Left Ventricular Assist Devices: A Primer for the Non-Mechanical Circulatory Support Provider
Abstract
:1. Introduction
2. The LVAD Patient Profile
3. Toward the Modern Continuous Flow Era
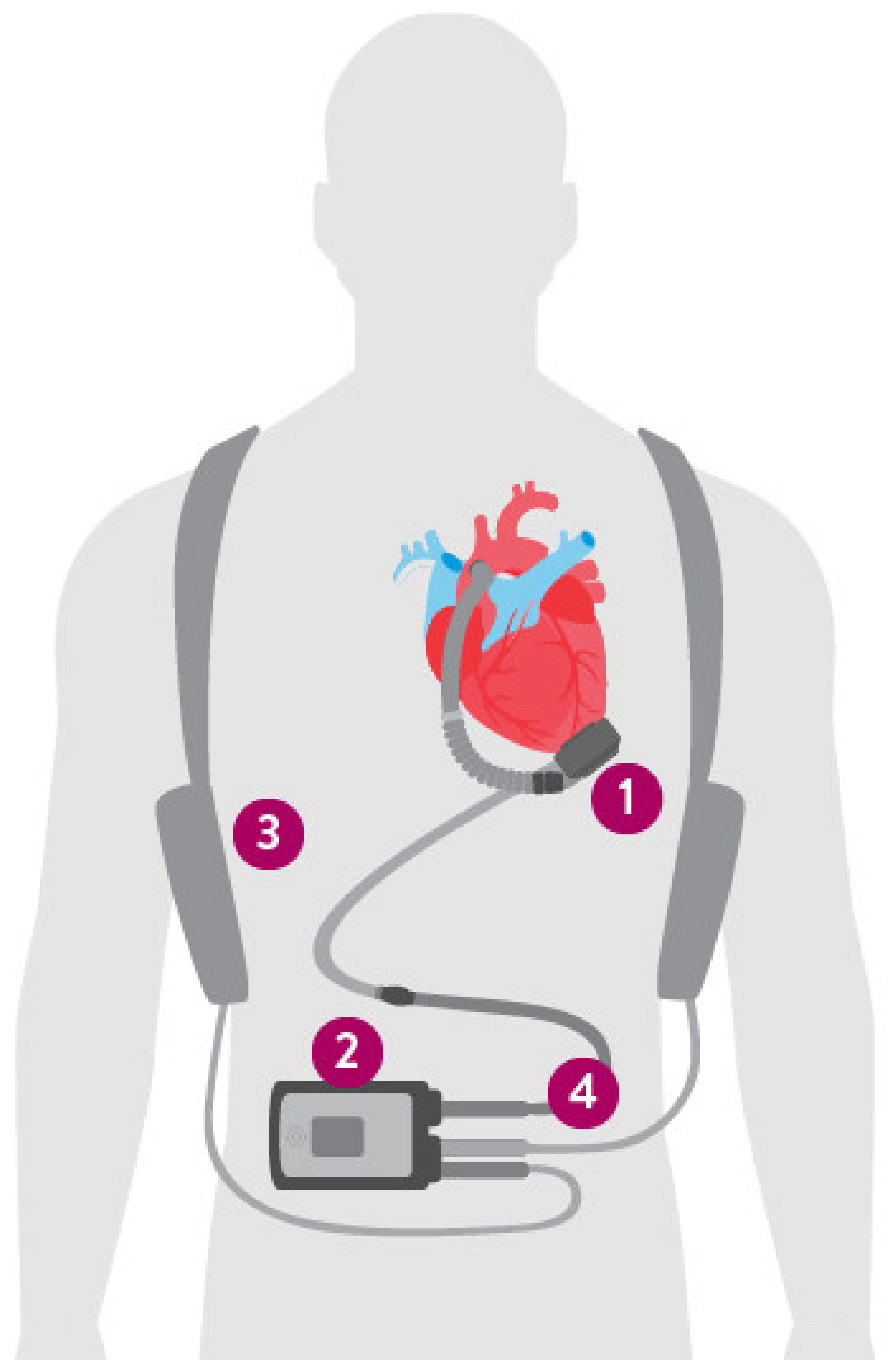
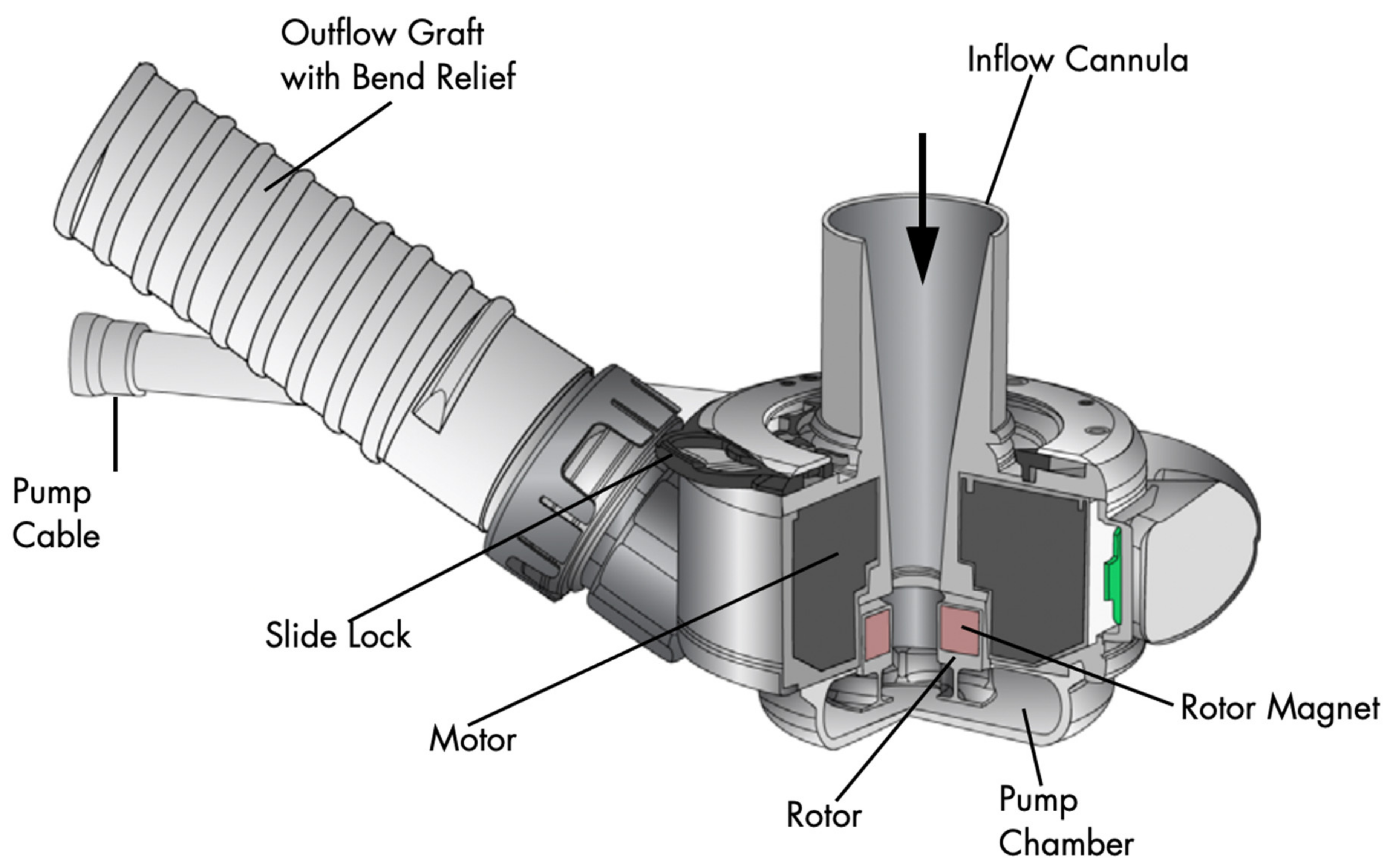
4. The Left Ventricular Assist Device Components
5. Clinical Scenarios and LVAD Emergencies
5.1. Hemodynamic Basics
5.2. Peri-Procedural Management
5.3. Acute Bleeding
5.4. Thrombosis
5.5. Infections
5.6. Right Ventricular Failure
5.7. Hypertension
5.8. The Non-Responsive LVAD Patient
6. Putting It Together: Approach to the LVAD Patient
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Ponikowski, P.; Dickstein, K.; McMurray, J.J.; Gavazzi, A.; Bergh, C.-H.; Fraser, A.G.; Jaarsma, T.; Pitsis, A.; Mohacsi, P.; et al. Advanced chronic heart failure: A position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2007, 9, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Butler, J.; Fonarow, G.C. Simultaneous or Rapid Sequence Initiation of Quadruple Medical Therapy for Heart Failure—Optimizing Therapy with the Need for Speed. JAMA Cardiol. 2021, 6, 743–744. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac Resynchronization in Chronic Heart Failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef] [Green Version]
- Hunt, S.A.; Baker, D.W.; Chin, M.H.; Cinquegrani, M.P.; Feldmanmd, A.M.; Francis, G.S.; Ganiats, T.G.; Goldstein, S.; Gregoratos, G.; Jessup, M.L.; et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). Circulation 2001, 104, 2996–3007. [Google Scholar] [CrossRef] [Green Version]
