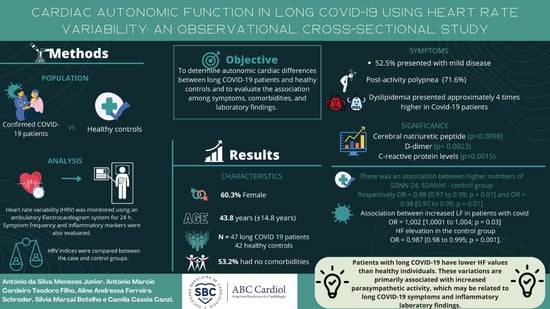
Cardiac Autonomic Function in Long COVID-19 Using Heart Rate Variability: An Observational Cross-Sectional Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design and Setting
2.2. Participants
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.2.3. Procedures
2.3. Heart Rate Variability Assessment
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Di Cristanziano, V.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 5 December 2022).
- Grubb, B.P. Autonomic Dysfunction as a Consequence of COVID-19 Infection. J. Am. Coll. Cardiol. 2022, 79, 2331–2332. [Google Scholar] [CrossRef] [PubMed]
- Barizien, N.; Le Guen, M.; Russel, S.; Touche, P.; Huang, F.; Vallée, A. Clinical characterization of dysautonomia in long COVID-19 patients. Sci. Rep. 2021, 11, 14042. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, W.C.; Fedewa, M.V.; MacDonald, H.V.; Holmes, C.J.; Cicone, Z.S.; Plews, D.J.; Esco, M.R. The Accuracy of Acquiring Heart Rate Variability from Portable Devices: A Systematic Review and Meta-Analysis. Sport. Med. 2019, 49, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Romero-Duarte, Á.; Rivera-Izquierdo, M.; de Alba, I.G.-F.; Pérez-Contreras, M.; Fernández-Martínez, N.F.; Ruiz-Montero, R.; Serrano-Ortiz, Á.; González-Serna, R.O.; Salcedo-Leal, I.; Jiménez-Mejías, E.; et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: The ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021, 19, 129. [Google Scholar] [CrossRef]
- Eshak, N.; Abdelnabi, M.; Ball, S.; Elgwairi, E.; Creed, K.; Test, V.; Nugent, K. Dysautonomia: An overlooked neurological manifestation in a critically ill COVID-19 patient. Am. J. Med. Sci. 2020, 360, 427–429. [Google Scholar] [CrossRef]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef]
- Stella, A.B.; Furlanis, G.; Frezza, N.A.; Valentinotti, R.; Ajcevic, M.; Manganotti, P. Autonomic dysfunction in post-COVID patients with and witfhout neurological symptoms: A prospective multidomain observational study. J. Neurol. 2022, 269, 587–596. [Google Scholar] [CrossRef]
- Kanjwal, K.; Jamal, S.; Kichloo, A.; Grubb, B.P. New-onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. J. Innov. Card. Rhythm Manag. 2020, 11, 4302–4304. [Google Scholar] [CrossRef]
- Nunan, D.; Sandercock, G.R.H.; Brodie, D.A. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin. Electrophysiol. 2010, 33, 1407–1417. [Google Scholar] [CrossRef]
- Jouven, X.; Empana, J.-P.; Schwartz, P.J.; Desnos, M.; Courbon, D.; Ducimetière, P. Heart-rate profile during exercise as a predictor of sudden death. N. Engl. J. Med. 2005, 352, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Ladlow, P.; O’Sullivan, O.; Houston, A.; Barker-Davies, R.; May, S.; Mills, D.; Dewson, D.; Chamley, R.; Naylor, J.; Mulae, J.; et al. Dysautonomia following COVID-19 is not associated with subjective limitations or symptoms but is associated with objective functional limitations. Heart Rhythm. 