Broken Heart Syndrome: Evolving Molecular Mechanisms and Principles of Management
Abstract
:1. Introduction
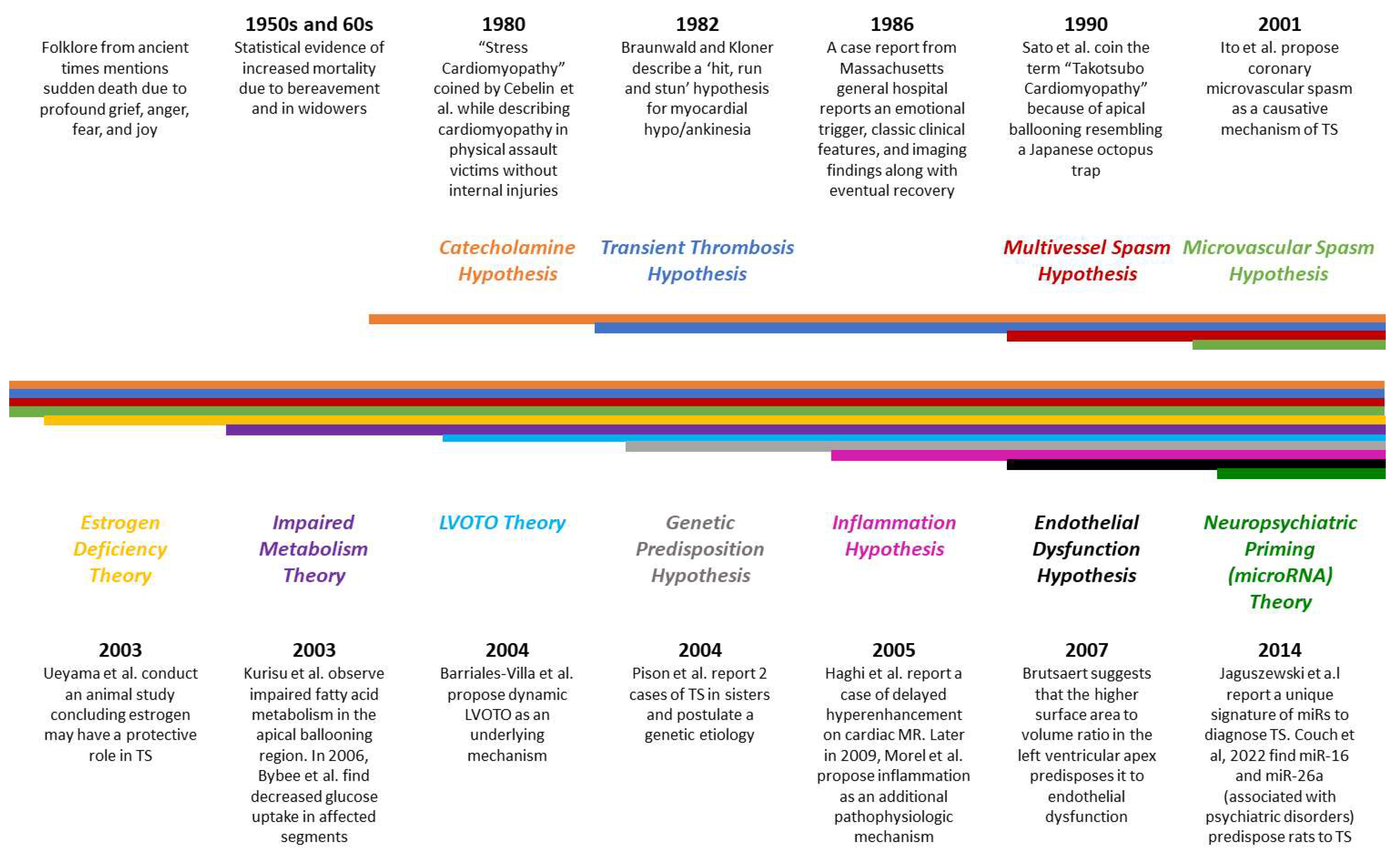
2. Etiopathogenesis of TS
2.1. Predisposing Factors
2.2. Role of Stressors
2.3. Pathogenic Mechanisms
2.3.1. Cardiovascular Mechanisms
2.3.2. Neuropsychiatry and TS
3. Underlying Molecular Mechanisms and Recent Updates
3.1. Role of the Adrenergic System and Myocardial Survival Pathways
3.2. Role of Estrogen
3.3. Genetic Polymorphisms
3.4. MicroRNAs and Brain Heart Axis
3.5. Cardiac Macrophages
3.6. Other Molecular Mechanisms
3.6.1. Microvascular Reactivity and Coronary Spasm
3.6.2. Perfusion of the Apex
3.6.3. Inflammatory Mechanisms
4. Broken Heart, Why Not Happy Heart Syndrome?
5. Clinical Presentation
6. Principles of Management
6.1. Diagnosis of Broken Heart Syndrome
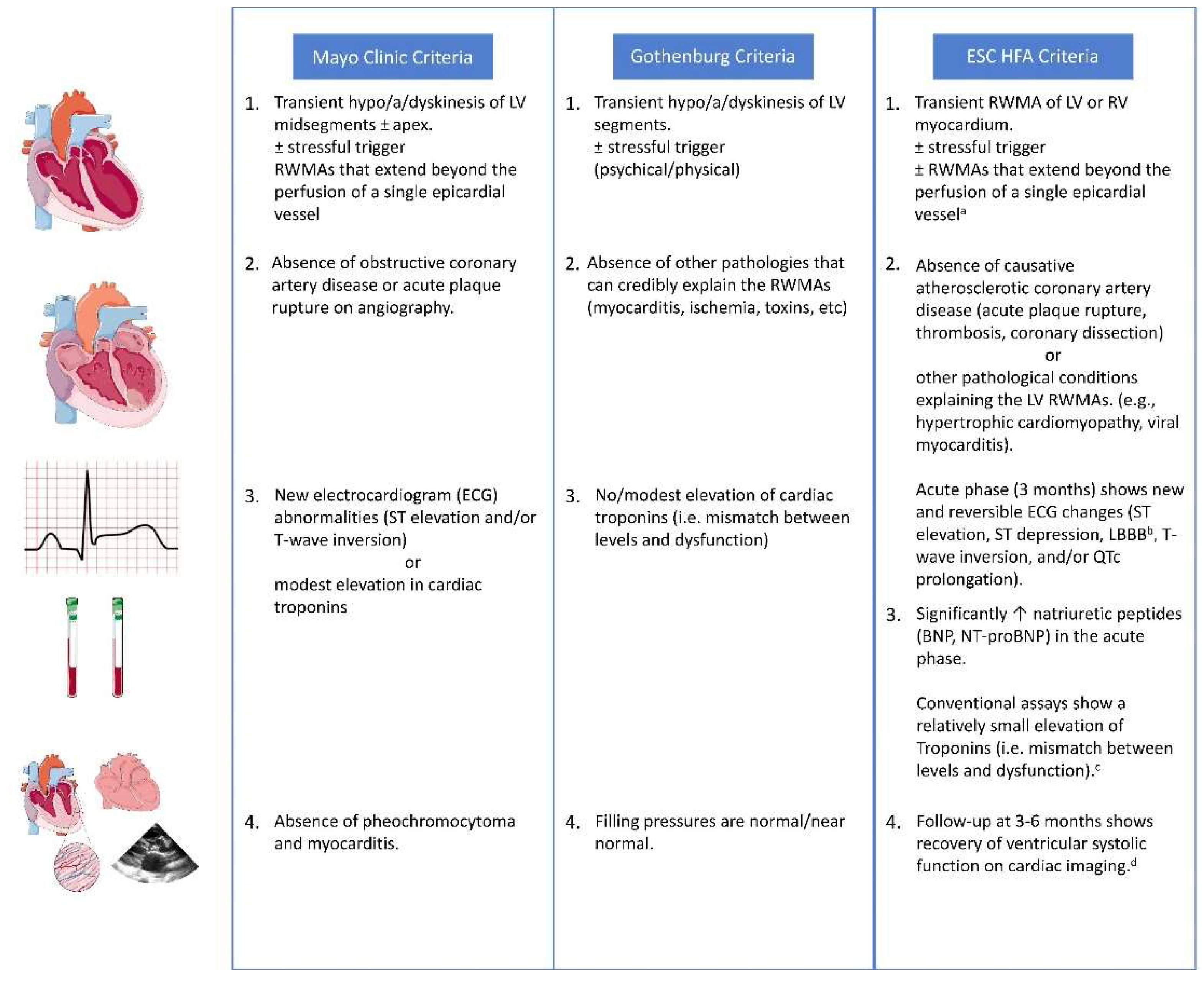
6.2. Differential Diagnosis
6.3. Risk Stratification
6.4. Biomarkers
6.4.1. Other Biomarkers
6.4.2. Novel Biomarkers
6.5. Electrocardiography
6.5.1. ST Segment
6.5.2. T Wave
6.5.3. QT Segment
6.6. Coronary Angiography and Ventriculography
6.7. Echocardiography
6.8. Cardiovascular Magnetic Resonance (CMR) Imaging
7. Treatment Modalities
7.1. Evolving Concepts for Heart Failure
7.2. Current Evidence for Pharmacotherapy
8. Complications
8.1. Cardiogenic Shock and Acute Heart Failure
8.2. Arrhythmias
8.3. Obstruction of the Left Ventricular Outflow Tract
8.4. Thrombo-Embolism
8.5. Intramyocardial Hemorrhage and Rupture
9. Prognosis
10. Future Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, T.; Khan, H.; Gamble, D.T.; Scally, C.; Newby, D.E.; Dawson, D. Takotsubo Syndrome: Pathophysiology, Emerging Concepts, and Clinical Implications. Circulation 2022, 145, 1002–1019. [Google Scholar] [CrossRef]
- Hassan, Y.-S.; Yamasaki, K. History of Takotsubo Syndrome: Is the Syndrome Really Described as a Disease Entity First in 1990? Some Inaccuracies. Int. J. Cardiol. 2013, 166, 736–737. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Cammann, V.L.; Napp, L.C.; Jurisic, S.; Diekmann, J.; Bataiosu, D.R.; Seifert, B.; Jaguszewski, M.; Sarcon, A.; Neumann, C.A.; et al. Differences in the Clinical Profile and Outcomes of Typical and Atypical Takotsubo Syndrome: Data from the International Takotsubo Registry. JAMA Cardiol. 2016, 1, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Akashi, Y.J.; Nef, H.M.; Lyon, A.R. Epidemiology and Pathophysiology of Takotsubo Syndrome. Nat. Rev. Cardiol. 2015, 12, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.S.; Hughey, A.B.; Kolias, T.J. Nationwide Trends in Reported Incidence of Takotsubo Cardiomyopathy from 2006 to 2012. Am. J. Cardiol. 2015, 116, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Murugiah, K.; Wang, Y.; Desai, N.R.; Spatz, E.S.; Nuti, S.V.; Dreyer, R.P.; Krumholz, H.M. Trends in Short- and Long-Term Outcomes for Takotsubo Cardiomyopathy Among Medicare Fee-for-Service Beneficiaries, 2007 to 2012. JACC Heart Fail. 2016, 4, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, S.W.; Windenburg, D.C.; Lesser, J.R.; Maron, M.S.; Hauser, R.G.; Lesser, J.N.; Haas, T.S.; Hodges, J.S.; Maron, B.J. Natural History and Expansive Clinical Profile of Stress (Tako-Tsubo) Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 55, 333–341. [Google Scholar] [CrossRef]
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current State of Knowledge on Takotsubo Syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef]
- Singh, K.; Carson, K.; Usmani, Z.; Sawhney, G.; Shah, R.; Horowitz, J. Systematic Review and Meta-Analysis of Incidence and Correlates of Recurrence of Takotsubo Cardiomyopathy. Int. J. Cardiol. 2014, 174, 696–701. [Google Scholar] [CrossRef]
