Efficacy and Safety of Naldemedine for Patients with Cancer with Opioid-Induced Constipation in Clinical Practice: A Real-World Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment
2.3. Assessment of Treatment Efficacy
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
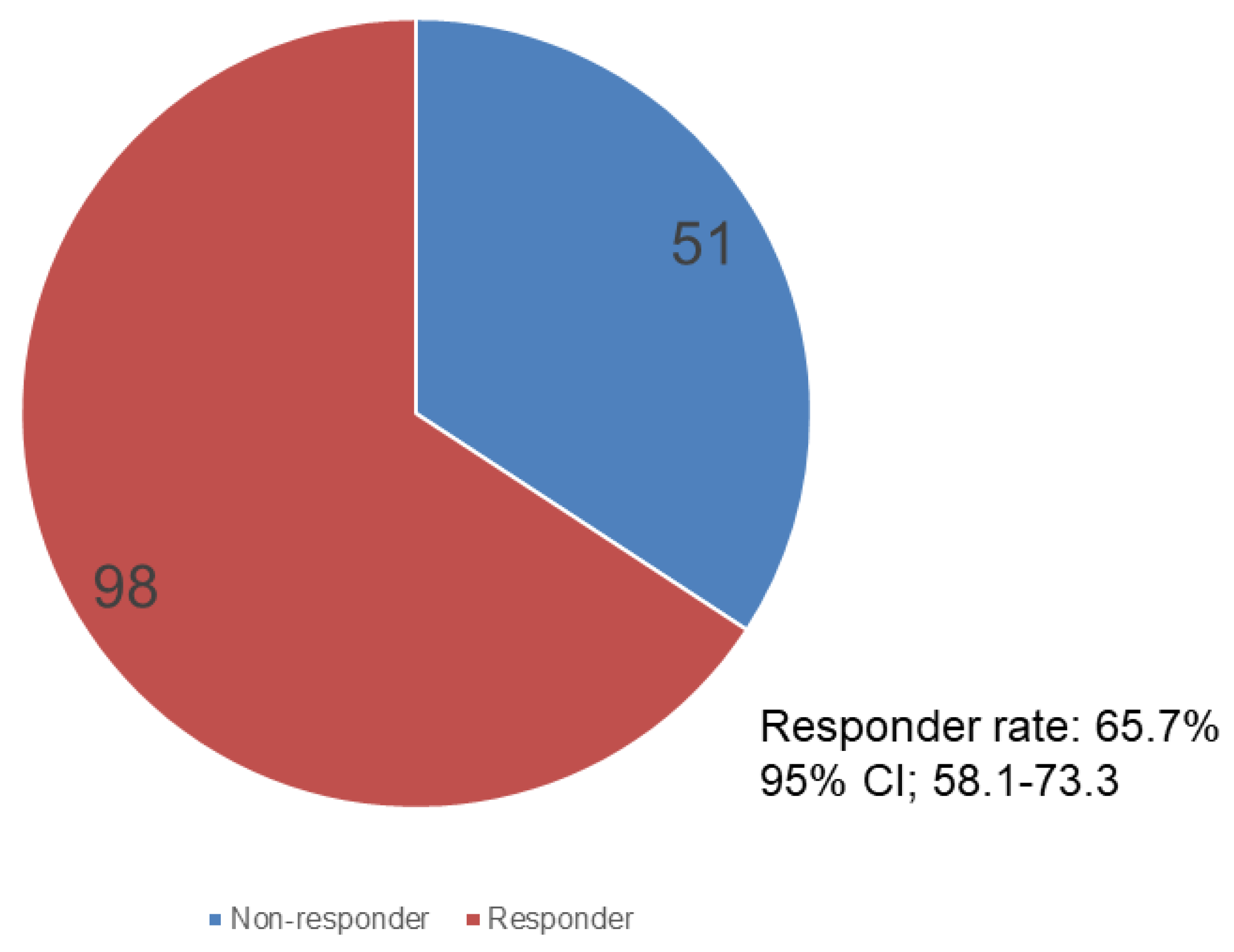
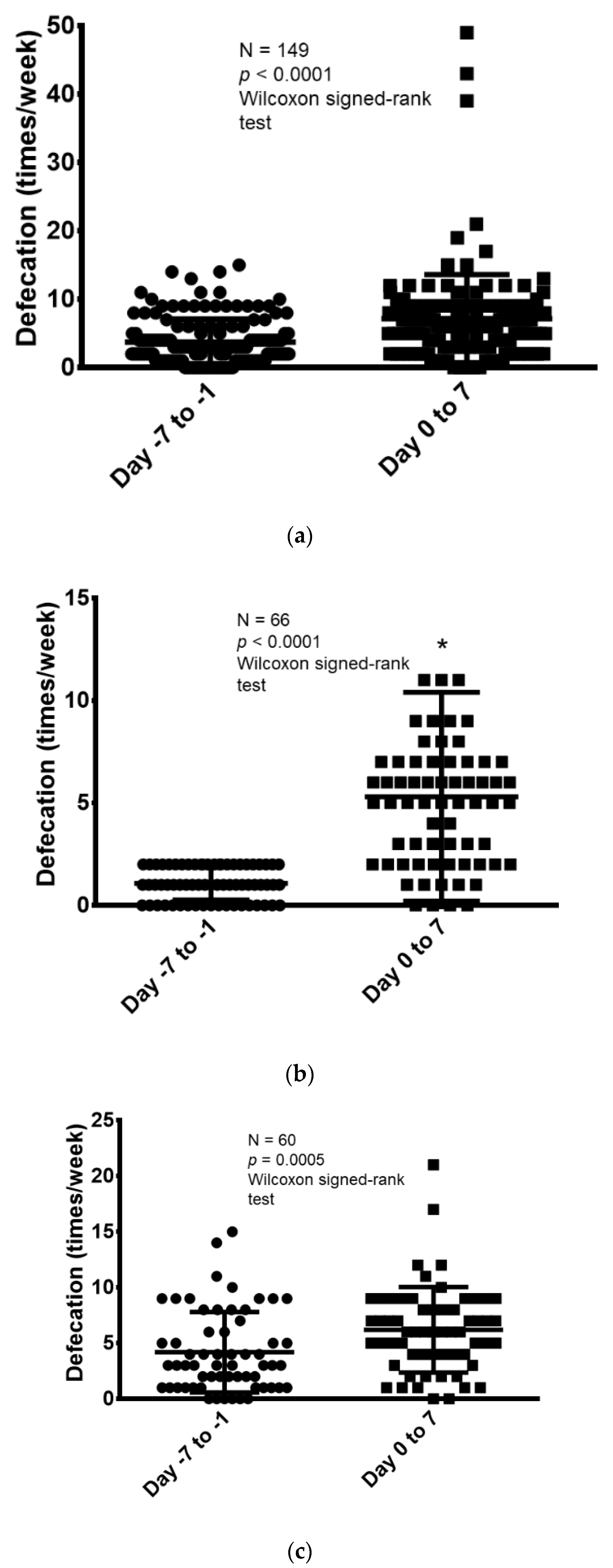
3.2. Treatment Efficacy and Safety
3.3. Clinical Factors Influencing Treatment Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campora, E.; Merlini, L.; Pace, M.; Bruzzone, M.; Luzzani, M.; Gottlieb, A.; Rosso, R. The Incidence of Narcotic-Induced Emesis. J. Pain Symptom Manag. 1991, 6, 428–430. [Google Scholar] [CrossRef]
- Hardy, J.; Daly, S.; McQuade, B.; Albertsson, M.; Chimontsi-Kypriou, V.; Stathopoulos, P.; Curtis, P.A. Double-Blind, Randomised, Parallel Group, Multinational, Multicentre Study Comparing a Single Dose of Ondansetron 24 mg p.o. with Placebo and Metoclopramide 10 mg t.d.s. p.o. in the Treatment of Opioid-Induced Nausea and Emesis in Cancer Patients. Support. Care Cancer 2002, 10, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [Green Version]
- Nosek, K.; Leppert, W.; Nosek, H.; Wordliczek, J.; Onichimowski, D. A Comparison of Oral Controlled-Release Morphine and Oxycodone with Transdermal Formulations of Buprenorphine and Fentanyl in the Treatment of Severe Pain in Cancer Patients. Drug Des. Devel. Ther. 2017, 11, 2409–2419. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Sawada, T.; Kawai, K.; Ishihara, Y. Pharmacological Profile of TAN-452, a Novel Peripherally Acting Opioid Receptor Antagonist for the Treatment of Opioid-Induced Bowel Syndromes. Life Sci. 2018, 215, 246–252. [Google Scholar] [CrossRef]
- Tokoro, A.; Imai, H.; Fumita, S.; Harada, T.; Noriyuki, T.; Gamoh, M.; Akashi, Y.; Sato, H.; Kizawa, Y. Incidence of Opioid-Induced Constipation in Japanese Patients with Cancer Pain: A Prospective Observational Cohort Study. Cancer Med. 2019, 8, 4883–4891. [Google Scholar] [CrossRef] [Green Version]
- Kanemasa, T.; Koike, K.; Takase, K.; Arai, T.; Nakamura, A.; Morioka, Y.; Hasegawa, M. Pharmacological Profile of Naldemedine, a Peripherally Acting µ-Opioid Receptor Antagonist: Comparison with Naloxone and Naloxegol. J. Pharmacol. Exp. Ther. 2020, 373, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Urits, I.; Patel, A.; Kiernan, H.C.; Clay, C.J.; Monteferrante, N.; Jung, J.W.; Berger, A.A.; Kassem, H.; Hasoon, J.; Kaye, A.D.; et al. Naldemedine for the Use of Management of Opioid Induced Constipation. Psychopharmacol. Bull. 2020, 50, 97–118. [Google Scholar]
