Safety and Efficacy of the Nit-Occlud® Coil for Percutaneous Closure of Various Sizes of PDA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ethics Statement
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmadi, A.; Sabri, M.; Bigdelian, H.; Dehghan, B.; Gharipour, M. Comparison of cost-effectiveness and postoperative outcome of device closure and open surgery closure techniques for treatment of patent ductus arteriosus. ARYA Atheroscler. 2014, 10, 37–40. [Google Scholar] [PubMed]
- Porstmann, W.; Wierny, L.; Warnke, H. Closure of persistent ductus arteriosus without thoracotomy. Ger. Med. Mon. 1967, 12, 259–261. [Google Scholar] [PubMed]
- Ghasemi, A.; Pandya, S.; Reddy, S.V.; Turner, D.R.; Du, W.; Navabi, M.A.; Mirzaaghayan, M.R.; Kiani, A.; Sloan, K.; Forbes, T.J. Trans-catheter closure of patent ductus arteriosus-What is the best device? Catheter. Cardiovasc. Interv. 2010, 76, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Celiker, A.; Aypar, E.; Karagoz, T.; Dilber, E.; Ceviz, N. Transcatheter closure of patent ductus arteriosus with Nit-Occlud coils. Catheter. Cardiovasc. Interv. 2005, 65, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Maksymenko, A.V.; Kuzmenko, Y.L.; Dovhaliuk, A.A.; Motrechko, O.O.; Herrmann, F.E.; Haas, N.A.; Lehner, A. Percutaneous closure of patent ductus arteriosus with the Nit-Occlud((R)) patent ductus arteriosus device in 268 consecutive cases. Ann. Pediatr. Cardiol. 2019, 12, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Alkashkari, W.; Albugami, S.; Alrahimi, J.; Althobaiti, M.; Kinsara, A.; Abousa, A.; Krimly, A.; Alzahrani, A.; Niazi, A.; Aburemish, H. Percutaneous Device Closure of Patent Ductus Arteriosus in Adult Patients with 10-Year Follow-up. Heart Views 2019, 20, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Salem, M.M.; Forbes, T.J.; Gordon, B.M.; Soriano, B.D.; Dimas, V.; Goldstein, B.H.; Owada, C.; Javois, A.; Bass, J.; et al. Results of the combined U.S. multicenter postapproval study of the Nit-Occlud PDA device for percutaneous closure of patent ductus arteriosus. Catheter. Cardiovasc. Interv. 