- Pilgrim, T.M.; Wyss, T.R. Takotsubo Cardiomyopathy or Transient Left Ventricular Apical Ballooning Syndrome: A Systematic Review. Int. J. Cardiol. 2008, 124, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Cebelin, M.S.; Hirsch, C.S. Human Stress Cardiomyopathy: Myocardial Lesions in Victims of Homicidal Assaults without Internal Injuries. Hum. Pathol. 1980, 11, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Barriales Villa, R.; Bilbao Quesada, R.; Iglesias Río, E.; Bayón Meleiro, N.; Mantilla González, R.; Penas Lado, M. Transient Left Ventricular Apical Ballooning without Coronary Stenoses Syndrome: Importance of the Intraventricular Pressure Gradient. Rev. Esp. Cardiol. 2004, 57, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Damian, G.; Dansoreanu, M. EPR study of non-covalent spin labeled serum albumin and hemoglobin. Biophys. Chem. 2002, 99, 181–188. [Google Scholar] [CrossRef]
- Spieker, L.E.; Hürlimann, D.; Ruschitzka, F.; Corti, R.; Enseleit, F.; Shaw, S.; Hayoz, D.; Deanfield, J.E.; Lüscher, T.F.; Noll, G. Mental Stress Induces Prolonged Endothelial Dysfunction via Endothelin—A Receptors. Circulation 2002, 105, 2817–2820. [Google Scholar] [CrossRef]
- Ueyama, T.; Hano, T.; Kasamatsu, K.; Yamamoto, K.; Tsuruo, Y.; Nishio, I. Estrogen Attenuates the Emotional Stress-Induced Cardiac Responses in the Animal Model of Tako-Tsubo (Ampulla) Cardiomyopathy. J. Cardiovasc. Pharm. 2003, 42 (Suppl. 1), S117–S120. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; Benezet-Mazuecos, J.; Navarro, F.; Farre, J. Takotsubo Syndrome: A Bayesian Approach to Interpreting Its Pathogenesis. Mayo Clin. Proc. 2006, 81, 732–735. [Google Scholar] [CrossRef]
- Pison, L.; de Vusser, P.; Mullens, W. Apical Ballooning in Relatives. Heart 2004, 90, e67. [Google Scholar] [CrossRef]
- Rees, W.D.; Lutkins, S.G. Mortality of Bereavement. Br. Med. J. 1967, 4, 13–16. [Google Scholar] [CrossRef]
- Parkes, C.M.; Benjamin, B.; Fitzgerald, R.G. Broken Heart: A Statistical Study of Increased Mortality among Widowers. Br. Med. J. 1969, 1, 740–743. [Google Scholar] [CrossRef]
- Braunwald, E.; Kloner, R.A. The Stunned Myocardium: Prolonged, Postischemic Ventricular Dysfunction. Circulation 1982, 66, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Cabot, R.C.; Scully, R.E.; Mark, E.J.; McNeely, B.U.; Ryan, T.J.; Fallon, J.T. Case Records of the Massachusetts General Hospital. Weekly Clinicopathological Exercises. Case 18-1986. A 44-Year-Old Woman with Substernal Pain and Pulmonary Edema after Severe Emotional Stress. N. Engl. J. Med. 1986, 314, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Tateishi, H.; Uchida, T.; Dote, K.; Ishihara, M.; Kodama, K.; Haze, K.; Hori, M. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure. Kagaku Hyoronsha 1990, 2, 55–64. [Google Scholar]
- Dote, K.; Sato, H.; Tateishi, H.; Uchida, T.; Ishihara, M. Myocardial Stunning Due to Simultaneous Multivessel Coronary Spasms: A Review of 5 Cases. J. Cardiol. 1991, 21, 203–214. [Google Scholar] [PubMed]
- Brutsaert, D.L. Tako-Tsubo Cardiomyopathy. Eur. J. Heart Fail. 2007, 9, 854. [Google Scholar] [CrossRef]
- Haghi, D.; Fluechter, S.; Suselbeck, T.; Borggrefe, M.; Papavassiliu, T. Delayed Hyperenhancement in a Case of Takotsubo Cardiomyopathy. J. Cardiovasc. Magn. Reson. 2005, 7, 845–847. [Google Scholar] [CrossRef]
- Morel, O.; Sauer, F.; Imperiale, A.; Cimarelli, S.; Blondet, C.; Jesel, L.; Trinh, A.; de Poli, F.; Ohlmann, P.; Constantinesco, A.; et al. Importance of Inflammation and Neurohumoral Activation in Takotsubo Cardiomyopathy. J. Card. Fail. 2009, 15, 206–213. [Google Scholar] [CrossRef]
