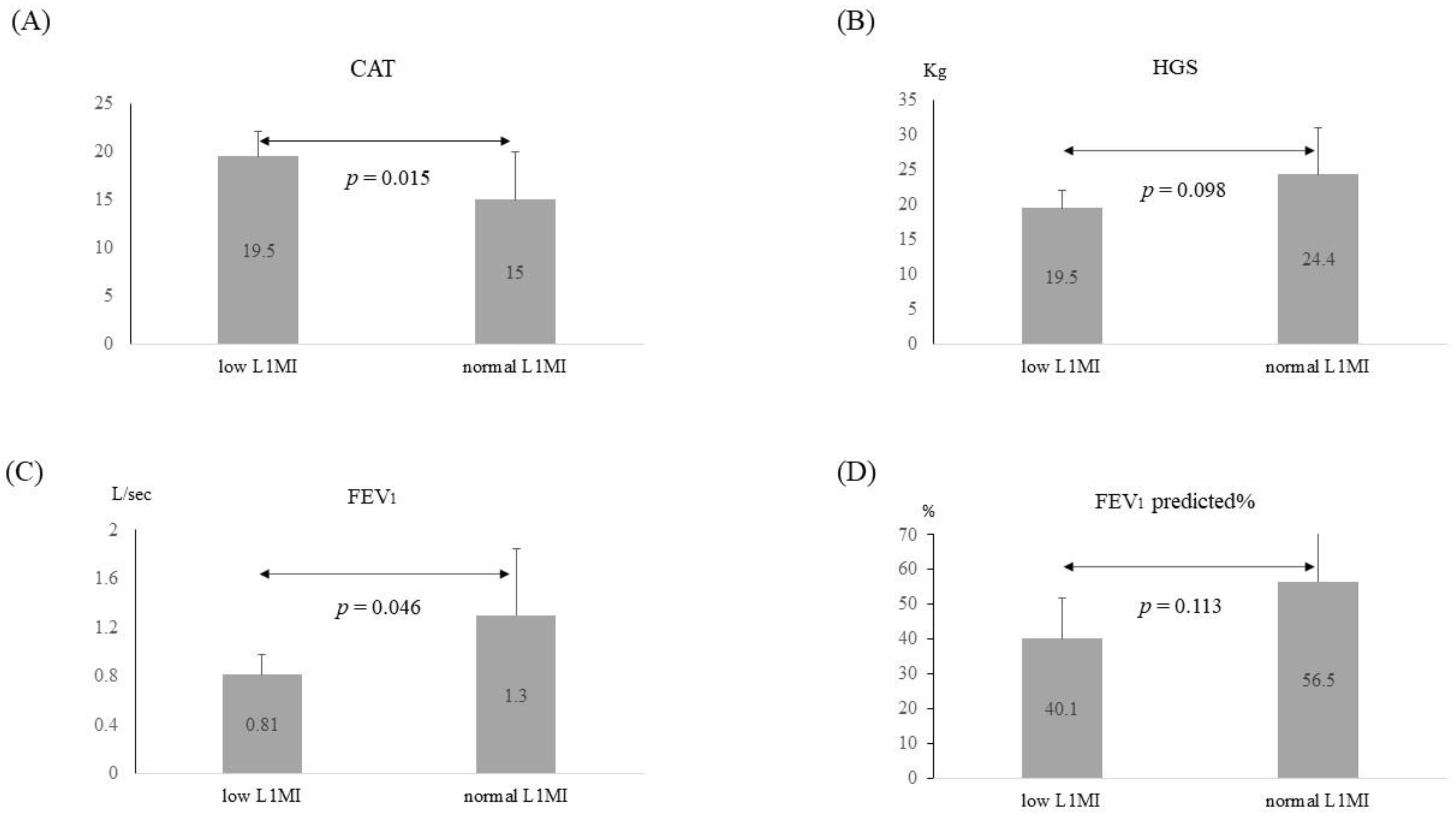
3.3. Application of the Diagnostic Criteria for Low L1MI in COPD Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
- Lung: FEV1 (forced expiratory volume in one second) predicted < 50%, mMRC (modified Medical Research Council dyspnea scale) ≥ 2, or oxygen dependence
- Heart: LVEF (left ventricle ejection fraction) < 50% or NYHA (New York heart association) function class ≥ 2
- Kidney: eGFR (estimated glomerular filtration rate) < 45 mL/min
- Liver: Total bilirubin level > 2 mg/dL or abdominal sonograph reported cirrhosis
- Neurologic: Barthel index score < 60
Appendix C
Appendix D
References
- Evans, W.J. Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010, 91, 1123S–1127S. [Google Scholar] [CrossRef] [Green Version]
- Rogers, M.A.; Evans, W.J. Changes in skeletal muscle with aging: Effects of exercise training. Exerc. Sport. Sci. Rev. 1993, 21, 65–102. [Google Scholar] [CrossRef]
- Pichard, C.; Kyle, U.G.; Morabia, A.; Perrier, A.; Vermeulen, B.; Unger, P. Nutritional assessment: Lean body mass depletion at hospital admission is associated with an increased length of stay. Am. J. Clin. Nutr. 2004, 79, 613–618. [Google Scholar] [CrossRef] [Green Version]
- Dahya, V.; Xiao, J.; Prado, C.M.; Burroughs, P.; McGee, D.; Silva, A.C.; Hurt, J.E.; Mohamed, S.G.; Noel, T.; Batchelor, W. Computed tomography-derived skeletal muscle index: A novel predictor of frailty and hospital length of stay after transcatheter aortic valve replacement. Am. Heart. J. 2016, 182, 21–27. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Gariballa, S.; Alessa, A. Sarcopenia: Prevalence and prognostic significance in hospitalized patients. Clin. Nutr. 2013, 32, 772–776. [Google Scholar] [CrossRef]
- Cosquéric, G.; Sebag, A.; Ducolombier, C.; Thomas, C.; Piette, F.; Weill-Engerer, S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br. J. Nutr. 2000, 96, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Maddocks, M.; Kon, S.S.; Canavan, J.L.; Jones, S.E.; Nolan, C.M.; Labey, A.; Polkey, M.I.; Man, W.D. Physical frailty and pulmonary rehabilitation in COPD: A prospective cohort study. Thorax 2016, 71, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Albano, D.; Messina, C.; Vitale, J.; Sconfienza, L.M. Imaging of sarcopenia: Old evidence and new insights. Eur. Radiol. 2020, 30, 2199–2208. [Google Scholar] [CrossRef]
