Bereaved Family Members’ Perspectives of Good Death and Quality of End-of-Life Care for Malignant Pleural Mesothelioma Patients: A Cross-Sectional Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design, Participants, and Setting
2.2. Outcomes
2.3. Instruments
2.3.1. Information of Patients and Bereaved Family Members
2.3.2. Good Death Inventory
2.3.3. Care Evaluation Scale
2.3.4. Symptoms
2.4. Missing Data
2.5. Comparison of Study Data
2.6. Statistical Analysis
2.7. Ethical Consideration
3. Results
3.1. Characteristics of Malignant Pleural Mesothelioma Patients and Bereaved Family Members
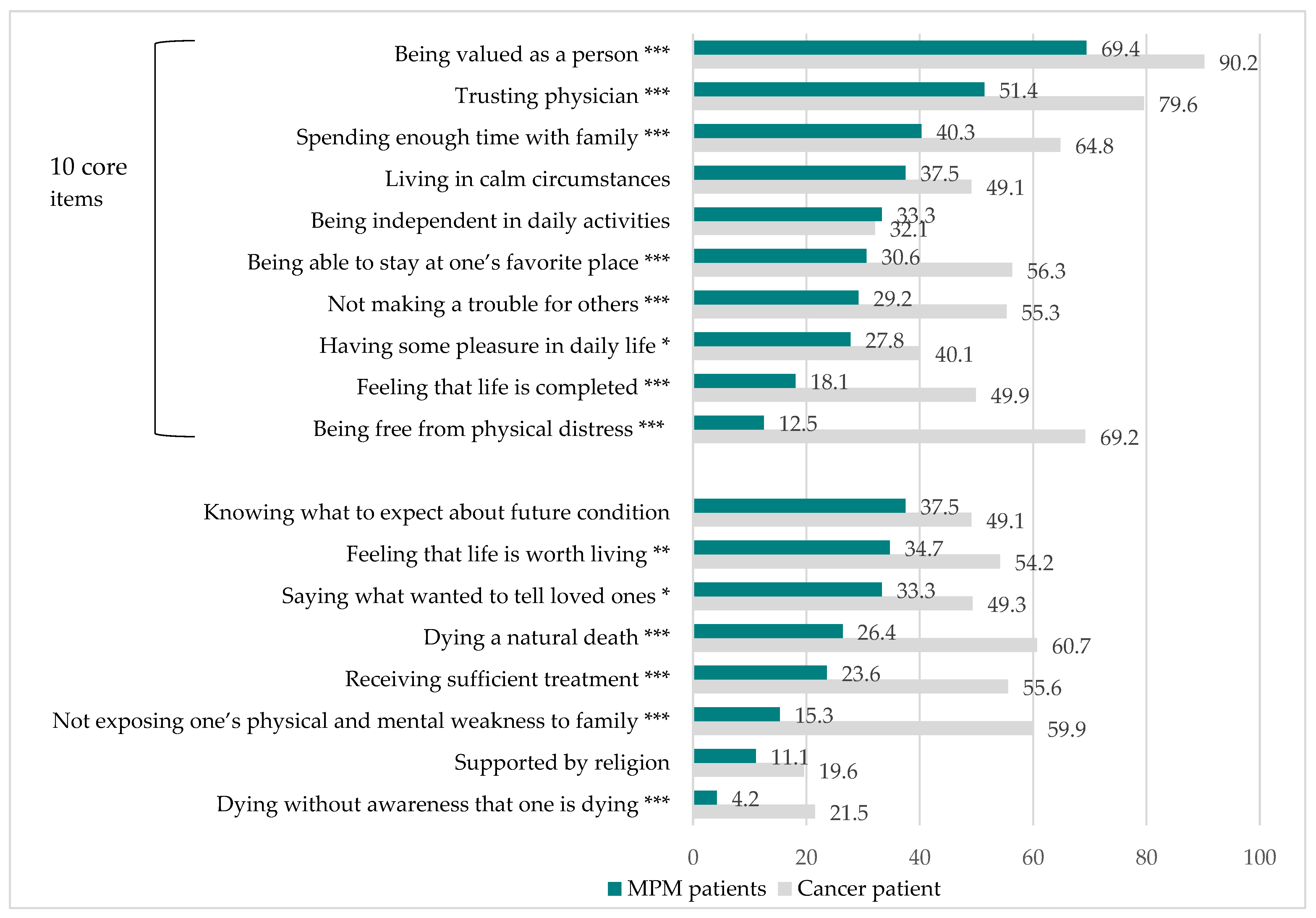
3.2. Achievement of Good Death
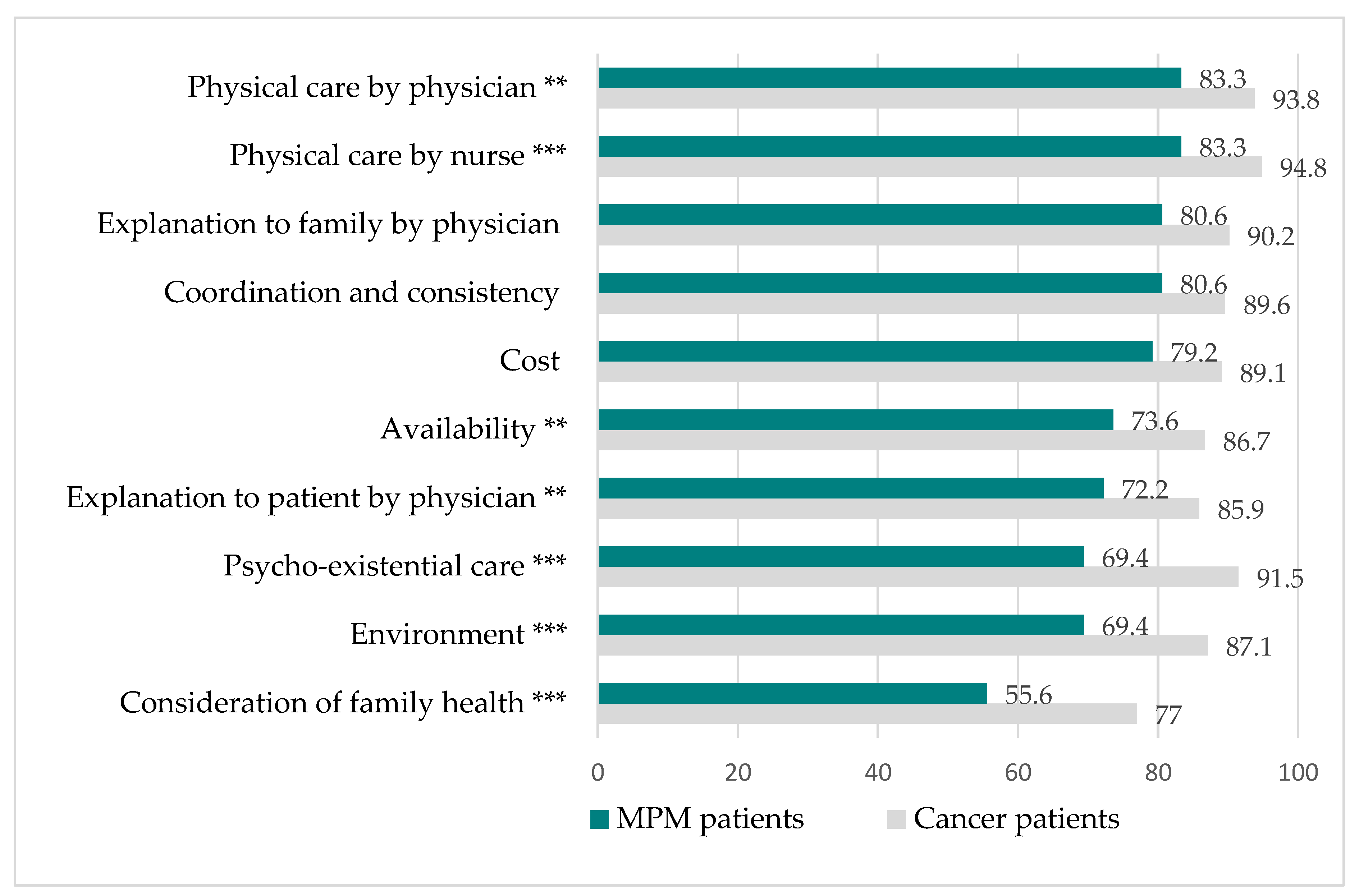
3.3. Quality of End-of-Life Care
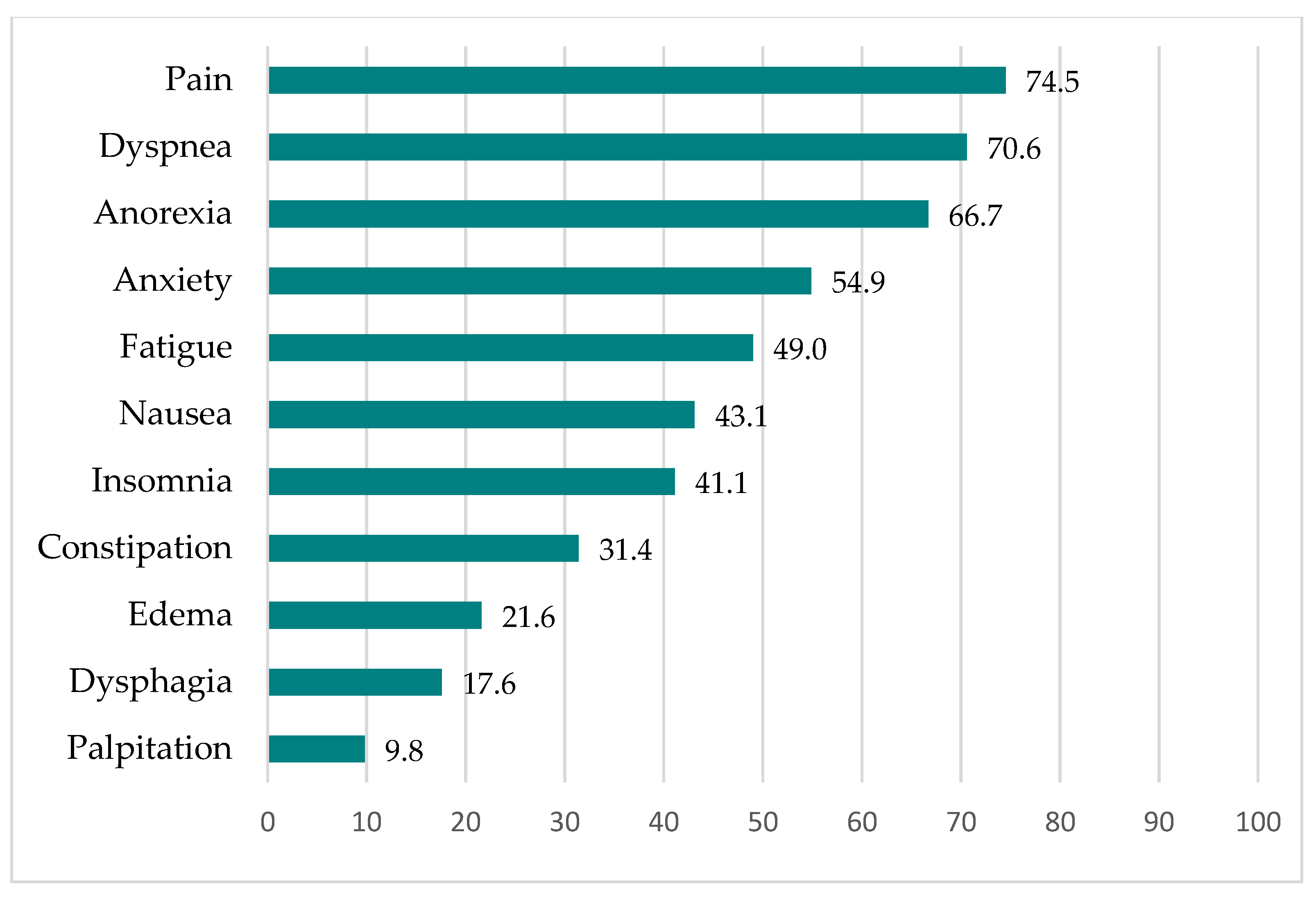
3.4. Symptoms
3.5. Factors Associated with a Good Death
3.6. Factors Associated with Quality of End-of-Life Care
4. Discussion
4.1. Poor Achievement of Good Death
4.2. Heavy Symptom Burden
4.3. Poor Quality of End-of-Life Care
4.4. Implications for Care and Further Research
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bianchi, C.; Bianchi, T. Malignant Mesothelioma: Global Incidence and Relationship with Asbestos. Ind. Health 2007, 45, 379–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Japan Ministry of Health Labor & Welfare. Yearly Changes (from 1995 to 2018) in Number of Death from Mesothelioma by Prefecture (Based on Vital Statistics). 2019. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/tokusyu/chuuhisyu17/index.html (accessed on 25 May 2021).