- Kurisu, S.; Inoue, I.; Kawagoe, T.; Ishihara, M.; Shimatani, Y.; Nishioka, K.; Umemura, T.; Nakamura, S.; Yoshida, M.; Sato, H. Myocardial Perfusion and Fatty Acid Metabolism in Patients with Tako-Tsubo-like Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2003, 41, 743–748. [Google Scholar] [CrossRef]
- Bybee, K.A.; Murphy, J.; Prasad, A.; Wright, R.S.; Lerman, A.; Rihal, C.S.; Chareonthaitawee, P. Acute Impairment of Regional Myocardial Glucose Uptake in the Apical Ballooning (Takotsubo) Syndrome. J. Nucl. Cardiol. 2006, 13, 244–250. [Google Scholar] [CrossRef]
- Jaguszewski, M.; Osipova, J.; Ghadri, J.R.; Napp, L.C.; Widera, C.; Franke, J.; Fijalkowski, M.; Nowak, R.; Fijalkowska, M.; Volkmann, I.; et al. A Signature of Circulating MicroRNAs Differentiates Takotsubo Cardiomyopathy from Acute Myocardial Infarction. Eur. Heart J. 2014, 35, 999–1006. [Google Scholar] [CrossRef]
- Couch, L.S.; Channon, K.; Thum, T. Molecular Mechanisms of Takotsubo Syndrome. Int. J. Mol. Sci. 2022, 23, 12262. [Google Scholar] [CrossRef]
- Pelliccia, F.; Greco, C.; Vitale, C.; Rosano, G.; Gaudio, C.; Kaski, J.C. Takotsubo Syndrome (Stress Cardiomyopathy): An Intriguing Clinical Condition in Search of Its Identity. Am. J. Med. 2014, 127, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P.; Poh, K.K.; Lee, C.H.; Tan, H.C.; Razak, A.; Chia, B.L.; Low, A.F. Diverse Clinical Spectrum of Stress-Induced Cardiomyopathy. Int. J. Cardiol. 2009, 133, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Parodi, G.; Greco, C.; Antoniucci, D.; Brenner, R.; Bossone, E.; Cacciotti, L.; Capucci, A.; Citro, R.; Delmas, C.; et al. Comorbidities Frequency in Takotsubo Syndrome: An International Collaborative Systematic Review Including 1109 Patients. Am. J. Med. 2015, 128, 654.e11–654.e19. [Google Scholar] [CrossRef] [PubMed]
- Summers, M.R.; Lennon, R.J.; Prasad, A. Pre-Morbid Psychiatric and Cardiovascular Diseases in Apical Ballooning Syndrome (Tako-Tsubo/Stress-Induced Cardiomyopathy): Potential Pre-Disposing Factors? J. Am. Coll. Cardiol. 2010, 55, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Vitale, C.; Marazzi, G.; Volterrani, M. Menopause and Cardiovascular Disease: The Evidence. Climacteric 2007, 10 (Suppl. 1), 19–24. [Google Scholar] [CrossRef]
- Martin, E.A.; Prasad, A.; Rihal, C.S.; Lerman, L.O.; Lerman, A. Endothelial Function and Vascular Response to Mental Stress Are Impaired in Patients with Apical Ballooning Syndrome. J. Am. Coll. Cardiol. 2010, 56, 1840. [Google Scholar] [CrossRef]
- Hinojosa-Laborde, C.; Chapa, I.; Lange, D.; Haywood, J.R. Gender Differences in Sympathetic Nervous System Regulation. Clin. Exp. Pharm. Physiol. 1999, 26, 122–126. [Google Scholar] [CrossRef]
- Sader, M.A.; Celermajer, D.S. Endothelial Function, Vascular Reactivity and Gender Differences in the Cardiovascular System. Cardiovasc. Res. 2002, 53, 597–604. [Google Scholar] [CrossRef]
- Redfors, B.; Shao, Y.; Omerovic, E. Stress-Induced Cardiomyopathy (Takotsubo)—Broken Heart and Mind? Vasc. Health Risk. Manag. 2013, 9, 149. [Google Scholar] [CrossRef]
- Yoshimura, S.; Toyoda, K.; Ohara, T.; Nagasawa, H.; Ohtani, N.; Kuwashiro, T.; Naritomi, H.; Minematsu, K. Takotsubo Cardiomyopathy in Acute Ischemic Stroke. Ann. Neurol. 2008, 64, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Ennezat, P.v.; Pesenti-Rossi, D.; Aubert, J.M.; Rachenne, V.; Bauchart, J.J.; Auffray, J.L.; Logeart, D.; Cohen-Solal, A.; Asseman, P. Transient Left Ventricular Basal Dysfunction without Coronary Stenosis in Acute Cerebral Disorders: A Novel Heart Syndrome (Inverted Takotsubo). Echocardiography 2005, 22, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Gonsalves, S.; Saha, A.; Ross, S.; Williams, G. Acute Subarachnoid Haemorrhage as a Precipitant for Takotsubo Cardiomyopathy: A Case Report and Discussion. Int. J. Cardiol. 2009, 132, 283–285. [Google Scholar] [CrossRef]