2019, 93, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Bouhanick, B.; Sosner, P.; Brochard, K.; Mounier-Vehier, C.; Plu-Bureau, G.; Hascoet, S.; Ranchin, B.; Pietrement, C.; Martinerie, L.; Boivin, J.M.; et al. Hypertension in Children and Adolescents: A Position Statement From a Panel of Multidisciplinary Experts Coordinated by the French Society of Hypertension. Front. Pediatr. 2021, 9, 680803. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, B.; Benson, L.N. Closure of persistently patent arterial duct and its impact on cerebral circulatory haemodynamics in children. Can. J. Anaesth. 1998, 45, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, K.; Toyono, M.; Tamura, M. Effects of coil closure of patent ductus arteriosus on left anterior descending coronary artery blood flow using transthoracic Doppler echocardiography. J. Am. Soc. Echocardiogr. 2004, 17, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Krichenko, A.; Benson, L.N.; Burrows, P.; Moes, C.A.; McLaughlin, P.; Freedom, R.M. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am. J. Cardiol. 1989, 63, 877–880. [Google Scholar] [CrossRef]
- Bilici, M.; Demir, F.; Akin, A.; Ture, M.; Balik, H.; Kuyumcu, M. Transcatheter Closure of Patent Ductus Arteriosus in Children with the Occlutech Duct Occluder. Pediatr. Cardiol. 2017, 38, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Liang, Y.M.; Wang, X.F.; Guo, B.J.; Zheng, K.; Gu, Y.; Lyu, Z.Y. A Retrospective Study of 1526 Cases of Transcatheter Occlusion of Patent Ductus Arteriosus. Chin. Med. J. 2015, 128, 2284–2289. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.; Waller, B.R., 3rd; Agrawal, V.; Wright, D.; Arevalo, A.; Zurakowski, D.; Sathanandam, S. Morphologic characterization of the patent ductus arteriosus in the premature infant and the choice of transcatheter occlusion device. Catheter. Cardiovasc. Interv. 