- Zwart, A.T.; van der Hoorn, A.; van Ooijen, P.M.A.; Steenbakkers, R.; de Bock, G.H.; Halmos, G.B. CT-measured skeletal muscle mass used to assess frailty in patients with head and neck cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 1060–1069. [Google Scholar] [CrossRef] [Green Version]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [Green Version]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016, 32, 1200–1205. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Prado, C.M.; Birdsell, L.A.; Baracos, V.E. The emerging role of computerized tomography in assessing cancer cachexia. Curr. Opin. Support. Palliat. Care 2009, 3, 269–275. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, Y.S.; Park, I.; Ahn, H.K.; Cho, E.K.; Jeong, Y.M.; Kim, J.H. Evaluation of sarcopenia in small-cell lung cancer patients by routine chest CT. Support. Care Cancer 2016, 24, 4721–4726. [Google Scholar] [CrossRef]
- Recio-Boiles, A.; Galeas, J.N.; Goldwasser, B.; Sanchez, K.; Man, L.M.W.; Gentzler, R.D.; Gildersleeve, J.; Hollen, P.J.; Gralla, R.J. Enhancing evaluation of sarcopenia in patients with non-small cell lung cancer (NSCLC) by assessing skeletal muscle index (SMI) at the first lumbar (L1) level on routine chest computed tomography (CT). Support. Care Cancer 2018, 26, 2353–2359. [Google Scholar] [CrossRef]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 2018, 8, 11369. [Google Scholar] [CrossRef] [Green Version]
- National Lung Screening Trial Research, T.; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P. MS16.04 National Lung Screening Program in Taiwan. J. Thorac. Oncol. 2018, 13, S274–S275. [Google Scholar] [CrossRef] [Green Version]
- Yoon, D.Y.; Moon, J.H.; Kim, H.K.; Choi, C.S.; Chang, S.K.; Yun, E.J.; Seo, Y.L. Comparison of low-dose CT and MR for measurement of intra-abdominal adipose tissue: A phantom and human study. Acad. Radiol. 2008, 15, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.; Tan, J.; Roller, B.; Chiles, C.; Weaver, A.A.; Boutin, R.D.; Kritchevsky, S.B.; Lenchik, L. Machine Learning for Automatic Paraspinous Muscle Area and Attenuation Measures on Low-Dose Chest CT Scans. Acad. Radiol. 2019, 26, 1686–1694. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, J.; Huang, Z. Low-dose chest CT: Optimizing radiation protection for patients. AJR Am. J. Roentgenol. 2004, 183, 809–816. [Google Scholar] [CrossRef]