- Myojin, T.; Azuma, K.; Okumura, J.; Uchiyama, I. Future trends of mesothelioma mortality in Japan bas ed on a risk function. Ind. Health 2012, 50, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murayama, T.; Takahashi, K.; Natori, Y.; Kurumatani, N. Estimation of future mortality from pleural malignant mesothelioma in Japan based on an age-cohort model. Am. J. Ind. Med. 2005, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gemba, K.; Fujimoto, N.; Kato, K.; Aoe, K.; Takeshima, Y.; Inai, K.; Kishimoto, T. National survey of malignant mesothelioma and asbestos exposure in Japan. Cancer Sci. 2012, 103, 483–490. [Google Scholar] [CrossRef]
- Hollen, P.J.; Gralla, R.J.; Liepa, A.M.; Symanowski, J.T.; Rusthoven, J.J. Adapting the lung cancer symptom scale (LCSS) to mesothelioma: Using the LCSS-Meso conceptual model for validation. Cancer 2004, 101, 587–595. [Google Scholar] [CrossRef]
- Clayson, H.; Seymour, J.; Noble, B. Mesothelioma from the Patient’s Perspective. Hematol. Clin. N. Am. 2005, 19, 1175–1190. [Google Scholar] [CrossRef]
- Moore, S.; Darlison, L.; Tod, A.M. Living with mesothelioma. A literature review. Eur. J. Cancer Care 2010, 19, 458–468. [Google Scholar] [CrossRef]
- Nagamatsu, Y.; Oze, I.; Aoe, K.; Hotta, K.; Kato, K.; Nakagawa, J.; Hara, K.; Kishimoto, T.; Fujimoto, N. Quality of life of survivors of malignant pleural mesothelioma in Japan: A cross sectional study. BMC Cancer 2018, 18, 350. [Google Scholar] [CrossRef] [Green Version]
- Woolhouse, I.; Bishop, L.; Darlison, L.; De Fonseka, D.; Edey, A.; Edwards, J.; Faivre-Finn, C.; Fennell, D.A.; Holmes, S.; Kerr, K.M.; et al. British Thoracic Society Guideline for the investigation and management of malignant pleural mesothelioma. Thorax 2018, 73, i1–i30. [Google Scholar] [CrossRef] [Green Version]
- Muers, M.F.; Stephens, R.J.; Fisher, P.; Darlison, L.; Higgs, C.M.; Lowry, E.; Nicholson, A.G.; O’Brien, M.; Peake, M.; Rudd, R.; et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): A multicentre randomised trial. Lancet 2008, 371, 1685–1694. [Google Scholar] [CrossRef] [Green Version]
- Arber, A.; Spencer, L.; Parker, A. ‘It’s all bad news’: The first 3 months following a diagnosis of malignant pleural mesothelioma. BMJ Support. Palliat. Care 2011, 1, A14–A15. [Google Scholar] [CrossRef]
- Innamorati, M.; Tamburello, S.; Tamburello, A.; Casale, S.; Cont, C.; Guglielmucci, F.; Granieri, A. Quality of life and personality traits in patients with malignant pleural mesothelioma and their first-degree caregivers. Neuropsychiatr. Dis. Treat. 2013, 9, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Hughes, N.; Arber, A. The lived experience of patients with pleural mesothelioma. Int. J. Palliat. Nurs. 2008, 14, 66–71. [Google Scholar] [CrossRef]
- Barak, Y.; Achiron, A.; Rotstein, Z.; Elizur, A.; Noy, S. Stress associated with asbestosis: The trauma of waiting for death. Psycho-Oncol. 1988, 7, 126–128. [Google Scholar] [CrossRef]