- Greco, C.; Cavalletti, C.; di Piero, V.; Argentino, C.; Scopinaro, F. Scintigraphic Demonstration of Myocardial Ischaemia. Lancet 1988, 2, 281. [Google Scholar] [CrossRef] [PubMed]
- Y-Hassan, S.; Tornvall, P. Epidemiology, Pathogenesis, and Management of Takotsubo Syndrome. Clin. Auton. Res. 2018, 28, 53–65. [Google Scholar] [CrossRef]
- Gianaros, P.J.; Jennings, J.R.; Sheu, L.K.; Greer, P.J.; Kuller, L.H.; Matthews, K.A. Prospective Reports of Chronic Life Stress Predict Decreased Grey Matter Volume in the Hippocampus. Neuroimage 2007, 35, 795–803. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Brinjikji, W.; Salka, S. Demographic and Co-Morbid Predictors of Stress (Takotsubo) Cardiomyopathy. Am. J. Cardiol. 2012, 110, 1368–1372. [Google Scholar] [CrossRef]
- Deshmukh, A.; Kumar, G.; Pant, S.; Rihal, C.; Murugiah, K.; Mehta, J.L. Prevalence of Takotsubo Cardiomyopathy in the United States. Am. Heart J. 2012, 164, 66–71. [Google Scholar] [CrossRef]
- Corrigan, F.E.; Kimmel, M.C.; Jayaram, G. Four Cases of Takotsubo Cardiomyopathy Linked with Exacerbations of Psychiatric Illness. Innov. Clin. Neurosci. 2011, 8, 50. [Google Scholar]
- Bristow, M.R.; Ginsburg, R.; Umans, V.; Fowler, M.; Minobe, W.; Rasmussen, R.; Zera, P.; Menlove, R.; Shah, P.; Jamieson, S. Beta 1- and Beta 2-Adrenergic-Receptor Subpopulations in Nonfailing and Failing Human Ventricular Myocardium: Coupling of Both Receptor Subtypes to Muscle Contraction and Selective Beta 1-Receptor down-Regulation in Heart Failure. Circ. Res. 1986, 59, 297–309. [Google Scholar] [CrossRef]
- Heubach, J.F.; Ravens, U.; Kaumann, A.J. Epinephrine Activates Both Gs and Gi Pathways, but Norepinephrine Activates Only the Gs Pathway through Human Β2-Adrenoceptors Overexpressed in Mouse Heart. Mol. Pharm. 2004, 65, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Paur, H.; Wright, P.T.; Sikkel, M.B.; Tranter, M.H.; Mansfield, C.; O’Gara, P.; Stuckey, D.J.; Nikolaev, V.O.; Diakonov, I.; Pannell, L.; et al. High Levels of Circulating Epinephrine Trigger Apical Cardiodepression in a β 2-Adrenergic Receptor/Gi-Dependent Manner: A New Model of Takotsubo Cardiomyopathy. Circulation 2012, 126, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Nef, H.M.; Möllmann, H.; Hilpert, P.; Troidl, C.; Voss, S.; Rolf, A.; Behrens, C.B.; Weber, M.; Hamm, C.W.; Elsässer, A. Activated Cell Survival Cascade Protects Cardiomyocytes from Cell Death in Tako-Tsubo Cardiomyopathy. Eur. J. Heart Fail. 2009, 11, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Rosano, G.M.C.; Kaski, J.C. Role of Coronary Microvascular Dysfunction in Takotsubo Cardiomyopathy. Circ. J. 2016, 80, 299–305. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef]
- Almeida, A.R. What Is the Role of Estrogen in Predisposition to Takotsubo Syndrome throughout a Woman’s Reproductive Life? Rev. Port. Cardiol. 2022, 41, 889–890. [Google Scholar] [CrossRef]
- Rodrigues Brás, D.; Semedo, P.; Cordeiro Piçarra, B.; Dionísio, P.; Carvalho, J.; Azevedo Guerreiro, R.; Congo, K.; Aguiar, J. Takotsubo Syndrome in a Breast-Feeding Young Woman: Highlighting the Protection of Oestrogens? Rev. Port. Cardiol. 2022, 41, 887.e1–887.e5. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Lubahn, D.; Liu, J.; Vannan, M.; Levin, E.R. Estrogen Inhibits Cardiac Hypertrophy: Role of Estrogen Receptor-β to Inhibit Calcineurin. Endocrinology 2008, 149, 3361–3369. [Google Scholar] [CrossRef]
- Möller, C.; Stiermaier, T.; Brabant, G.; Graf, T.; Thiele, H.; Eitel, I. Comprehensive Assessment of Sex Hormones in Takotsubo Syndrome. Int. J. Cardiol. 2018, 250, 11–15. [Google Scholar] [CrossRef]