- Blair, H.A. Naldemedine: A Review in Opioid-Induced Constipation. Drugs 2019, 79, 1241–1247. [Google Scholar] [CrossRef]
- Katakami, N.; Harada, T.; Murata, T.; Shinozaki, K.; Tsutsumi, M.; Yokota, T.; Arai, M.; Tada, Y.; Narabayashi, M.; Boku, N. Randomized Phase III and Extension Studies of Naldemedine in Patients with Opioid-Induced Constipation and Cancer. J. Clin. Oncol. 2017, 35, 3859–3866. [Google Scholar] [CrossRef]
- Katakami, N.; Harada, T.; Murata, T.; Shinozaki, K.; Tsutsumi, M.; Yokota, T.; Arai, M.; Tada, Y.; Narabayashi, M.; Boku, N. Randomized phase III and Extension Studies: Efficacy and Impacts on Quality of Life of Naldemedine in Subjects with Opioid-Induced Constipation and Cancer. Ann. Oncol. 2018, 29, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Nakazawa, M.; Honda, K.; Hashimoto, S. Post-Marketing Surveillance of the Safety and Effectiveness of Naldemedine in the Management of Opioid-Induced Constipation in Patients with Cancer Pain in Japan. Support. Care Cancer 2022, 30, 3943–3954. [Google Scholar] [CrossRef] [PubMed]
- Hiruta, E.; Fujita, Y.; Imai, H.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Kamiya, T.; Ito, M.; Takei, S.; Matsuura, M.; et al. Real-World Patient Characteristics and Treatment Patterns of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Cancer: A Multicenter Retrospective Chart Review Study. Medicina 2021, 57, 1233. [Google Scholar] [CrossRef] [PubMed]
- Kubota, R.; Fukumura, K.; Wajima, T. Population Pharmacokinetics and Exposure–Response Relationships of Naldemedine. Pharm. Res. 2018, 35, 225. [Google Scholar] [CrossRef] [PubMed]
- Osaka, I.; Ishiki, H.; Yokota, T.; Tada, Y.; Sato, H.; Okamoto, M.; Satomi, E. Safety and Efficacy of Naldemedine in Cancer Patients with Opioid-Induced Constipation: A Pooled, Subgroup Analysis of Two Randomised Controlled Studies. ESMO Open 2019, 4, e000527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokunaga, K.; Matsuzawa, Y.; Kotani, K.; Keno, Y.; Kobatake, T.; Fujioka, S.; Tarui, S. Ideal Body Weight Estimated from the Body Mass Index with the Lowest Morbidity. Int. J. Obes. 1991, 15, 1–5. [Google Scholar]