2022, 19, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Agnello, L.; Ciaccio, A.M. Biomarkers for prognosis and treatment response in COVID-19 patients. Ann. Lab. Med. 2021, 41, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart rate variability and cardiac vagal tone in psychophysiological research—Recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Shinu, P.; Morsy, M.A.; Deb, P.K.; Nair, A.B.; Goyal, M.; Shah, J.; Kotta, S. SARS CoV-2 organotropism associated pathogenic relationship of gut-brain axis and illness. Front. Mol. Biosci. 2020, 7, 606779. [Google Scholar] [CrossRef] [PubMed]
- Swai, J.; Hu, Z.; Zhao, X.; Rugambwa, T.; Ming, G. Heart rate and heart rate variability comparison between postural orthostatic tachycardia syndrome versus healthy participants; a systematic review and meta-analysis. BMC Cardiovasc. Disord. 2019, 19, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.A.; Lipsitz, L.A. Heart rate variability standards. Circulation 1997, 95, 280–281. [Google Scholar]
- Tan, C.; Huang, Y.; Shi, F.; Tan, K.; Ma, Q.; Chen, Y.; Jiang, X.; Li, X. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J. Med. Virol. 2020, 92, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Hasty, F.; García, G.; Dávila, C.H.; Wittels, S.H.; Hendricks, S.; Chong, S. Heart rate variability as a possible predictive marker for acute inflammatory response in COVID-19 patients. Mil. Med. 2020, 186, e34–e38. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Laghi-Pasini, F.; Boutjdir, M.; Capecchi, P.L. Cardioimmunology of arrhythmias: The role of autoimmune and inflammatory cardiac channelopathies. Nat. Rev. Immunol. 2019, 19, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; Garg, R.; Ritch, A.; Sarkar, P. Postural orthostatic tachycardia syndrome. Postgrad. Med. J. 2007, 83, 478–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, S.-T.; Yu, W.-C.; Mok, N.-S.; Tsui, P.-T.; Tong, W.-L.; Stella, W.C. Tachycardia amongst subjects recovering from severe acute respiratory syndrome (SARS). Int. J. Cardiol. 2005, 100, 167–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaliyaperumal, D.; Rk, K.; Alagesan, M.; Ramalingam, S. Characterization of cardiac autonomic function in COVID-19 using heart rate variability: A hospital based preliminary observational study. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Benedí, C.; Oliver-Forniés, P.; Galluccio, F.; Altinpulluk, E.Y.; Ergonenc, T.; Allam, A.E.S.; Salazar, C.; Fajardo-Pérez, M. Is the heart rate variability monitoring using the analgesia nociception index a predictor of illness severity and mortality in critically ill patients with COVID-19? A pilot study. PLoS ONE 2021, 16, e0249128. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Z.; Yuan, Y.; Han, J.; Wang, Z.; Chen, H.; Wang, S.; Wang, Z.; Hu, H.; Zhou, L.; et al. Alteration of autonomic nervous system is associated with severity and outcomes in patients with COVID-19. Front. Physiol. 2021, 12, 630038. [Google Scholar] [CrossRef]
- Desforges, M.; Le Coupanec, A.; Stodola, J.K.; Meessen-Pinard, M.; Talbot, P.J. Human coronaviruses: Viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014, 194, 145–158. [Google Scholar] [CrossRef]