- Rose, E.A.; Gelijns, A.C.; Moskowitz, A.; Heitjan, D.F.; Stevenson, L.W.; Dembitsky, W.P.; Long, J.W.; Ascheim, D.D.; Tierney, A.R.; Levitan, R.G.; et al. Long-Term Use of a Left Ventricular Assist Device for End-Stage Heart Failure. N. Engl. J. Med. 2001, 345, 1435–1443. [Google Scholar] [CrossRef]
- NCA—Artificial Hearts and Related Devices, including Ventricular Assist Devices for Bridge-to-Transplant and Destination Therapy (CAG-00453N). Available online: https://www.cms.gov/medicare-coverage-database/view/nca.aspx?NCAId=298 (accessed on 23 April 2022).
- Molina, E.J.; Shah, P.; Kiernan, M.S.; Cornwell, W.K.; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann. Thorac. Surg. 2021, 111, 778–792. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar]
- Baumwol, J. “I Need Help”—A mnemonic to aid timely referral in advanced heart failure. J. Heart Lung Transplant. 2017, 36, 593–594. [Google Scholar] [CrossRef]
- Stevenson, L.W.; Pagani, F.; Young, J.B.; Jessup, M.; Miller, L.; Kormos, R.L.; Naftel, D.C.; Ulisney, K.; Desvigne-Nickens, P.; Kirklin, J.K. INTERMACS Profiles of Advanced Heart Failure: The Current Picture. J. Heart Lung Transplant. 2009, 28, 535–541. [Google Scholar] [CrossRef]
- Alba, A.C.; Rao, V.; Ivanov, J.; Ross, H.J.; Delgado, D.H. Usefulness of the INTERMACS Scale to Predict Outcomes after Mechanical Assist Device Implantation. J. Heart Lung Transplant. 2009, 28, 827–833. [Google Scholar] [CrossRef]
- Glynn, P.; Lloyd-Jones, D.M.; Feinstein, M.J.; Carnethon, M.; Khan, S.S. Disparities in Cardiovascular Mortality Related to Heart Failure in the United States. J. Am. Coll. Cardiol. 2019, 73, 2354–2355. [Google Scholar] [CrossRef]