2016, 87, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Forsey, J.T.; Elmasry, O.A.; Martin, R.P. Patent arterial duct. Orphanet J. Rare Dis. 2009, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes Sarmento, J.; Correia-Costa, A.; Goncalves, E.; Baptista, M.J.; Silva, J.C.; Moreira, J. Percutaneous patent ductus arteriosus closure: Twelve years of experience. Rev. Port. Cardiol. 2021, 40, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montes, J.A.; Zabal Cerdeira, C.; Calderon-Colmenero, J.; Juanico Enriquez, A.; Cardona Garza, A.; Colin Ortiz, J.L.; Buendia Hernandez, A. Patent ductus arteriosus in the adult: Transcatheher treatment immediate and medium term results. Arch. Cardiol. Mex. 2006, 76, 163–168. [Google Scholar] [PubMed]
- Zabal, C.; Garcia-Montes, J.A.; Buendia-Hernandez, A.; Calderon-Colmenero, J.; Patino-Bahena, E.; Juanico-Enriquez, A.; Attie, F. Percutaneous closure of hypertensive ductus arteriosus. Heart 2010, 96, 625–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
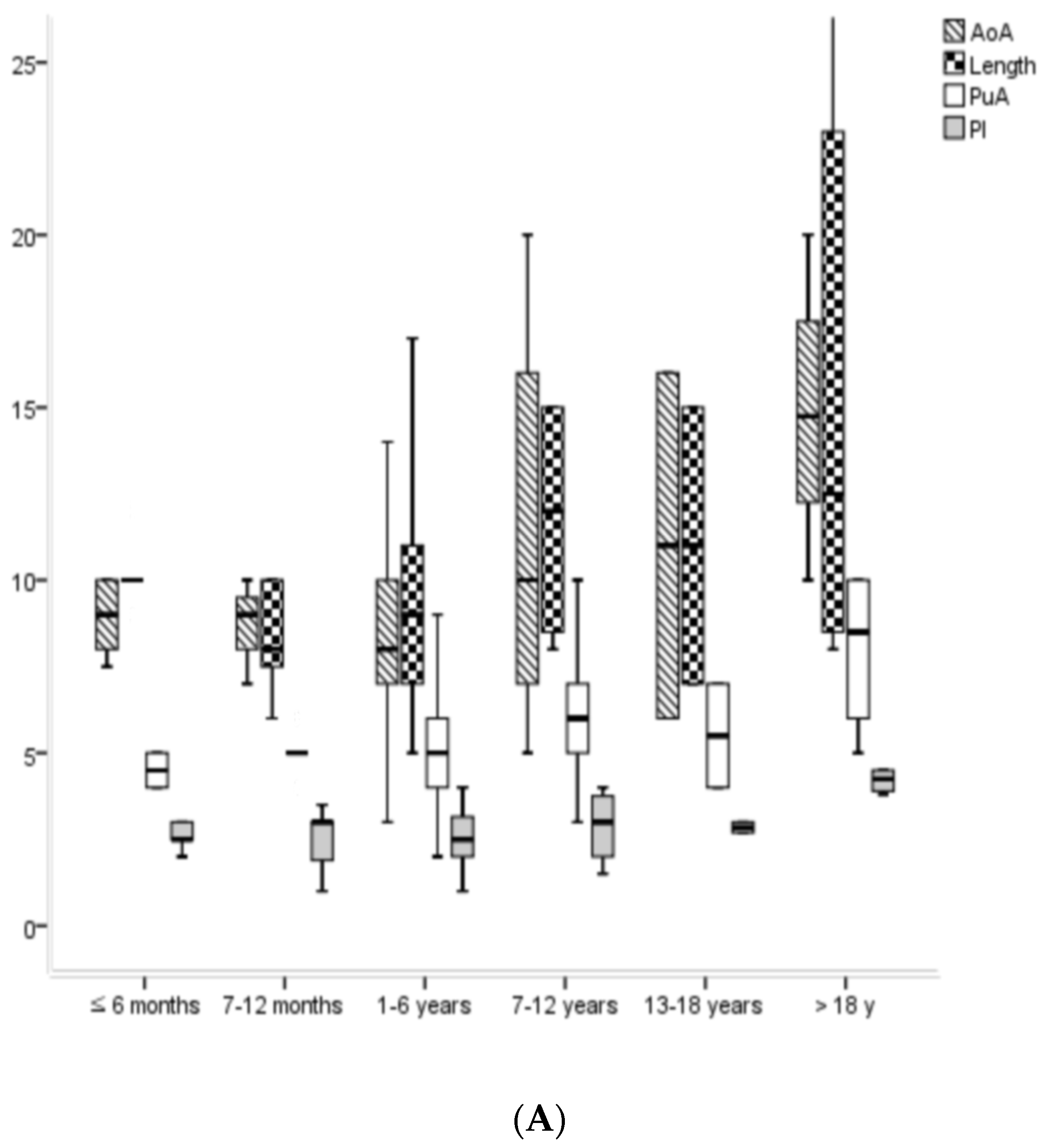

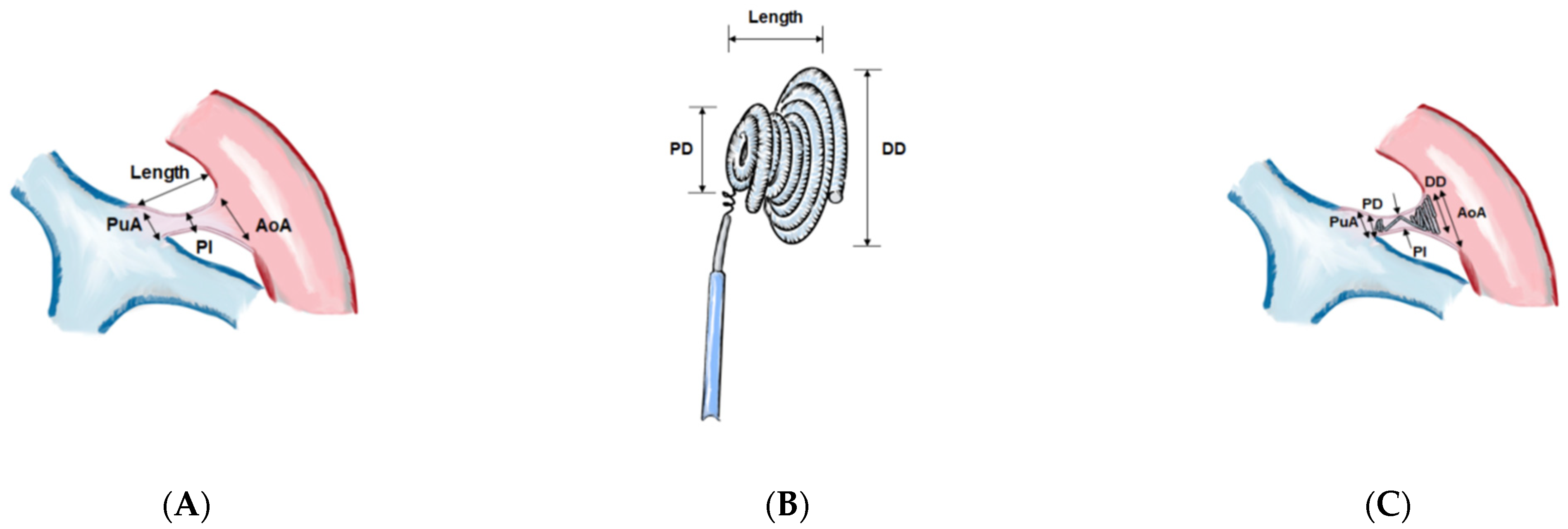

| Pediatrics (0–18 y) | Age ≤ 6 Months | Age 7–12 Months | Age 1–6 Years | Age 7–12 Years | Age 13–18 Years | p Value (Among Pediatrics) | Adults (>18 y) | |
|---|---|---|---|---|---|---|---|---|
| Number | 325 | 26 | 57 | 175 | 57 | 10 | 36 | |
| Age (months) | 41 ± 40 | 4 ± 2 | 10 ± 2 | 34 ± 18 | 103 ± 23 | 178 ± 21 | <0.001 | 451 ± 185 |
| Body weight (kg) | 16 ± 11 | 7.0 ± 2.6 | 9.0 ± 1.6 | 14 ± 5 | 30 ± 10 | 56 ± 13 | <0.001 | 57 ± 9 |
| Pre-Ao Pr (mmHg) | ||||||||
| Systole | 111 ± 17 | 100 ± 10 | 102 ± 15 | 112 ± 16 | 125 ± 21 | 121 ± 13 | <0.001 | 129 ± 26 |
| Diastole | 63 ± 14 | 54 ± 10 | 54 ± 10 | 64 ± 13 | 76 ± 15 | 76 ± 10 | <0.001 | 66 ± 11 |
| Mean | 83 ± 15 | 72 ± 10 | 74 ± 13 | 84 ± 14 | 96 ± 16 | 93 ± 15 | <0.001 | 89 ± 11 |
| Pulse Pr | 50 ± 15 | 48 ± 10 | 49 ± 13 | 48 ± 10 | 49 ± 12 | 45 ± 7 | 0.748 | 67 ± 14 |
| Pre-MPA Pr | ||||||||