- Yu, H.; Sun, T.; Feng, J. Complications and Pathophysiology of COVID-19 in the Nervous System. Front. Neurol. 2020, 11, 573421. [Google Scholar] [CrossRef]
- Acanfora, C.; Casucci, G. Neprilysin inhibitor-angiotensin II receptor blocker combination (sacubitril/valsartan): Rationale for adoption in SARS-CoV-2 patients. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 135–136. [Google Scholar] [CrossRef] [Green Version]
- Shah, B.; Kunal, S.; Bansal, A.; Jain, J.; Poundrik, S.; Shetty, M.K.; Batra, V.; Chaturvedi, V.; Yusuf, J.; Mukhopadhyay, S.; et al. Heart rate variability as a marker of cardiovascular dysautonomia in post-COVID-19 syndrome using artificial intelligence. Indian Pacing Electrophysiol. J. 2022, 22, 70–76. [Google Scholar] [CrossRef]
- Acanfora, D.; Nolano, M.; Acanfora, C.; Colella, C.; Provitera, V.; Caporaso, G.; Rodolico, G.R.; Bortone, A.S.; Galasso, G.; Casucci, G. Impaired Vagal Activity in Long-COVID-19 Patients. Viruses 2022, 14, 1035. [Google Scholar] [CrossRef] [PubMed]
- Soliński, M.; Pawlak, A.; Petelczyc, M.; Buchner, T.; Aftyka, J.; Gil, R.; Król, Z.J.; Żebrowski, J.J. Heart rate variability comparison between young males after 4–6 weeks from the end of SARS-CoV-2 infection and controls. Sci. Rep. 2022, 12, 8832. [Google Scholar] [CrossRef] [PubMed]
- Marques, K.C.; Silva, C.C.; Trindade, S.D.S.; Santos, M.C.D.S.; Rocha, R.S.B.; Vasconcelos, P.F.D.C.; Quaresma, J.A.S.; Falcão, L.F.M. Reduction of Cardiac Autonomic Modulation and Increased Sympathetic Activity by Heart Rate Variability in Patients with Long COVID. Front. Cardiovasc. Med. 2022, 9, 862001. [Google Scholar] [CrossRef] [PubMed]
- Asarcikli, L.D.; Hayiroglu, M.I.; Osken, A.; Keskin, K.; Kolak, Z.; Aksu, T. Heart rate variability and cardiac autonomic functions in post-COVID period. J. Interv. Card. Electrophysiol. 2022, 63, 715–721. [Google Scholar] [CrossRef]
| Acquisition | System of Measurement | Category | Autonomic Reflection |
|---|---|---|---|
| Time-domain | SDNN | The standard deviation of all normal–normal (R–R) intervals | PNS and SNS activity |
| pNN50 | Percentage of consecutive N–N intervals that deviate from one another by more than 50 ms | PNS activity | |
| RMSSD | The square root of the mean squared differences between normal adjacent R–R intervals | PNS activity | |
| Frequency-domain | TP | Total power (<0.4 Hz) | Variability in autonomic function as a complete |
| VLF | Very low frequency (<0.04 Hz) | Thermoregulatory cycles | |
| LF | Low frequency (0.05–0.15 Hz) | Combined action of the PNS and SNS | |
| HF | High frequency (0.15–0.4 Hz) | PNS activity | |
| LF: HF | The ratio of low-frequency to high frequency | SNS-to-PNS balance |
| Variable | Case Group (n = 47) | Control Group (n = 42) | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Comorbidities | |||||
| Arterial hypertension | 8 | 17.0 | 10 | 23.8 | |
| Dyslipidemia | 7 | 14.9 | 3 | 7.1 | |
| Obesity | 4 | 8.5 | 5 | 11.9 | |
| Diabetes mellitus | 2 | 4.3 | 2 | 4.8 | |
| Chagas | 1 | 2.1 | 1 | 2.4 | |