- Nayak, A.; Hicks, A.J.; Morris, A.A. Understanding the Complexity of Heart Failure Risk and Treatment in Black Patients. Circ. Heart Fail. 2020, 13, e007264. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Hellkamp, A.S.; Hernandez, A.F.; Fonarow, G.C.; Thomas, K.L.; Al-Khalidi, H.R.; Heidenreich, P.A.; Hammill, S.; Yancy, C.; Peterson, E.D.; et al. Trends in Use of Implantable Cardioverter-Defibrillator Therapy among Patients Hospitalized for Heart Failure: Have the Previously Observed Sex and Racial Disparities Changed over Time? Circulation 2012, 125, 1094–1101. [Google Scholar] [CrossRef]
- Mwansa, H.; Lewsey, S.; Mazimba, S.; Breathett, K. Racial/Ethnic and Gender Disparities in Heart Failure with Reduced Ejection Fraction. Curr. Heart Fail. Rep. 2021, 18, 41–51. [Google Scholar] [CrossRef]
- DeBakey, M.E. Left ventricular bypass pump for cardiac assistance: Clinical experience. Am. J. Cardiol. 1971, 27, 3–11. [Google Scholar] [CrossRef]
- Gemmato, C.J.; Forrester, M.D.; Myers, T.J.; Frazier, O.H.; Cooley, D.A. Thirty-five years of mechanical circulatory support at the Texas Heart Institute: An updated overview. Tex. Heart Inst. J. 2005, 32, 168–177. [Google Scholar]
- Pennington, D.G.; McBride, L.R.; Peigh, P.S.; Miller, L.W.; Swartz, M.T. Eight years’ experience with bridging to cardiac transplantation. J. Thorac. Cardiovasc. Surg. 1994, 107, 472–480. [Google Scholar] [CrossRef]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M.; Long, J.W.; et al. Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist Device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef] [Green Version]