- Ischaki, E.; Papatheodorou, G.; Gaki, E.; Papa, I.; Koulouris, N.; Loukides, S. Body mass and fat-free mass indices in COPD: Relation with variables expressing disease severity. Chest 2007, 132, 164–169. [Google Scholar] [CrossRef]
- McDonald, M.L.; Diaz, A.A.; Ross, J.C.; San Jose Estepar, R.; Zhou, L.; Regan, E.A.; Eckbo, E.; Muralidhar, N.; Come, C.E.; Cho, M.H.; et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann. Am. Thorac. Soc. 2014, 11, 326–334. [Google Scholar] [CrossRef]
- Van Der Werf, A.; Langius, J.A.E.; De Van Der Schueren, M.A.E.; Nurmohamed, S.A.; Van Der Pant, K.A.M.I.; Blauwhoff-Buskermolen, S.; Wierdsma, N.J. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur. J. Clin. Nutr. 2018, 72, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Broughman, J.R.; Williams, G.R.; Deal, A.M.; Yu, H.; Nyrop, K.A.; Alston, S.M.; Gordon, B.B.; Sanoff, H.K.; Muss, H.B. Prevalence of sarcopenia in older patients with colorectal cancer. J. Geriatr. Oncol. 2015, 6, 442–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, N.; Ando, Y.; Gyawali, B.; Shimokata, T.; Maeda, O.; Fukaya, M.; Goto, H.; Nagino, M.; Kodera, Y. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol. Rep. 2016, 35, 1727–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.H.; Kim, K.W.; Shin, Y.; Lee, J.; Ko, Y.; Kim, Y.-J.; Lee, M.J.; Bae, S.-J.; Park, S.W.; Choe, J.; et al. Reference data and T-scores of lumbar skeletal muscle area and its skeletal muscle indices measured by CT scan in a healthy Korean population. J. Gerontol. Ser. A 2021, 76, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Song, H.; Kim, S.H. The association between L1 skeletal muscle index derived from routine CT and in-hospital mortality in CAP patients in the ED. Am. J. Emerg. Med. 2021, 42, 49–54. [Google Scholar] [CrossRef]
- Woo, J.; Arai, H.; Ng, T.P.; Sayer, A.A.; Wong, M.; Syddall, H.; Yamada, M.; Zeng, P.; Wu, S.; Zhang, T.M. Ethnic and geographic variations in muscle mass, muscle strength and physical performance measures. Eur. Geriatr. Med. 2014, 5, 155–164. [Google Scholar] [CrossRef]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of skeletal muscle: A 12-yr longitudinal study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [CrossRef]
- Wen, X.; Wang, M.; Jiang, C.M.; Zhang, Y.M. Are current definitions of sarcopenia applicable for older Chinese adults? J. Nutr. Health. Aging. 2011, 15, 847–851. [Google Scholar] [CrossRef]