| Systole | 29 ± 9 | 29 ± 9 | 26 ± 6 | 29 ± 7 | 34 ± 13 | 36 ± 19 | 0.001 | 30 ± 8 |
| Diastole | 12 ± 6 | 11 ± 6 | 11 ± 5 | 12 ± 5 | 15 ± 8 | 15 ± 12 | 0.012 | 11 ± 4 |
| Mean | 19 ± 7 | 18 ± 6 | 18 ± 5 | 19 ± 6 | 23 ± 11 | 24 ± 16 | 0.003 | 19 ± 6 |
| Pulse Pr | 17 ± 6 | 17 ± 7 | 15 ± 5 | 16 ± 5 | 19 ± 7 | 21 ± 10 | 0.002 | 19 ± 5 |
| Pr gaps of Pre-Ao and pre-MPA | ||||||||
| Systole | 82 ± 17 | 71 ± 15 | 76 ± 14 | 83 ± 16 | 95 ± 15 | 84 ± 23 | <0.001 | 100 ± 22 |
| Diastole | 50 ± 14 | 42 ± 13 | 43 ± 11 | 51 ± 13 | 64 ± 12 | 60 ± 11 | <0.001 | 55 ± 11 |
| Mean | 63 ± 15 | 53 ± 14 | 56 ± 13 | 64 ± 14 | 75 ± 11 | 68 ± 18 | <0.001 | 71 ± 10 |
| Post-Ao Pr | ||||||||
| Systole | 114 ± 15 | 95 ± 9 | 113 ± 6 | 115 ± 15 | 112 ± 19 | 123 ± 15 | 0.027 | 140 ± 17 |
| Diastole | 67 ± 12 | 47 ± 10 | 63 ± 6 | 68 ± 10 | 72 ± 24 | 73 ± 10 | 0.027 | 73 ± 3 |
| Mean | 86 ± 11 | 67 ± 6 | 83 ± 3 | 87 ± 10 | 87 ± 19 | 93 ± 16 | 0.035 | 90 ± 10 |
| Pulse Pr | 48 ± 14 | 47 ± 13 | 50 ± 10 | 47 ± 13 | 40 ± 9 | 50 ± 18 | 0.874 | 64 ± 25 |
| PDA sizes (mm) | ||||||||
| AoA | 7.9 ± 3.6 | 7.3 ± 1.7 | 7.4 ± 2.6 | 7.6 ± 3.4 | 9.8 ± 5.5 | 10.5 ± 4.9 | 0.003 | 14.7 ± 5.2 |
| PuA | 5.3 ± 1.7 | 4.9 ± 1.2 | 5.0 ± 0.6 | 5.3 ± 1.8 | 6.4 ± 2.3 | 5.5 ± 2.1 | 0.061 | 8.0 ± 2.4 |
| Length | 9.2 ± 3.5 | 8.3 ± 2.8 | 8.7 ± 3.2 | 8.9 ± 3.1 | 11.9 ± 5.1 | 12.1 ± 3.4 | <0.001 | 14.8 ± 5.9 |
| PI | 2.3 ± 1.0 | 2.3 ± 1.2 | 2.1 ± 0.9 | 2.3 ± 0.9 | 2.6 ± 1.2 | 2.9 ± 0.8 | 0.023 | 4.4 ± 1.6 |
| Size gaps of PDA and NOC (mm) | ||||||||
| AoA—DD | 1.7 ± 3.4 | 0.8 ± 3.5 | 1.7 ± 2.1 | 1.3 ± 3.1 | 3.9 ± 5.2 | 2.8 ± 4.9 | 0.001 | 5.1 ± 5.8 |
| PuA—PD | −0.3 ± 1.6 | −0.5 ± 1.2 | −0.4 ± 0.8 | −0.4 ± 1.8 | 0.6 ± 1.5 | −0.5 ± 2.1 | 0.707 | 1.3 ± 2.5 |
| PI—DD | −3.5 ± 1.4 | −3.6 ± 1.2 | −3.4 ± 1.2 | −3.6 ± 1.4 | −3.2 ± 1.6 | −4.4 ± 1.8 | 0.192 | −5.0 ± 2.4 |
| PI—PD | −2.6 ± 1.0 | −2.7 ± 1.0 | −2.6 ± 0.9 | −2.6 ± 1.0 | −2.2 ± 1.4 | −2.6 ± 0.8 | 0.365 | −1.8 ± 1.9 |