- Sayer, G.; Naka, Y.; Jorde, U.P. Ventricular Assist Device Therapy. Cardiovasc. Ther. 2009, 27, 140–150. [Google Scholar] [CrossRef]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device—Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.G.; Pagani, F.D.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Boyce, S.W.; Najjar, S.S.; Jeevanandam, V.; Anderson, A.S.; et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N. Engl. J. Med. 2017, 376, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Health C for D and R. Stop New Implants of the Medtronic HVAD System—Letter to Health Care Providers. FDA. Published online 12 August 2021. Available online: https://www.fda.gov/medical-devices/letters-health-care-providers/stop-new-implants-medtronic-hvad-system-letter-health-care-providers (accessed on 4 March 2022).
- HeartMate 3 LVAD|Abbott. Available online: https://www.cardiovascular.abbott/us/en/hcp/products/heart-failure/left-ventricular-assist-devices/heartmate-3/about.html (accessed on 8 March 2022).
- Burkhoff, D.; Sayer, G.; Doshi, D.; Uriel, N. Hemodynamics of Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2015, 66, 2663–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, D.; Pamboukian, S.V.; Teuteberg, J.J.; Birks, E.; Lietz, K.; Moore, S.A.; Morgan, J.A.; Arabia, F.; Bauman, M.E.; Buchholz, H.W.; et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: Executive summary. J. Heart Lung Transplant. 2013, 32, 157–187. [Google Scholar] [CrossRef]
- The ARIES HeartMate 3 Pump IDE Study—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04069156 (accessed on 2 April 2022).