- Limongelli, G.; Masarone, D.; Maddaloni, V.; Rubino, M.; Fratta, F.; Cirillo, A.; Ludovica, S.B.; Pacileo, R.; Fusco, A.; Coppola, G.R.; et al. Genetics of Takotsubo Syndrome. Heart Fail. Clin. 2016, 12, 499–506. [Google Scholar] [CrossRef]
- Vriz, O.; Minisini, R.; Citro, R.; Guerra, V.; Zito, C.; de Luca, G.; Pavan, D.; Pirisi, M.; Limongelli, G.; Bossone, E. Analysis of Β1 and Β2-Adrenergic Receptors Polymorphism in Patients with Apical Ballooning Cardiomyopathy. Acta Cardiol. 2017, 66, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Small, K.M.; Forbes, S.L.; Rahman, F.F.; Bridges, K.M.; Liggett, S.B. A Four Amino Acid Deletion Polymorphism in the Third Intracellular Loop of the Human α(2C)-Adrenergic Receptor Confers Impaired Coupling to Multiple Effectors. J. Biol. Chem. 2000, 275, 23059–23064. [Google Scholar] [CrossRef] [PubMed]
- Figtree, G.A.; Bagnall, R.D.; Abdulla, I.; Buchholz, S.; Galougahi, K.K.; Yan, W.; Tan, T.; Neil, C.; Horowitz, J.D.; Semsarian, C.; et al. No Association of G-Protein-Coupled Receptor Kinase 5 or β-Adrenergic Receptor Polymorphisms with Takotsubo Cardiomyopathy in a Large Australian Cohort. Eur. J. Heart Fail. 2013, 15, 730–733. [Google Scholar] [CrossRef]
- Citro, R.; D’Avenia, M.; de Marco, M.; Giudice, R.; Mirra, M.; Ravera, A.; Silverio, A.; Farina, R.; Silvestri, F.; Gravina, P.; et al. Polymorphisms of the Antiapoptotic Protein Bag3 May Play a Role in the Pathogenesis of Tako-Tsubo Cardiomyopathy. Int. J. Cardiol. 2013, 168, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, E.; Saliba-Gustafsson, P.; Ehrenborg, E.; Tornvall, P. Lack of Genetic Susceptibility in Takotsubo Cardiomyopathy: A Case-Control Study. BMC Med. Genet. 2018, 19, 1–7. [Google Scholar] [CrossRef]
- Ferradini, V.; Vacca, D.; Belmonte, B.; Mango, R.; Scola, L.; Novelli, G.; Balistreri, C.R.; Sangiuolo, F. Genetic and Epigenetic Factors of Takotsubo Syndrome: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9875. [Google Scholar] [CrossRef]
- Couch, L.S.; Fiedler, J.; Chick, G.; Clayton, R.; Dries, E.; Wienecke, L.M.; Fu, L.; Fourre, J.; Pandey, P.; Derda, A.A.; et al. Circulating MicroRNAs Predispose to Takotsubo Syndrome Following High-Dose Adrenaline Exposure. Cardiovasc. Res. 2022, 118, 1758–1770. [Google Scholar] [CrossRef]
- d’Avenia, M.; Citro, R.; de Marco, M.; Veronese, A.; Rosati, A.; Visone, R.; Leptidis, S.; Philippen, L.; Vitale, G.; Cavallo, A.; et al. A Novel MiR-371a-5p-Mediated Pathway, Leading to BAG3 Upregulation in Cardiomyocytes in Response to Epinephrine, Is Lost in Takotsubo Cardiomyopathy. Cell Death Dis. 2015, 6, e1948. [Google Scholar] [CrossRef]
- Oliveri, F.; Goud, H.K.; Mohammed, L.; Mehkari, Z.; Javed, M.; Althwanay, A.; Ahsan, F.; Rutkofsky, I.H. Role of Depression and Anxiety Disorders in Takotsubo Syndrome: The Psychiatric Side of Broken Heart. Cureus 2020, 12, e10400. [Google Scholar] [CrossRef]
- Hiestand, T.; Hänggi, J.; Klein, C.; Topka, M.S.; Jaguszewski, M.; Ghadri, J.R.; Lüscher, T.F.; Jäncke, L.; Templin, C. Takotsubo Syndrome Associated with Structural Brain Alterations of the Limbic System. J. Am. Coll. Cardiol. 2018, 71, 809–811. [Google Scholar] [CrossRef]
- Radfar, A.; Abohashem, S.; Osborne, M.T.; Wang, Y.; Dar, T.; Hassan, M.Z.O.; Ghoneem, A.; Naddaf, N.; Patrich, T.; Abbasi, T.; et al. Stress-Associated Neurobiological Activity Associates with the Risk for and Timing of Subsequent Takotsubo Syndrome. Eur. Heart J. 2021, 42, 1898–1908. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Chang, E.; Tang, X.; Watanabe, I.; Zhang, R.; Jeong, H.W.; Adams, R.H.; Jain, M.K. Cardiac Macrophages Regulate Isoproterenol-Induced Takotsubo-like Cardiomyopathy. JCI Insight 2022, 7, e156236. [Google Scholar] [CrossRef] [PubMed]
- Möller, C.; Stiermaier, T.; Meusel, M.; Jung, C.; Graf, T.; Eitel, I. Microcirculation in Patients with Takotsubo Syndrome—The Prospective CIRCUS-TTS Study. J. Clin. Med. 2021, 10, 2127. [Google Scholar] [CrossRef] [PubMed]