- Lau, E.M.C.; Lynn, H.S.H.; Woo, J.W.; Kwok, T.C.Y.; Melton, L.J. Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Mialich, M.S.; Sicchieri, J.M.F.; Jordao Junior, A.A. Analysis of body composition: A critical review of the use of bioelectrical impedance analysis. Internat. J. Clin. Nutri. 2014, 2, 1–10. [Google Scholar]
- Větrovská, R.; Vilikus, Z.; Klaschka, J.; Stránská, Z.; Svačina, Š.; Svobodová, Š.; Matoulek, M. Does impedance measure a functional state of the body fat? Physiol. Res. 2014, 63 (Suppl. 2), S309–S320. [Google Scholar] [CrossRef] [PubMed]
- Van Vugt, J.L.A.; Levolger, S.; Gharbharan, A.; Koek, M.; Niessen, W.J.; Burger, J.W.A.; Willemsen, S.P.; De Bruin, R.W.; Ijzermans, J.N. A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J. Cachexia Sarcopenia Muscle 2017, 8, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Rollins, K.E.; Awwad, A.; Macdonald, I.A.; Lobo, D.N. A comparison of two different software packages for analysis of body composition using computed tomography images. Nutrition 2019, 57, 92–96. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 1780) | Male (n = 1129) | Female (n = 651) | |
|---|---|---|---|
| Age (years) | 51.2 ± 11.1 | 51.4 ± 11.2 | 50.8 ± 11.0 |
| 20–29 n (%) | 3 (0.2) | 3 (0.3) | 0 (0) |
| 30–39 n (%) | 330 (18.1) | 202 (17.9) | 120 (18.4) |
| 40–49 n(%) | 515 (28.3) | 307 (27.2) | 194 (29.8) |
| 50–59 n (%) | 579 (31.8) | 362 (32.1) | 205 (31.5) |
| 60–69 n (%) | 332 (18.2) | 221 (19.6) | 106 (16.3) |
| 70–79 n (%) | 57 (3.1) | 31 (2.7) | 25 (3.8) |
| ≥80 n (%) | 4 (0.2) | 3 (0.3) | 1 (0.2) |
| Height (cm) | 165.6 ± 8.8 | 170.8 ± 6.4 | 157.9 ± 5.7 * |
| Weight (kg) | 68.3 ± 13.5 | 74.5 ± 11.4 | 58.3 ± 10.2 * |
| BMI | 24.7 ± 3.7 | 25.5 ± 3.4 | 23.4 ± 3.8 * |
| <18.5 n (%) | 58 (3.2) | 16 (1.4) | 38 (5.8) |
| 18.5–25 n (%) | 975 (53.6) | 520 (46.1) | 435 (66.8) |
| 25–30 n (%) | 637 (35.0) | 481 (42.6) | 144 (22.1) |
| 30–35 n (%) | 130 (7.1) | 101 (8.9) | 28 (4.3) |
| >35 n (%) | 20 (1.1) | 11 (1.0) | 6 (0.9) |
| SMI (kg/m2) | 16.0 ± 5.7 | 17.3 ± 5.9 | 13.8 ± 4.7 * |
| L1MI (cm2/m2) | 35.5 ± 7.2 | 38.4 ± 6.1 | 29.7 ± 4.4 * |
| Male | p | Female | p | |||
|---|---|---|---|---|---|---|
| Reference Group (Age: 20–60) (n = 874) | Older Group (Age > 60) (n = 255) | Reference Group (Age: 20–60) (n = 519) | Older Group (Age > 60) (n = 132) | |||