- Potapov, E.V.; Antonides, C.; Crespo-Leiro, M.G.; Combes, A.; Färber, G.; Hannan, M.M.; Kukucka, M.; De Jonge, N.; Loforte, A.; Lund, L.H.; et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur. J. Cardio-Thorac. Surg. 2019, 56, 230–270. [Google Scholar] [CrossRef]
- Dalia, A.A.; Cronin, B.; Stone, M.E.; Turner, K.; Hargrave, J.; Melo, M.F.V.; Essandoh, M. Anesthetic Management of Patients with Continuous-Flow Left Ventricular Assist Devices Undergoing Noncardiac Surgery: An Update for Anesthesiologists. J. Cardiothorac. Vasc. Anesth. 2018, 32, 1001–1012. [Google Scholar] [CrossRef]
- Mehra, M.R. The burden of haemocompatibility with left ventricular assist systems: A complex weave. Eur. Heart J. 2017, 40, 673–677. [Google Scholar] [CrossRef]
- Netuka, I.; Kvasnička, T.; Kvasnička, J.; Hrachovinová, I.; Ivak, P.; Mareček, F.; Bílková, J.; Malikova, I.; Jančová, M.; Malý, J.; et al. Evaluation of von Willebrand factor with a fully magnetically levitated centrifugal continuous-flow left ventricular assist device in advanced heart failure. J. Heart Lung Transplant. 2016, 35, 860–867. [Google Scholar] [CrossRef] [Green Version]
- Heilmann, C.; Trummer, G.; Beyersdorf, F.; Brehm, K.; Berchtold-Herz, M.; Schelling, J.; Geisen, U.; Zieger, B. Acquired Von Willebrand syndrome in patients on long-term support with HeartMate II. Eur. J. Cardio-Thorac. Surg. 2016, 51, 587–590. [Google Scholar] [CrossRef]
- Crow, S.; Chen, D.; Milano, C.; Thomas, W.; Joyce, L.; Piacentino, V.; Sharma, R.; Wu, J.; Arepally, G.; Bowles, D.; et al. Acquired von Willebrand Syndrome in Continuous-Flow Ventricular Assist Device Recipients. Ann. Thorac. Surg. 2010, 90, 1263–1269. [Google Scholar] [CrossRef]
- Pagani, F.D.; Miller, L.W.; Russell, S.D.; Aaronson, K.D.; John, R.; Boyle, A.J.; Conte, J.V.; Bogaev, R.C.; MacGillivray, T.E.; Naka, Y.; et al. Extended Mechanical Circulatory Support with a Continuous-Flow Rotary Left Ventricular Assist Device. J. Am. Coll. Cardiol. 2009, 54, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Teuteberg, J.J.; Slaughter, M.S.; Rogers, J.G.; McGee, E.C.; Pagani, F.; Gordon, R.; Rame, E.; Acker, M.; Kormos, R.L.; Salerno, C.; et al. The HVAD Left Ventricular Assist Device. JACC Heart Fail. 2015, 3, 818–828. [Google Scholar] [CrossRef]
- Vidula, H.; Altintas, O.; McNitt, S.; DeVore, A.D.; Birati, E.Y.; Genuardi, M.V.; Sheikh, F.H.; Polonsky, B.; Alexis, J.D.; Gosev, I.; et al. Low Blood Pressure Threshold for Adverse Outcomes During Left Ventricular Assist Device Support. Am. J. Cardiol. 2022, 169, 78–85. [Google Scholar] [CrossRef]
- Barac, Y.; Schroder, J.N.; Daneshmand, M.A.; Patel, C.B.; Milano, C.A. Heartmate III Replacement for Recurring Left Ventricular Assist Device Pump Thrombosis. ASAIO J. 2018, 64, 424–426. [Google Scholar] [CrossRef]
- Jung, M.H.; Gustafsson, F.; Houston, B.; Russell, S.D. Ramp Study Hemodynamics, Functional Capacity, and Outcome in Heart Failure Patients with Continuous-Flow Left Ventricular Assist Devices. ASAIO J. 2016, 62, 442–446. [Google Scholar] [CrossRef]
- Posada, J.G.D.; Moayedi, Y.; Alhussein, M.; Rodger, M.; Alvarez, J.; Wintersperger, B.J.; Ross, H.J.; Butany, J.; Billia, F.; Rao, V. Outflow Graft Occlusion of the HeartMate 3 Left Ventricular Assist Device. Circ. Heart Fail. 2017, 10, e004275. [Google Scholar] [CrossRef]