- Nagamatsu, Y.; Horiuchi, S.; Natori, Y. The stages and difficulties of patients with malignant pleural mesothelioma. J. Hum. Care Stud. 2012, 12, 69–81. [Google Scholar]
- Miyashita, M.; Morita, T.; Sato, K.; Hirai, K.; Shima, Y.; Uchitomi, Y. Good Death Inventory: A Measure for Evaluating Good Death from the Bereaved Family Member’s Perspective. J. Pain Symptom Manag. 2008, 35, 486–498. [Google Scholar] [CrossRef]
- Addington-Hall, J.; O’Callaghan, A. A comparison of the quality of care provided to cancer patients in the UK in the last three months of life in in-patient hospices compared with hospitals, from the perspective of bereaved relatives: Results from a survey using the VOICES questionnaire. Palliat. Med. 2009, 23, 190–197. [Google Scholar] [CrossRef]
- Rhodes, R.L.; Mitchell, S.L.; Miller, S.C.; Connor, S.R.; Teno, J.M. Bereaved family members’ evaluation of hospice care: What factors influence overall satisfaction with services? J. Pain Symptom Manag. 2008, 35, 365–371. [Google Scholar] [CrossRef]
- Downey, L.; Curtis, J.R.; Lafferty, W.E.; Herting, J.R.; Engelberg, R.A. The quality of dying and death questionnaire (QODD): Empirical domains and theoretical perspectives. J. Pain Symptom Manag. 2010, 39, 9–22. [Google Scholar] [CrossRef]
- Miyashita, M.; Morita, T.; Sato, K.; Tsuneto, S.; Shima, Y. A Nationwide Survey of Quality of End-of-Life Cancer Care in Designated Cancer Centers, Inpatient Palliative Care Units, and Home Hospices in Japan: The J-HOPE Study. J. Pain Symptom Manag. 2015, 50, 38–47.e3. [Google Scholar] [CrossRef]
- Shear, M.K.; Simon, N.; Wall, M.; Zisook, S.; Neimeyer, R.; Duan, N.; Reynolds, C.; Lebowitz, B.; Sung, S.; Ghesquiere, A.; et al. Complicated grief and related bereavement issues for DSM-5. Depress. Anxiety 2011, 28, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, M.; Aoyama, M.; Nakahata, M.; Yamada, Y.; Abe, M.; Yanagihara, K.; Shirado, A.; Shutoh, M.; Okamoto, Y.; Hamano, J.; et al. Development the Care Evaluation Scale Version 2.0: A modified version of a measure for bereaved family members to evaluate the structure and process of palliative care for cancer patient. BMC Palliat. Care 2017, 16, 8. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, M.; Morita, T.; Tsuneto, S.; Sato, K.; Shima, Y. The Japan HOspice and Palliative Care Evaluation Study (J-HOPE Study): Study Design and Characteristics of Participating Institutions. Am. J. Hosp. Palliat. Med. 2008, 25, 223–232. [Google Scholar] [CrossRef]
- Clayson, H. The Experience of Mesothelioma in Northern England; University of Sheffield: Sheffield, UK, 2007. [Google Scholar]
- Mercadante, S.; Degiovanni, D.; Casuccio, A. Symptom burden in mesothelioma patients admitted to home palliative care. Curr. Med. Res. Opin. 2016, 32, 1985–1988. [Google Scholar] [CrossRef]