| Pre-Qp/Qs | 1.4 ± 1.0 | 1.6 ± 0.5 | 1.4 ± 0.4 | 1.3 ± 0.3 | 1.3 ± 0.2 | 1.3 ± 0.2 | 0.023 | 1.5 ± 0.4 |
| Post-Qp/Qs | 1.0 ± 0.8 | 1.1 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.0 | 0.716 | 1.0 ± 0.0 |
| Size Groups of PDA Isthmus (PI, mm) at Catheterization | ||||
|---|---|---|---|---|
| Small (0.5 ≤ Sizes < 2) | Moderate (2 ≤ Sizes < 4) | Large (4 ≤ Sizes ≤ 9) | p Value | |
| Numbers (n = 361) | 101 | 210 | 50 | |
| Age, m | 28 ± 24 | 59 ± 106 | 202 ± 220 | <0.001 |
| Body weight, kg | 13 ± 7 | 19 ± 16 | 38 ± 21 | <0.001 |
| Pre-Ao Pr, mmHg | ||||
| Systole | 109 ± 19 | 112 ± 18 | 123 ± 22 | <0.001 |
| Diastole | 64 ± 15 | 62 ± 13 | 67 ± 13 | 0.191 |
| Mean | 82 ± 17 | 83 ± 15 | 88 ± 13 | 0.111 |
| Pulse Pr | 46 ± 11 | 50 ± 12 | 56 ± 19 | <0.001 |
| Pre-MPA Pr | ||||
| Systole | 27 ± 7 | 29 ± 8 | 32 ± 11 | 0.010 |
| Diastole | 11 ± 4 | 13 ± 6 | 14 ± 7 | 0.004 |
| Mean | 18 ± 5 | 20 ± 7 | 21 ± 9 | 0.009 |
| Pulse Pr | 16 ± 6 | 17 ± 6 | 18 ± 7 | 0.284 |
| Pr gaps of Pre-Ao and pre-MPA | ||||
| Systole | 81 ± 19 | 83 ± 16 | 94 ± 20 | <0.001 |
| Diastole | 52 ± 15 | 49 ± 13 | 54 ± 13 | 0.055 |
| Mean | 64 ± 17 | 63 ± 13 | 69 ± 12 | 0.069 |
| Post-Ao Pr | ||||
| Systole | 117 ± 12 | 113 ± 16 | 124 ± 18 | 0.197 |
| Diastole | 63 ± 6 | 66 ± 11 | 73 ± 12 | 0.167 |
| Mean | 88 ± 6 | 85 ± 11 | 91 ± 12 | 0.270 |
| Pulse Pr | 53 ± 15 | 47 ± 13 | 51 ± 15 | 0.656 |
| PDA sizes, mm | ||||
| AoA | 5.8 ± 2.7 | 8.5 ± 3.6 | 13.2 ± 5.2 | <0.001 |
| PuA | 4.4 ± 1.5 | 5.2 ± 1.6 | 7.2 ± 2 | <0.001 |
| Length | 7.8 ± 3.1 | 9.9 ± 3.7 | 13.2 ± 5.2 | <0.001 |
| PI | 1.2 ± 0.3 | 2.6 ± 0.5 | 4.6 ± 1.1 | <0.001 |
| Size gaps of PDA and NOC, mm | ||||
| AoA—DD | 0.9 ± 2.5 | 2 ± 3 | 4 ± 6 | <0.001 |
| PuA—PD | −0.3 ± 1.5 | −0.4 ± 1.7 | 0.6 ± 1.8 | 0.130 |
| PI—DD | −3 ± 0.9 | −4 ± 2 | −4 ± 2 | 0.201 |
| PI—PD | −3 ± 0.6 | −3 ± 1.0 | −1 ± 1 | <0.001 |
| Pre-Qp/Qs * | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.5 ± 0.4 | <0.001 |
| Post-Qp/Qs * | 1.0 ± 0.1 | 1.0 ± 0.9 | 1.0 ± 0.0 | 0.658 |