- Mehra, M.R.; Salerno, C.; Naka, Y.; Uriel, N.; Cleveland, J.C.; Horstmanshof, D.; Goldstein, D.J. A tale of the twist in the outflow graft: An analysis from the MOMENTUM 3 trial. J. Heart Lung Transplant. 2018, 37, 1281–1284. [Google Scholar] [CrossRef]
- Givertz, M.M.; DeFilippis, E.M.; Colvin, M.; Darling, C.E.; Elliott, T.; Hamad, E.; Hiestand, B.C.; Martindale, J.L.; Pinney, S.P.; Shah, K.B.; et al. HFSA/SAEM/ISHLT Clinical Expert Consensus Document on the Emergency Management of Patients with Ventricular Assist Devices. J. Card. Fail. 2019, 25, 494–515. [Google Scholar] [CrossRef]
- Kusne, S.; Mooney, M.; Danziger-Isakov, L.; Kaan, A.; Lund, L.H.; Lyster, H.; Wieselthaler, G.; Aslam, S.; Cagliostro, B.; Chen, J.; et al. An ISHLT consensus document for prevention and management strategies for mechanical circulatory support infection. J. Heart Lung Transplant. 2017, 36, 1137–1153. [Google Scholar] [CrossRef]
- Kormos, R.L.; Teuteberg, J.J.; Pagani, F.; Russell, S.D.; John, R.; Miller, L.W.; Massey, T.; Milano, C.A.; Moazami, N.; Sundareswaran, K.S.; et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: Incidence, risk factors, and effect on outcomes. J. Thorac. Cardiovasc. Surg. 2010, 139, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Drakos, S.G.; Janicki, L.; Horne, B.D.; Kfoury, A.G.; Reid, B.B.; Clayson, S.; Horton, K.; Haddad, F.; Li, D.Y.; Renlund, D.G.; et al. Risk Factors Predictive of Right Ventricular Failure after Left Ventricular Assist Device Implantation. Am. J. Cardiol. 2010, 105, 1030–1035. [Google Scholar] [CrossRef]
- Grandin, E.W.; Zamani, P.; Mazurek, J.A.; Troutman, G.; Birati, E.Y.; Vorovich, E.; Chirinos, J.A.; Tedford, R.J.; Margulies, K.B.; Atluri, P.; et al. Right ventricular response to pulsatile load is associated with early right heart failure and mortality after left ventricular assist device. J. Heart Lung Transplant. 2016, 36, 97–105. [Google Scholar] [CrossRef]
- LaRue, S.J.; Raymer, D.S.; Pierce, B.R.; Nassif, M.E.; Sparrow, C.T.; Vader, J.M. Clinical outcomes associated with INTERMACS-defined right heart failure after left ventricular assist device implantation. J. Heart Lung Transplant. 2016, 36, 475–477. [Google Scholar] [CrossRef]
- Grandin, E.W.; Troutman, G.S.; Gulati, A.A.; Zamani, P.; Mazurek, J.A.; Atluri, P.; Rame, J.E. A Modified Grading System for Early Right Heart Failure Matches Functional Outcomes and Survival after Left Ventricular Assist Devices. ASAIO J. 2020, 67, 185–191. [Google Scholar] [CrossRef]
- Nassif, M.E.; Tibrewala, A.; Raymer, D.S.; Andruska, A.; Novak, E.; Vader, J.M.; Itoh, A.; Silvestry, S.C.; Ewald, G.A.; LaRue, S.J. Systolic blood pressure on discharge after left ventricular assist device insertion is associated with subsequent stroke. J. Heart Lung Transplant. 2014, 34, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Saeed, O.; Jermyn, R.; Kargoli, F.; Madan, S.; Mannem, S.; Gunda, S.; Nucci, C.; Farooqui, S.; Hassan, S.; Mclarty, A.; et al. Blood Pressure and Adverse Events during Continuous Flow Left Ventricular Assist Device Support. Circ. Heart Fail. 2015, 8, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Validity and Reliability of a Novel Slow Cuff-Deflation System for Noninvasive Blood Pressure Monitoring in Patients with Continuous-Flow Left Ventricular Assist Device—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23811966/ (accessed on 2 April 2022).