- Ahmedzai, S.; Clayson, H. Supportive and Palliative Care in Mesothelioma; University Press: New York, NY, USA, 2006. [Google Scholar]
- Abrahm, J.L. Palliative Care for the Patient with Mesothelioma. Semin. Thorac. Cardiovasc. Surg. 2009, 21, 164–171. [Google Scholar] [CrossRef]
- Ando, M.; Kawamura, R.; Morita, T.; Hirai, K.; Miyashita, M.; Okamoto, T.; Shima, Y. Value of religious care for relief of psycho-existential suffering in Japanese terminally ill cancer patients: The perspective of bereaved family members. Psycho-Oncol. 2010, 19, 750–755. [Google Scholar] [CrossRef]
- Carr, D. Death and dying in the contemporary United States: What are the psychological implications of anticipated death? Soc. Pers. Psychol. Compass 2012, 6, 184–195. [Google Scholar] [CrossRef]
- Bibby, A.C.; De Fonseka, D.; Morley, A.J.; Keenan, E.; Addeo, A.; Smith, S.; Edey, A.J.; Maskell, N.A. Exploring the characteristics of patients with mesothelioma who chose active symptom control over chemotherapy as first-line treatment: A prospective, observational, single centre study. BMC Palliat. Care 2017, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysham, N.G.; Cox, C.E.; Wolf, S.P.; Kamal, A.H. Symptom Burden of Chronic Lung Disease Compared with Lung Cancer at Time of Referral for Palliative Care Consultation. Ann. Am. Thorac. Soc. 2015, 12, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Van Zandwijk, N.; Clarke, C.; Henderson, D.; Musk, A.W.; Fong, K.; Nowak, A.; Loneragan, R.; McCaughan, B.; Boyer, M.; Feigen, M.; et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J. Thorac. Dis. 2013, 5, E254–E307. [Google Scholar] [PubMed]
- Miyashita, M.; Hirai, K.; Morita, T.; Sanjo, M.; Uchitomi, Y. Barriers to referral to inpatient palliative care units in Japan: A qualitative survey with content analysis. Support. Care Cancer 2007, 16, 217–222. [Google Scholar] [CrossRef]
- Horne, G.; Payne, S.; Seymour, J. Do patients with lung cancer recall physician-initiated discussions about planning for end-of-life care following disclosure of a terminal prognosis? BMJ Support. Palliat. Care 2016, 9, 197–201. [Google Scholar] [CrossRef]
- Nagamatsu, Y.; Oze, I.; Aoe, K.; Hotta, K.; Kato, K.; Nakagawa, J.; Hara, K.; Kishimoto, T.; Fujimoto, N. Physician requests by patients with malignant pleural mesothelioma in Japan. BMC Cancer 2019, 19, 383. [Google Scholar] [CrossRef]

| Disease | MPM | Cancer * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 72 | Place of Death | ||||||||||
| Designated Cancer Center (n = 2794) | Palliative Care Unit (n = 5312) | Home Hospice (n = 292) | |||||||||
| Patients | n | % | n | % | n | % | n | % | |||
| Sex | Men | 59 | 81.9 | 1820 | 65.1 | 2906 | 54.7 | 181 | 62 | ||
| Women | 13 | 18.1 | 973 | 34.8 | 2364 | 44.5 | 111 | 38 | |||
| Primary cancer site | Pleura ** | 72 | 100 | - | - | - | - | - | - | ||
| Lung | 0 | 0 | 688 | 24.6 | 1246 | 23.5 | 63 | 21.6 | |||