- Lemaire, A.; Pointreau, Y.; Narciso, B.; Piloquet, F.X.; Braniste, V.; Sabaté, J.M. Effectiveness of Naloxegol in Patients with Cancer Pain Suffering from Opioid-Induced Constipation. Support. Care Cancer 2021, 29, 7577–7586. [Google Scholar] [CrossRef]
- Wild, J.; Webster, L.; Yamada, T.; Hale, M. Safety and Efficacy of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Chronic Non-Cancer Pain Receiving Opioid Therapy: A Subgroup Analysis of Patients ≥ 65 Years of Age >/= 65 Years of Age. Drugs Aging 2020, 37, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Katakami, N.; Oda, K.; Tauchi, K.; Nakata, K.; Shinozaki, K.; Yokota, T.; Suzuki, Y.; Narabayashi, M.; Boku, N. Phase IIb, Randomized, Double-Blind, Placebo-Controlled Study of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Cancer. J. Clin. Oncol. 2017, 35, 1921–1928. [Google Scholar] [CrossRef]
- Müller-Lissner, S.; Bassotti, G.; Coffin, B.; Drewes, A.M.; Breivik, H.; Eisenberg, E.; Emmanuel, A.; Laroche, F.; Meissner, W.; Morlion, B. Opioid-Induced Constipation and Bowel Dysfunction: A Clinical Guideline. Pain Med. 2017, 18, 1837–1863. [Google Scholar] [CrossRef] [Green Version]
- Rentz, A.M.; Yu, R.; Müller-Lissner, S.; Leyendecker, P. Validation of the Bowel Function Index to Detect Clinically Meaningful Changes in Opioid-Induced Constipation. J. Med. Econ. 2009, 12, 371–383. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | N = 149 (%) |
|---|---|
| Sex | |
| Male | 89 (59.7) |
| Female | 60 (40.3) |
| Median age at treatment (years) (range) | 72 (38–96) |
| Performance Status (PS) | |
| 0/1/2/3/4 | 15/25/38/50/21 (10.1)/(16.8)/(25.5)/(33.6)/(14.1) |
| Primary tumor | |
| Thoracic cancer | 37 (24.9) |
| Liver, biliary, and pancreatic cancer | 34 (22.8) |
| Gastrointestinal cancer | 33 (22.1) |
| Urinary tract, renal cell, and prostate cancer | 17 (11.4) |
| Hematologic cancer | 10 (6.7) |
| Gynecological cancer | 8 (5.4) |
| Head and neck cancer | 4 (2.7) |
| Breast cancer | 4 (2.7) |
| Others | 2 (1.3) |
| Therapy before and during naldemedine administration *, ** | |
| Chemotherapy | 40 (26.8) |
| Radiotherapy | 19 (12.6) |
| Chemoradiotherapy | 3 (2.0) |
| Surgery | 1 (0.6) |
| Best supportive care alone | 95 (63.8) |
| Central nervous system metastases | |
| Yes | 14 (9.4) |
| No | 135 (90.6) |