- Garg, S.; Ayers, C.R.; Fitzsimmons, C.; Meyer, D.; Peltz, M.; Bethea, B.; Cornwell, W.; Araj, F.; Thibodeau, J.; Drazner, M.H. In-Hospital Cardiopulmonary Arrests in Patients with Left Ventricular Assist Devices. J. Card. Fail. 2014, 20, 899–904. [Google Scholar] [CrossRef]
- Peberdy, M.A.; Gluck, J.A.; Ornato, J.P.; Bermudez, C.A.; Griffin, R.E.; Kasirajan, V.; Kerber, R.E.; Lewis, E.F.; Link, M.S.; Miller, C.; et al. Cardiopulmonary Resuscitation in Adults and Children with Mechanical Circulatory Support: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e1115–e1134. [Google Scholar] [CrossRef] [Green Version]
- Characteristics and Outcomes of COVID-19 in Patients on Left Ventricular Assist Device Support—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33813838/ (accessed on 2 April 2022).
- Teuteberg, J.J.; Cleveland, J.C.; Cowger, J.; Higgins, R.S.; Goldstein, D.J.; Keebler, M.; Kirklin, J.K.; Myers, S.L.; Salerno, C.T.; Stehlik, J.; et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann. Thorac. Surg. 2020, 109, 649–660. [Google Scholar] [CrossRef] [Green Version]

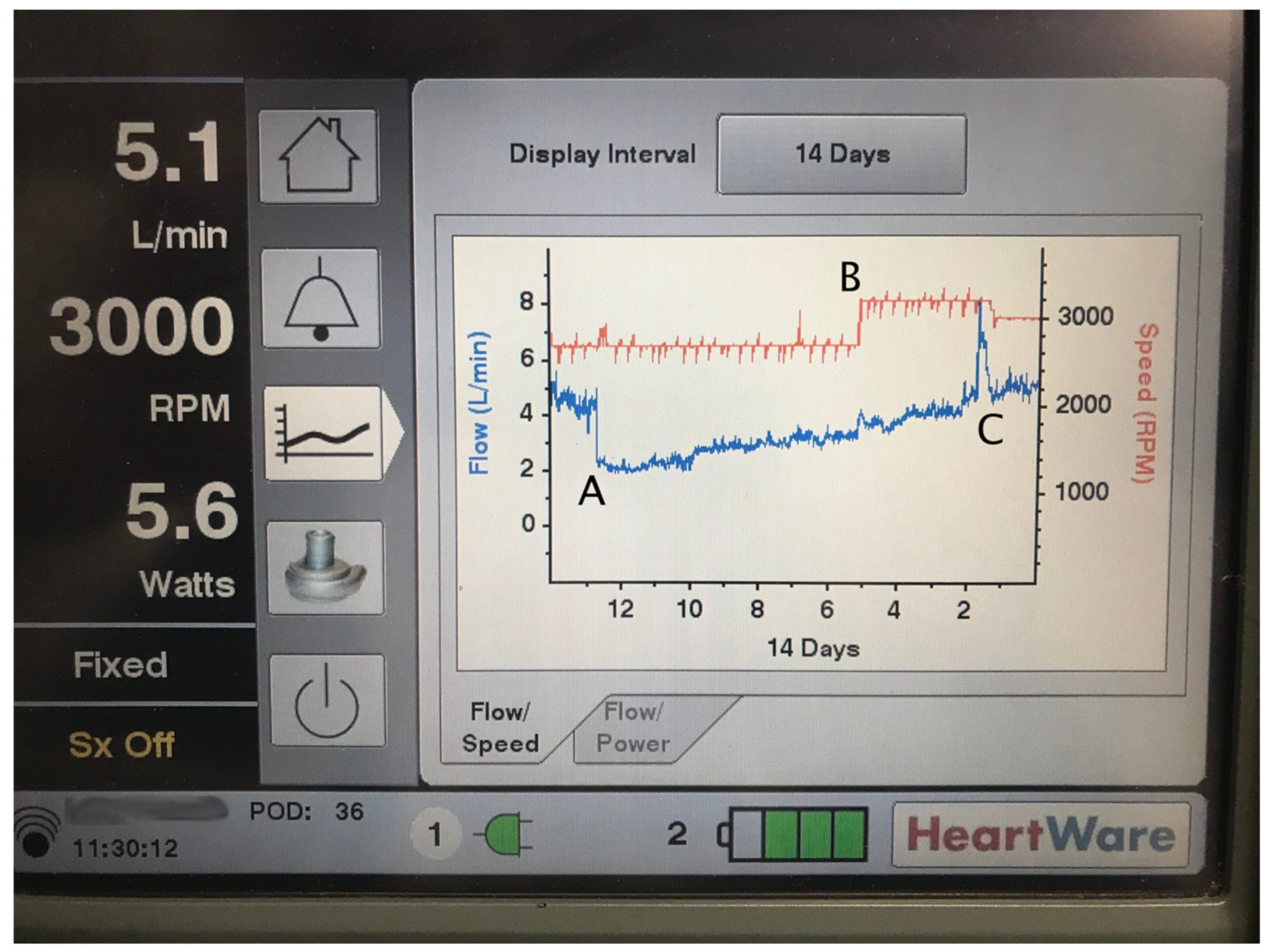
| Condition | Flow | Pulsatility/PI | MAP | Comments |
|---|---|---|---|---|
| Hypovolumia | Decreased LV filling leads to decreased LV contractility and lower pulsatility. Extreme hypovolemia leads to suction, and sudden increase in pulsatility as flow intermittently drops to zero during suck-down. | |||
| mild | ⇔/⇓ | ⇓ | ⇔ | |
| severe (with suction) | ⇓ | ⇑⇑ | ⇔/⇓ | |