| Stomach | 0 | 0 | 395 | 14.1 | 635 | 12 | 36 | 12.3 | |||
| Colorectum/rectum | 0 | 0 | 260 | 9.3 | 651 | 12.3 | 54 | 18.5 | |||
| Liver | 0 | 0 | 279 | 10 | 281 | 5.3 | 18 | 6.2 | |||
| Gall bladder/bile duct | 0 | 0 | 165 | 5.9 | 201 | 3.8 | 14 | 4.8 | |||
| Pancreas | 0 | 0 | 243 | 8.7 | 398 | 7.5 | 18 | 6.2 | |||
| Esophagus | 0 | 0 | 112 | 4 | 184 | 3.5 | 8 | 2.7 | |||
| Breast | 0 | 0 | 83 | 3 | 266 | 5 | 8 | 2.7 | |||
| Others | - | - | 513 | 18.4 | 1389 | 26.2 | 69 | 23.7 | |||
| Source of asbestos exposure | Occupation | 49 | 68.1 | ||||||||
| Neighboring factory | 17 | 23.6 | |||||||||
| School | 1 | 1.4 | |||||||||
| Family | 1 | 1.4 | |||||||||
| Unknown | 4 | 5.4 | |||||||||
| Treatment | Surgery | 14 | 19.4 | ||||||||
| (includes multiple treatments) | Extrapleural pneumonectomy | 12 | 16.7 | ||||||||
| Pleurectomy decoration | 2 | 2.8 | |||||||||
| Chemotherapy | 51 | 70.8 | |||||||||
| Radiotherapy | 15 | 20.8 | |||||||||
| Palliative care | 41 | 56.9 | |||||||||
| Compensated | Workmen’s accident compensation insurance | 47 | 65.3 | ||||||||
| (some had both types) | Asbestos-related health damage relief system | 56 | 77.8 | ||||||||
| Place of death | Respiratory ward | 35 | 48.6 | ||||||||
| Palliative care unit/hospice | 24 | 33.3 | |||||||||
| Home | 10 | 13.9 | |||||||||
| Other | 3 | 4.2 | |||||||||
| Age at diagnosis (years) | Range: | 36–92 | Mean ± SD | 66.9 ± 9.6 | 69.8 ± 11.5 | 70.9 ± 12.1 | 71.8 ± 13.0 | ||||
| Survival (months) | 0.5–69 | 14.5 ± 14.1 | |||||||||
| Bereaved family members | n | % | n | % | n | % | n | % | |||
| Sex | Men | 15 | 20.8 | 825 | 29.5 | 1694 | 31.9 | 60 | 20.6 | ||
| Women | 57 | 79.2 | 1696 | 60.7 | 3556 | 67.1 | 228 | 78.1 | |||
| Relationship with patient | Spouse | 52 | 72.2 | 1535 | 54.9 | 2506 | 47.2 | 165 | 56.5 | ||
| Child | 20 | 17.8 | 672 | 24.1 | 1809 | 34.1 | 78 | 26.7 | |||
| Son/daughter-in-law | 0 | 0 | 181 | 6.5 | 353 | 6.7 | 34 | 11.6 | |||
| Parent | 0 | 0 | 49 | 1.8 | 100 | 1.9 | 4 | 1.4 | |||
| Sibling | 0 | 0 | 56 | 2 | 310 | 5.8 | 6 | 2.1 | |||
| Others | 0 | 0 | 32 | 1.2 | 188 | 3.5 | 4 | 1.4 | |||
| Experience of end-of-life discussion with patient | Yes | 27 | 37.5 | ||||||||
| No | 44 | 61.1 | |||||||||
| Timing of patient’s death | Much sooner than expected | 31 | 43.1 | ||||||||
| Sooner than expected | 25 | 34.7 | |||||||||
| Moderate | 9 | 12.5 | |||||||||
| Later than expected | 5 | 6.9 | |||||||||
| Much later than expected | 2 | 2.8 | |||||||||
| Satisfaction with care | |||||||||||
| on diagnosis | Satisfied | 29 | 40.3 | ||||||||
| Not satisfied | 43 | 59.7 | |||||||||
| When patient became critical | Satisfied | 31 | 38.9 | ||||||||
| Not satisfied | 41 | 61.1 | |||||||||
| When patient died | Satisfied | 47 | 65.3 | ||||||||
| Not satisfied | 25 | 34.7 | |||||||||
| Financial impact of patient’s MPM on family | Significant impact | 12 | 16.7 | ||||||||