| Cancerous peritonitis | |
| Yes | 19 (12.8) |
| No | 130 (87.2) |
| Gastrointestinal obstruction | |
| Yes | 0 (0) |
| No | 149 (100) |
| History of abdominal surgery before starting naldemedine | |
| Yes | 52 (34.9) |
| No | 97 (65.1) |
| History of radiation to the abdomen and pelvic region before starting naldemedine | |
| Yes | 22 (14.8) |
| No | 127 (85.2) |
| Presence of diabetes mellitus | |
| Yes | 21 (14.1) |
| No | 128 (85.9) |
| Body mass index (BMI) (kg/m2) | |
| <22/≥22 | 106/43 (71.1)/(28.9) |
| Median BMI (range) | 20.4 (13.7–34.8) |
| Discontinuation within 7 days | |
| Yes | 19 (12.8) |
| No | 130 (87.2) |
| Use of laxatives before starting naldemedine administration | |
| Yes | 123 (82.6) |
| No | 26 (17.4) |
| Use of laxatives after starting naldemedine administration | |
| Yes | 118 (79.2) |
| No | 31 (20.8) |
| Regular use of antiemetic medication after initiation of naldemedine | |
| Yes | 44 (29.5) |
| No or unknown | 105 (70.5) |
| Irregular use of antiemetic agents after starting naldemedine | |
| Yes | 25 (16.8) |
| No or unknown | 124 (83.2) |
| Survival status at data-cutoff date | |
| Dead | 117 (78.5) |
| Alive | 32 (21.5) |
| Period from naldemedine initiation to death | |
| Median period (days) (range) | 35 (7–790) |
| Parameter | N = 149 (%) |
|---|---|
| Daily dose of opioids | |
| <20 mg | 52 (34.9) |
| 20–49 | 53 (35.6) |
| 50–99 | 19 (12.7) |
| ≥100 | 25 (16.8) |
| Regular use of opioids | |
| Oxycodone | 87 (58.4) |
| Morphine | 15 (10.0) |
| Fentanyl | 32 (21.5) |
| Hydromorphone | 11 (7.4) |
| Others | 3 (2.0) |
| No regular use | 1 (0.7) |
| Days from first opioid administration to initial naldemedine use | |
| <4 | 22 (14.8) |
| 4–7 | 15 (10.0) |
| 8–29 | 69 (46.3) |
| 30–99 | 26 (17.5) |
| ≥100 | 17 (11.4) |
| Concomitant laxatives ** | |
| Magnesium oxide | 93 (62.4) |
| Sennoside | 37 (24.8) |
| Bisacodyl | 15 (10.1) |
| Lubiprostone | 22 (14.8) |
| Sodium picosulfate hydrate | 15 (10.1) |
| Sodium bicarbonate, sodium dihydrogen phosphate anhydrous suppository | 10 (6.8) |
| Others | 9 (0.6) |
| Concomitant antiemetic (regular and abbreviated use) ** | |
| Metoclopramide | 22 (14.8) |
| Domperidone | 12 (0.8) |
| Prochlorperazine | 20 (13.4) |
| Olanzapine | 11 (7.4) |
| Others | 4 (2.7) |
| No use | 31 (20.8) |