| Hypervolemia | Increased LV end diastolic pressure leads to increased LV contractility and pulsatility. Unchecked, however, the patient may progress to RV failure, increased LV-RV interdependence, impaired LV contractility and blood return. | |||
| mild | ⇔/⇑ | ⇑ | ⇔ | |
| severe (with RV failure) | ⇓ | ⇓ | ⇔/⇓ | |
| Hypertension | ⇓ | ⇑ | ⇑ | Increased afterload leads to substantial decrease in diastolic >> systolic flow, leading to larger pulsatility/PI. |
| Inflow/outflow obstruction | ⇓ | ⇑ | ⇔/⇓ | Creates a high afterload condition analogous to hypertension. |
| Rotor thrombus | “⇑⇑” | ⇔/⇓ | ⇔/⇓ | Increased power consumption from rotor weighted down by thrombus causes “power spikes” and high flow/power alarms. High flow is an artifact—effective flow is low. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troutman, G.S.; Genuardi, M.V. Left Ventricular Assist Devices: A Primer for the Non-Mechanical Circulatory Support Provider. J. Clin. Med. 2022, 11, 2575. https://doi.org/10.3390/jcm11092575
Troutman GS, Genuardi MV. Left Ventricular Assist Devices: A Primer for the Non-Mechanical Circulatory Support Provider. Journal of Clinical Medicine. 2022; 11(9):2575. https://doi.org/10.3390/jcm11092575
Chicago/Turabian StyleTroutman, Gregory S., and Michael V. Genuardi. 2022. "Left Ventricular Assist Devices: A Primer for the Non-Mechanical Circulatory Support Provider" Journal of Clinical Medicine 11, no. 9: 2575. https://doi.org/10.3390/jcm11092575
APA StyleTroutman, G. S., & Genuardi, M. V. (2022). Left Ventricular Assist Devices: A Primer for the Non-Mechanical Circulatory Support Provider. Journal of Clinical Medicine, 11(9), 2575. https://doi.org/10.3390/jcm11092575