| Some impact | 15 | 20.8 | |||||||||
| Moderate impact | 20 | 27.8 | |||||||||
| Minor impact | 15 | 20.8 | |||||||||
| No impact | 10 | 13.9 | |||||||||
| Level of anger toward asbestos | Very angry | 56 | 77.8 | ||||||||
| Angry | 11 | 15.3 | |||||||||
| Moderately angry | 4 | 5.6 | |||||||||
| Slightly angry | 1 | 1.4 | |||||||||
| Not angry at all | 0 | 0 | |||||||||
| Age (in years) | Range: | 32–82 | Mean ± SD | 62.5 ± 12.2 | 60.4 ± 12.5 | 59.3 ± 12.8 | 60.6 ± 12.1 | ||||
| Time since bereavement (months) | 9–110 | 45.2 ± 27.2 | 12.4 ± 3.5 | 11.8 ± 3.7 | 12.2 ± 6.6 | ||||||
| Dependent Variable: GDI Total Score (F = 9.098, p = 0.0001, Adjusted R2 = 0.260) | ||||||
|---|---|---|---|---|---|---|
| Model | B | SE | β | t | 95% CI | p-Value |
| Constant | 41.724 | 4.769 | 8.794 | 32.202–51.246 | 0.001 | |
| Satisfied with care received when patient became critical | 11.597 | 3.278 | 0.370 | 3.538 | 5.053–18.141 | 0.001 |
| Female bereaved family member | 11.061 | 4.028 | 0.284 | 2.746 | 3.018–19.103 | 0.008 |
| Patient died later than expected | 3.270 | 1.556 | 0.220 | 2.102 | 0.164–6.376 | 0.039 |
| Dependent Variable: CES Total Score (F = 34.558, p = 0.0001, Adjusted R2 = 0.493) | ||||||
|---|---|---|---|---|---|---|
| Model | B | SE | β | t | 95% CI | p-Value |
| Constant | 30.545 | 1.807 | 16.907 | 26.939–34.152 | 0.001 | |
| Satisfied with the care received when the patient died | 13.272 | 1.727 | 0.664 | 7.683 | 9.824–16.720 | 0.001 |
| Received chemotherapy | 4.048 | 1.832 | 0.191 | 2.209 | 0.391–7.705 | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagamatsu, Y.; Sakyo, Y.; Barroga, E.; Koni, R.; Natori, Y.; Miyashita, M. Bereaved Family Members’ Perspectives of Good Death and Quality of End-of-Life Care for Malignant Pleural Mesothelioma Patients: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 2541. https://doi.org/10.3390/jcm11092541
Nagamatsu Y, Sakyo Y, Barroga E, Koni R, Natori Y, Miyashita M. Bereaved Family Members’ Perspectives of Good Death and Quality of End-of-Life Care for Malignant Pleural Mesothelioma Patients: A Cross-Sectional Study. Journal of Clinical Medicine. 2022; 11(9):2541. https://doi.org/10.3390/jcm11092541
Chicago/Turabian StyleNagamatsu, Yasuko, Yumi Sakyo, Edward Barroga, Riwa Koni, Yuji Natori, and Mitsunori Miyashita. 2022. "Bereaved Family Members’ Perspectives of Good Death and Quality of End-of-Life Care for Malignant Pleural Mesothelioma Patients: A Cross-Sectional Study" Journal of Clinical Medicine 11, no. 9: 2541. https://doi.org/10.3390/jcm11092541
APA StyleNagamatsu, Y., Sakyo, Y., Barroga, E., Koni, R., Natori, Y., & Miyashita, M. (2022). Bereaved Family Members’ Perspectives of Good Death and Quality of End-of-Life Care for Malignant Pleural Mesothelioma Patients: A Cross-Sectional Study. Journal of Clinical Medicine, 11(9), 2541. https://doi.org/10.3390/jcm11092541