| None | 25 | 53.2 | 21 | 50.0 | 0.8426 |
| Sex | |||||
| Female | 28 | 59.6 | 30 | 71.4 | |
| Male | 19 | 40.4 | 12 | 28.6 | 0.2413 |
| Variables (n = 47) | Mild (n = 20) | Moderate (n = 19) | Severe (n = 6) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 41.2 | 10.3 | 46.3 | 11.7 | 47.5 | 17.0 | 0.3132 |
| Time * (months) | 4.2 | 2.3 | 5.2 | 2.2 | 4.7 | 1.9 | 0.5787 |
| Chest CT ** (%) | 4.3 | 6.3 | 14.2 | 9.3 | 25.8 | 10.2 | <0.0001 |
| Echocardiography LVEF (%) | 63.2 | 5.0 | 58.7 | 6.4 | 58.0 | 8.9 | 0.0481 |
| BNP (pg/mL) | 15.2 | 12.4 | 35.0 | 43.2 | 44.8 | 20.9 | 0.0098 |
| Calcitonin (pg/mL) | 2.7 | 1.0 | 3.2 | 1.8 | 3.9 | 2.3 | 0.3642 |
| D-dimer (ng/mL) | 180.8 | 121.2 | 312.9 | 221.0 | 454.4 | 179.5 | 0.0023 |
| Ferritin (pmol/L) | 209.4 | 164.6 | 302.4 | 232.5 | 365.8 | 274.9 | 0.2102 |
| CRP (mg/L) | 3.4 | 2.8 | 3.7 | 2.9 | 8.9 | 4.5 | 0.0015 |
| Procalcitonin (ng/mL) | 0.4 | 0.7 | 1.3 | 1.9 | 0.4 | 0.3 | 0.1976 |
| Fibrinogen (mg/dL) | 358.5 | 163.5 | 368.4 | 179.3 | 454.2 | 183.6 | 0.5100 |
| IL-6 (pg/mL) | 3.4 | 1.4 | 4.1 | 1.5 | 4.0 | 1.8 | 0.5921 |
| Variable | Case (n = 47) | Control (n = 44) | p-Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age | 44.4 | 12.2 | 39.6 | 12.9 | 0.0709 |
| HR | 82.3 | 9.2 | 75.8 | 10.0 | 0.0018 |
| Min HR | 52.4 | 11.5 | 48.1 | 9.3 | 0.0253 |
| Max HR | 130.9 | 18.9 | 125.6 | 19.2 | 0.1862 |
| VE | 267.7 | 1533.6 | 126.6 | 494.3 | 0.7060 |
| SVE | 90.6 | 419.1 | 12.3 | 36.5 | 0.9335 |
| SDNN-24 | 111.6 | 38.7 | 133.4 | 37.8 | 0.0078 |
| SDANNi | 99.7 | 38.5 | 122.3 | 39.9 | 0.0072 |
| rMSSD | 41.8 | 86.3 | 34.3 | 12.2 | 0.0310 |
| pNN50 | 18.3 | 66.7 | 11.8 | 8.6 | 0.0442 |
| Max QTc | 544.6 | 101.8 | 518.9 | 44.0 | 0.0389 |
| Max QT | 503.9 | 77.2 | 467.5 | 40.5 | 0.0086 |
| VLF | 2225.7 | 1631.9 | 2342.1 | 1183.8 | 0.2398 |
| LF | 780.4 | 513.1 | 828.5 | 417.7 | 0.6262 |
| HF | 233.6 | 172.2 | 307.3 | 196.1 | 0.0297 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes Junior, A.d.S.; Schröder, A.A.; Botelho, S.M.; Resende, A.L. Cardiac Autonomic Function in Long COVID-19 Using Heart Rate Variability: An Observational Cross-Sectional Study. J. Clin. Med. 2023, 12, 100. https://doi.org/10.3390/jcm12010100
Menezes Junior AdS, Schröder AA, Botelho SM, Resende AL. Cardiac Autonomic Function in Long COVID-19 Using Heart Rate Variability: An Observational Cross-Sectional Study. Journal of Clinical Medicine. 2023; 12(1):100. https://doi.org/10.3390/jcm12010100
Chicago/Turabian StyleMenezes Junior, Antonio da Silva, Aline Andressa Schröder, Silvia Marçal Botelho, and Aline Lazara Resende. 2023. "Cardiac Autonomic Function in Long COVID-19 Using Heart Rate Variability: An Observational Cross-Sectional Study" Journal of Clinical Medicine 12, no. 1: 100. https://doi.org/10.3390/jcm12010100
APA StyleMenezes Junior, A. d. S., Schröder, A. A., Botelho, S. M., & Resende, A. L. (2023). Cardiac Autonomic Function in Long COVID-19 Using Heart Rate Variability: An Observational Cross-Sectional Study. Journal of Clinical Medicine, 12(1), 100. https://doi.org/10.3390/jcm12010100