| Adverse Events * | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Diarrhea | 41 | 11 | 5 | 0 |
| Abdominal pain | 3 | 1 | 0 | - |
| Nausea | 9 | 4 | 1 | - |
| Anorexia | 16 | 4 | 1 | 0 |
| Vomiting | 2 | 2 | 1 | 0 |
| Fatigue | 14 | 1 | - | - |
| Variables | OR | 95% CI | p-Value |
|---|---|---|---|
| Sex | |||
| Male/female | 0.92 | 0.44–1.92 | 0.82 |
| Age | |||
| <75/≥75 | 1.35 | 0.63–2.956 | 0.42 |
| PS | |||
| 0–2/≥3 | 0.96 | 0.46–1.98 | 0.91 |
| BMI | |||
| <22/≥22 | 0.77 | 0.35–1.69 | 0.51 |
| Use of concomitant laxatives before starting naldemedine | |||
| Yes/no | 1.58 | 0.59–4.76 | 0.37 |
| Regular dose of opioids (morphine equivalent) | |||
| <30/≥30 | 2.08 | 1.01–4.32 | 0.042 |
| History of chemotherapy within 21 days prior to naldemedine administration | |||
| Yes/no | 0.92 | 0.40–2.05 | 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiba, H.; Imai, H.; Fujita, Y.; Hiruta, E.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Kamiya, T.; Ito, M.; Takei, S.; et al. Efficacy and Safety of Naldemedine for Patients with Cancer with Opioid-Induced Constipation in Clinical Practice: A Real-World Retrospective Study. J. Clin. Med. 2022, 11, 2672. https://doi.org/10.3390/jcm11092672
Nishiba H, Imai H, Fujita Y, Hiruta E, Masuno T, Yamazaki S, Tanaka H, Kamiya T, Ito M, Takei S, et al. Efficacy and Safety of Naldemedine for Patients with Cancer with Opioid-Induced Constipation in Clinical Practice: A Real-World Retrospective Study. Journal of Clinical Medicine. 2022; 11(9):2672. https://doi.org/10.3390/jcm11092672
Chicago/Turabian StyleNishiba, Hiromi, Hisao Imai, Yukiyoshi Fujita, Eriko Hiruta, Takashi Masuno, Shigeki Yamazaki, Hajime Tanaka, Teruhiko Kamiya, Masako Ito, Satoshi Takei, and et al. 2022. "Efficacy and Safety of Naldemedine for Patients with Cancer with Opioid-Induced Constipation in Clinical Practice: A Real-World Retrospective Study" Journal of Clinical Medicine 11, no. 9: 2672. https://doi.org/10.3390/jcm11092672
APA StyleNishiba, H., Imai, H., Fujita, Y., Hiruta, E., Masuno, T., Yamazaki, S., Tanaka, H., Kamiya, T., Ito, M., Takei, S., Matsuura, M., Mogi, J., Minato, K., & Obayashi, K. (2022). Efficacy and Safety of Naldemedine for Patients with Cancer with Opioid-Induced Constipation in Clinical Practice: A Real-World Retrospective Study. Journal of Clinical Medicine, 11(9), 2672. https://doi.org/10.3390/jcm11092672