- Godsman, N.; Kohlhaas, M.; Nickel, A.; Cheyne, L.; Mingarelli, M.; Schweiger, L.; Hepburn, C.; Munts, C.; Welch, A.; Delibegovic, M.; et al. Metabolic Alterations in a Rat Model of Takotsubo Syndrome. Cardiovasc. Res. 2022, 118, 1932–1946. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, I.S. Why Age Matters in Takotsubo Syndrome. J. Am. Coll. Cardiol. 2020, 75, 1878–1881. [Google Scholar] [CrossRef]
- Chandler, S.; Joshi, H.; Hoff, E.; Patel, K.; Ohanyan, V.; Kiedrowski, M.; Chilian, W.M.; Dong, F. Molecular Basis of Takotsubo Syndrome. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Scally, C.; Abbas, H.; Ahearn, T.; Srinivasan, J.; Mezincescu, A.; Rudd, A.; Spath, N.; Yucel-Finn, A.; Yuecel, R.; Oldroyd, K.; et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2019, 139, 1581–1592. [Google Scholar] [CrossRef]
- Wilson, H.M.; Cheyne, L.; Brown, P.A.J.; Kerr, K.; Hannah, A.; Srinivasan, J.; Duniak, N.; Horgan, G.; Dawson, D.K. Characterization of the Myocardial Inflammatory Response in Acute Stress-Induced (Takotsubo) Cardiomyopathy. JACC Basic Transl. Sci. 2018, 3, 766–778. [Google Scholar] [CrossRef]
- Qin, D.; Patel, S.M.; Champion, H.C. “happiness” and Stress Cardiomyopathy (Apical Ballooning Syndrome/Takotsubo Syndrome). Int. J. Cardiol. 2014, 172, e182–e183. [Google Scholar] [CrossRef]
- Allen, D.; Parmar, G.; Ravandi, A.; Hussain, F.; Kass, M. Happiness Can Break Your Heart: A Rare Case of Takotsubo Cardiomyopathy after Good News. Can. J. Cardiol. 2015, 31, 228.e1–228.e2. [Google Scholar] [CrossRef]
- Pressman, S.D.; Cohen, S. Does Positive Affect Influence Health? Psychol. Bull. 2005, 131, 925–971. [Google Scholar] [CrossRef] [PubMed]
- Saposnik, G.; Baibergenova, A.; Dang, J.; Hachinski, V. Does a Birthday Predispose to Vascular Events? Neurology 2006, 67, 300–304. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Sarcon, A.; Diekmann, J.; Bataiosu, D.R.; Cammann, V.L.; Jurisic, S.; Napp, L.C.; Jaguszewski, M.; Scherff, F.; Brugger, P.; et al. Happy Heart Syndrome: Role of Positive Emotional Stress in Takotsubo Syndrome. Eur. Heart J. 2016, 37, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Medina de Chazal, H.; del Buono, M.G.; Keyser-Marcus, L.; Ma, L.; Moeller, F.G.; Berrocal, D.; Abbate, A. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 1955–1971. [Google Scholar] [CrossRef] [PubMed]
- Prokudina, E.S.; Kurbatov, B.K.; Zavadovsky, K.V.; Vrublevsky, A.v.; Naryzhnaya, N.V.; Lishmanov, Y.B.; Maslov, L.N.; Oeltgen, P.R. Takotsubo Syndrome: Clinical Manifestations, Etiology and Pathogenesis. Curr. Cardiol. Rev. 2021, 17, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Y-Hassan, S. Takotsubo Syndrome Triggered by Acute Coronary Syndrome in a Cohort of 20 Patients: An Often Missed Diagnosis. Int. J. Cardiol. Res. 2015, 2, 28–33. [Google Scholar] [CrossRef]
- Y-Hassan, S.; Themudo, R.; Maret, E. Spontaneous Coronary Artery Dissection and Takotsubo Syndrome: The Chicken or the Egg Causality Dilemma. Catheter. Cardiovasc. Interv. 2017, 89, 1215–1218. [Google Scholar] [CrossRef]
- Y-Hassan, S.; Böhm, F. The Causal Link between Spontaneous Coronary Artery Dissection and Takotsubo Syndrome: A Case Presented with Both Conditions. Int. J. Cardiol. 2016, 203, 828–831. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Srichai, M.B.; Iqbal, S.N.; Slater, J.N.; Mancini, G.B.J.; Feit, F.; Pena-Sing, I.; Axel, L.; Attubato, M.J.; Yatskar, L.; et al. Mechanisms of Myocardial Infarction in Women without Angiographically Obstructive Coronary Artery Disease. Circulation 2011, 124, 1414–1425. [Google Scholar] [CrossRef]