| PDA types A/B/C/D/E/U, n | 45/6/5/6/34/5 | 130/16/11/4/24/25 | 37/4/5/0/4/0 | |
| RS, n | Total RS/COc | |||
| Immediate time | 12 (12%) | 65 (31%) | 13 (26%) | 90 (25%)/271 (75%) |
| 1 day | 11 (11%) | 53 (25%) | 10 (20%) | 74 (20%)/287 (80%) |
| 1 month | 4 (4%) | 26 (12%) | 5 (10%) | 35 (10%)/326 (90%) |
| 6 months | 3 (3%) | 8 (4%) | 4 (8%) | 15 (4%)/346 (96%) |
| 1 year | 1 (1%) | 4 (2%) | 3 (6%) | 8 (2%)/353 (98%) |
| Pediatric Ages | Adults | Total Ages | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤6 Months | 7–12 Months | 1–6 Years | 7–12 Years | 13–18 Years | Subtotal | Subtotal | ||
| PDA Types | n = 26 | n = 57 | n = 175 | n = 57 | n = 10 | n = 325 | n = 36 | n = 361 |
| A (conical) | 16 (62%) | 35 (60%) | 115 (65%) | 16 (28%) | 6 (60%) | 188 (58%) | 21 (58%) | 209 (58%) |
| B (window) | 1 (4%) | 3 (4%) | 12 (7%) | 4 (7%) | 1 (10%) | 21 (6%) | 5 (14%) | 26 (7%) |
| C (tubular) | 1 (4%) | 2 (4%) | 14 (8%) | 1 (2%) | 1 (10%) | 19 (6%) | 2 (6%) | 21 (6%) |
| D (complex) | 0 (0%) | 2 (4%) | 5 (3%) | 1 (2%) | 2 (20%) | 10 (3%) | 0 (0%) | 10 (3%) |
| E (elongated) | 6 (23%) | 13 (21%) | 29 (17%) | 8 (14%) | 0 | 56 (17%) | 2 (6%) | 58 (16%) |
| U (unclassified) | 2 (7%) | 2 (7%) | 0 | 27 (47%) | 0 | 31 (10%) | 6 (16%) | 37 (10%) |
| Pediatrics | p | Adults | p | |||
|---|---|---|---|---|---|---|
| Before | After | Wilcoxon Test | Before | After | Wilcoxon Test | |
| Systole Ao Pr | 111 ± 17 | 114 ± 15 | 0.031 | 129 ± 26 | 140 ± 17 | 0.317 |
| Diastole Ao Pr | 63 ± 14 | 67 ± 12 | 0.130 | 66 ± 11 | 73 ± 3 | 0.421 |
| Mean Ao Pr | 83 ± 15 | 86 ± 11 | 0.535 | 89 ± 11 | 90 ± 10 | 0.125 |
| Pulse Pr of Ao | 48 ± 10 | 47 ± 13 | 0.004 | 67 ± 14 | 64 ± 25 | 0.321 |
| Qp/Qs | 1.4 ± 1.0 | 1.0 ± 0.8 | <0.001 | 1.5 ± 0.4 | 1.0 ± 0.0 | <0.001 |
| The CHDs Accompanying PDA | Numbers (n = 361) |
|---|---|
| Simple patent ductus arteriosus | 329 |
| Secundum atrial septal defect | 11 |
| Ventricular septal defect | 8 |