| Age (years) | 47.1 ± 8.7 | 66.0 ± 4.5 | <0.001 | 47.0 ± 8.6 | 65.9 ± 4.9 | <0.001 |
| Height (cm) | 171.8 ± 6.3 | 167.2 ± 5.7 | <0.001 | 158.8 ± 5.3 | 154.4 ± 5.6 | <0.001 |
| Weight (kg) | 75.5 ± 11.7 | 70.8 ± 9.6 | <0.001 | 58.2 ± 10.0 | 58.4 ± 10.9 | 0.990 |
| BMI | 25.5 ± 3.5 | 25.3 ± 3.1 | 0.348 | 23.1 ± 3.7 | 24.5 ± 4.0 | 0.001 |
| <18.5 n (%) | 11 (1.3) | 5 (2.0) | 32 (6.2) | 6 (4.5) | ||
| 18.5–25 n (%) | 403 (46.1) | 117 (45.9) | 365 (70.3) | 70 (53.0) | ||
| 25–30 n (%) | 363 (41.5) | 118 (46.3) | 98 (18.9) | 46 (34.8) | ||
| 30–35 n (%) | 87 (10.0) | 14 (5.5) | 19 (3.7) | 9 (6.8) | ||
| >35 n (%) | 10 (1.1) | 1 (0.4) | 5 (1.0) | 1 (0.8) | ||
| SMI (kg/m2) | 17.4 ± 5.9 | 17.1 ± 5.9 | 0.396 | 13.7 ± 4.6 | 13.9 ± 4.7 | 0.903 |
| L1MI (cm2/m2) | 38.3 ± 6.0 | 38.6 ± 6.5 | 0.510 | 29.6 ± 4.4 | 31.1 ± 6.2 | 0.133 |
| Normal L1MI (n = 38) | Low L1MI (n = 6) | |
|---|---|---|
| Male n (%) | 33 (86.8) | 5 (83.3) |
| Age (years) | 74.4 ± 8.3 | 75.8 ± 5.0 |
| Height (cm) | 162.6 ± 8.3 | 160.8 ± 9.6 |
| Weight (kg) | 66.1 ± 11.7 | 49.1 ± 6.2 * |
| BMI | 25.0 ± 4.2 | 19.0 ± 2.5 * |
| Hypertension n (%) | 18 (47.4) | 3 (50) |
| DM n (%) | 7 (18.4) | 2 (33.3) |
| Heart disease n (%) | 15 (39.5) | 2 (33.3) |
| CKD n (%) | 5 (13.2) | 0 (0) |
| CVA n (%) | 3 (7.9) | 0 (0) |
| Cancer n (%) | 8 (21.1) | 1 (16.7) |
| Cirrhosis n (%) | 0 (0) | 0 (0) |
| SMI (kg/m2) | 18.2 ± 1.8 | 14.4 ± 1.2 * |
| L1MI (cm2/m2) | 36.5 ± 5.9 | 25.4 ± 1.8 * |
| Frequent exacerbation | 9 (23.7) | 3 (50) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.-H.; Gow, C.-H.; Chiu, Y.-L.; Li, T.-C. Determination of Low Muscle Mass by Muscle Surface Index of the First Lumbar Vertebra Using Low-Dose Computed Tomography. J. Clin. Med. 2022, 11, 2429. https://doi.org/10.3390/jcm11092429
Wang P-H, Gow C-H, Chiu Y-L, Li T-C. Determination of Low Muscle Mass by Muscle Surface Index of the First Lumbar Vertebra Using Low-Dose Computed Tomography. Journal of Clinical Medicine. 2022; 11(9):2429. https://doi.org/10.3390/jcm11092429
Chicago/Turabian StyleWang, Ping-Huai, Chien-Hung Gow, Yen-Ling Chiu, and Tien-Chi Li. 2022. "Determination of Low Muscle Mass by Muscle Surface Index of the First Lumbar Vertebra Using Low-Dose Computed Tomography" Journal of Clinical Medicine 11, no. 9: 2429. https://doi.org/10.3390/jcm11092429