- Eitel, I.; von Knobelsdorff-Brenkenhoff, F.; Bernhardt, P.; Carbone, I.; Muellerleile, K.; Aldrovandi, A.; Francone, M.; Desch, S.; Gutberlet, M.; Strohm, O.; et al. Clinical Characteristics and Cardiovascular Magnetic Resonance Findings in Stress (Takotsubo) Cardiomyopathy. JAMA 2011, 306, 277–286. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur. Heart J. 2018, 39, 2047. [Google Scholar] [CrossRef]
- Madhavan, M.; Rihal, C.S.; Lerman, A.; Prasad, A. Acute Heart Failure in Apical Ballooning Syndrome (TakoTsubo/Stress Cardiomyopathy): Clinical Correlates and Mayo Clinic Risk Score. J. Am. Coll. Cardiol. 2011, 57, 1400–1401. [Google Scholar] [CrossRef] [PubMed]
- Dagrenat, C.; von Hunolstein, J.J.; Matsushita, K.; Thebaud, L.; Greciano, S.; Tuzin, N.; Meyer, N.; Trinh, A.; Jesel, L.; Ohlmann, P.; et al. Value of Cardiac Biomarkers in the Early Diagnosis of Takotsubo Syndrome. J. Clin. Med. 2020, 9, 2985. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Neil, C.J.; Sverdlov, A.L.; Mahadavan, G.; Chirkov, Y.Y.; Kucia, A.M.; Stansborough, J.; Beltrame, J.F.; Selvanayagam, J.B.; Zeitz, C.J.; et al. N-Terminal pro-Brain Natriuretic Protein Levels in Takotsubo Cardiomyopathy. Am. J. Cardiol. 2011, 108, 1316–1321. [Google Scholar] [CrossRef]
- Cavalu, S.; Damian, G. Rotational correlation times of 3-carbamoyl-2,2,5,5-tetramethyl-3-pyrrolin-1-yloxy spin label with respect to heme and nonheme proteins. Biomacromolecules 2003, 6, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.C.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral Features of Myocardial Stunning Due to Sudden Emotional Stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef]
- Budnik, M.; Kochanowski, J.; Piatkowski, R.; Wojtera, K.; Peller, M.; Gaska, M.; Glowacka, P.; Karolczak, P.; Ochijewicz, D.; Opolski, G. Simple Markers Can Distinguish Takotsubo Cardiomyopathy from ST Segment Elevation Myocardial Infarction. Int. J. Cardiol. 2016, 219, 417–420. [Google Scholar] [CrossRef]
- Santoro, F.; Costantino, M.D.; Guastafierro, F.; Triggiani, G.; Ferraretti, A.; Tarantino, N.; Saguner, A.; di Biase, M.; Brunetti, N.D. Inflammatory Patterns in Takotsubo Cardiomyopathy and Acute Coronary Syndrome: A Propensity Score Matched Analysis. Atherosclerosis 2018, 274, 157–161. [Google Scholar] [CrossRef]
- Schieffer, B.; Schieffer, E.; Hilfiker-Kleiner, D.; Hilfiker, A.; Kovanen, P.T.; Kaartinen, M.; Nussberger, J.; Harringer, W.; Drexler, H. Expression of Angiotensin II and Interleukin 6 in Human Coronary Atherosclerotic Plaques: Potential Implications for Inflammation and Plaque Instability. Circulation 2000, 101, 1372–1378. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M. Leukocyte Behavior in Atherosclerosis, Myocardial Infarction, and Heart Failure. Science 2013, 339, 161–166. [Google Scholar] [CrossRef]
- Amariles, P.; Cifuentes, L. Drugs as Possible Triggers of Takotsubo Cardiomyopathy: A Comprehensive Literature Search—Update 2015. Curr. Clin. Pharm. 2016, 11, 95–109. [Google Scholar] [CrossRef]
- Rémond, C.; Leporati, J.; Proeschel, M.; Deroche, E.; Brière, F. de la Catecholamine-Induced Acute Myocardial Stunning after Accidental Intra-Operative Noradrenaline Bolus. Anaesth. Rep. 2022, 10, e12187. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Mirza, N.; Ali, R.; Rayad, M.N.; Ahmad, A.; Khan, A. Takotsubo Cardiomyopathy After Cocaine Intoxication. Eur. J. Case Rep. Intern. Med. 2022, 9. [Google Scholar] [CrossRef]