| Double outlet right ventricle with sub-pulmonary ventricular septal defect (Taussig-Bing anomaly) | 3 |
| Endocardial cushion defect | 3 |
| Pulmonary atresia with intact ventricular septum | 2 |
| Total anomalous pulmonary venous return, pulmonary atresia with complete endocardial cushion defect | 1 |
| Ebstein anomaly | 1 |
| Partial fusion of right coronary cusp and non-coronary cusp without significant aortic stenosis | 1 |
| Pulmonary valve stenosis | 1 |
| Hypertrophic cardiomyopathy | 1 |
| Numbers | Small-Sized PDAs | Moderate-Sized PDAs | Large-Sized PDAs | |
|---|---|---|---|---|
| Residual shunts | 8 | |||
| Persistent residual shunts | 6 | 4 | 2 | |
| Double NOCs to occlude residual shunts | 2 | 2 (7/5 + 4/3, 9/6 + 7/6) | ||
| Flow disturbances in the PA by NOC | 7 | |||
| Mild narrowing | 5 | 3 | 2 | |
| Stenosis of PA (particularly left PA) | 2 | 2 | ||
| Flow disturbances in the aorta by NOC | ||||
| Mild narrowing | 2 | 1 | 1 | |
| Atrial fibrillation | 1 | 1 (42 years old) | ||
| Total events | 18/361 = 5% | 8/361 = 2% | 7/361 = 2% | 3/361 = 1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Seol, J.; Choi, J.; Ha, K. Safety and Efficacy of the Nit-Occlud® Coil for Percutaneous Closure of Various Sizes of PDA. J. Clin. Med. 2022, 11, 2469. https://doi.org/10.3390/jcm11092469
Jung S, Seol J, Choi J, Ha K. Safety and Efficacy of the Nit-Occlud® Coil for Percutaneous Closure of Various Sizes of PDA. Journal of Clinical Medicine. 2022; 11(9):2469. https://doi.org/10.3390/jcm11092469
Chicago/Turabian StyleJung, Seyong, Jaehee Seol, Jaeyoung Choi, and Keesoo Ha. 2022. "Safety and Efficacy of the Nit-Occlud® Coil for Percutaneous Closure of Various Sizes of PDA" Journal of Clinical Medicine 11, no. 9: 2469. https://doi.org/10.3390/jcm11092469
APA StyleJung, S., Seol, J., Choi, J., & Ha, K. (2022). Safety and Efficacy of the Nit-Occlud® Coil for Percutaneous Closure of Various Sizes of PDA. Journal of Clinical Medicine, 11(9), 2469. https://doi.org/10.3390/jcm11092469