- Akashi, Y.J.; Musha, H.; Kida, K.; Itoh, K.; Inoue, K.; Kawasaki, K.; Hashimoto, N.; Miyake, F. Reversible Ventricular Dysfunction Takotsubo Cardiomyopathy. Eur. J. Heart Fail. 2005, 7, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased MicroRNA-1 and MicroRNA-133a Levels in Serum of Patients with Cardiovascular Disease Indicate Myocardial Damage. Circ. Cardiovasc. Genet. 2011, 4, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Ferdinande, B.; Kayaert, P.; Wiyono, S.; Goetschalkx, K.; Dubois, C.; Sinnaeve, P.; Adriaenssens, T.; Coosemans, M.; Desmet, W. Time Course of Electrocardiographic Changes in Transient Left Ventricular Ballooning Syndrome. Int. J. Cardiol. 2013, 169, 276–280. [Google Scholar] [CrossRef]
- Mitsuma, W.; Kodama, M.; Ito, M.; Tanaka, K.; Yanagawa, T.; Ikarashi, N.; Sugiura, K.; Kimura, S.; Yagihara, N.; Kashimura, T.; et al. Serial Electrocardiographic Findings in Women with Takotsubo Cardiomyopathy. Am. J. Cardiol. 2007, 100, 106–109. [Google Scholar] [CrossRef]
- Bybee, K.A.; Motiei, A.; Syed, I.S.; Kara, T.; Prasad, A.; Lennon, R.J.; Murphy, J.G.; Hammill, S.C.; Rihal, C.S.; Wright, R.S. Electrocardiography Cannot Reliably Differentiate Transient Left Ventricular Apical Ballooning Syndrome from Anterior ST-Segment Elevation Myocardial Infarction. J. Electrocardiol. 2007, 40, 38.e1–38.e6. [Google Scholar] [CrossRef]
- Frangieh, A.H.; Obeid, S.; Ghadri, J.R.; Imori, Y.; D’Ascenzo, F.; Kovac, M.; Ruschitzka, F.; Lüscher, T.F.; Duru, F.; Templin, C.; et al. ECG Criteria to Differentiate between Takotsubo (Stress) Cardiomyopathy and Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e003418. [Google Scholar] [CrossRef]
- Johnson, N.P.; Chavez, J.F.; Mosley, W.J.; Flaherty, J.D.; Fox, J.M. Performance of Electrocardiographic Criteria to Differentiate Takotsubo Cardiomyopathy from Acute Anterior ST Elevation Myocardial Infarction. Int. J. Cardiol. 2013, 164, 345–348. [Google Scholar] [CrossRef]
- Mugnai, G.; Pasqualin, G.; Benfari, G.; Bertagnolli, L.; Mugnai, F.; Vassanelli, F.; Marchese, G.; Pesarini, G.; Menegatti, G. Acute Electrocardiographic Differences between Takotsubo Cardiomyopathy and Anterior ST Elevation Myocardial Infarction. J. Electrocardiol. 2015, 48, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Piackova1, E.; Kaufmann, C.C.; Weihs, V.; El-Razek, A.; Geppert, A.; Nürnberg, M.; Wessely, E.; Smetana, P.; Weiss, T.; Huber, K. ECG Changes and Their Prognostic Impact in Patients with Takotsubo Syndrome. Cardiol. Cardiovasc. Med. 2019, 3, 438–449. [Google Scholar] [CrossRef]
- Ogura, R.; Hiasa, Y.; Takahashi, T.; Yamaguchi, K.; Fujiwara, K.; Ohara, Y.; Nada, T.; Ogata, T.; Kusunoki, K.; Yuba, K.; et al. Specific Findings of the Standard 12-Lead ECG in Patients with “Takotsubo” Cardiomyopathy: Comparison with the Findings of Acute Anterior Myocardial Infarction. Circ. J. 2003, 67, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, M.; Ebina, T.; Hibi, K.; Iwahashi, N.; Tsukahara, K.; Endo, M.; Maejima, N.; Nagashima, Z.; Suzuki, H.; Morita, S.; et al. Differences in Negative T Waves between Takotsubo Cardiomyopathy and Reperfused Anterior Acute Myocardial Infarction. Circ. J. 2012, 76, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Madias, J.E. Transient Attenuation of the Amplitude of the QRS Complexes in the Diagnosis of Takotsubo Syndrome. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 28–36. [Google Scholar] [CrossRef]
- Madias, J.E.; Bazaz, R.; Agarwal, H.; Win, M.; Medepalli, L. Anasarca-Mediated Attenuation of the Amplitude of Electrocardiogram Complexes: A Description of a Heretofore Unrecognized Phenomenon. J. Am. Coll. Cardiol. 2001, 38, 756–764. [Google Scholar] [CrossRef]
- Kurisu, S.; Inoue, I.; Kawagoe, T.; Ishihara, M.; Shimatani, Y.; Nakamura, S.; Yoshida, M.; Mitsuba, N.; Hata, T.; Sato, H. Time Course of Electrocardiographic Changes in Patients with Tako-Tsubo Syndrome: Comparison with Acute Myocardial Infarction with Minimal Enzymatic Release. Circ. J. 2004, 68, 77–81. [Google Scholar] [CrossRef]
- Schneider, B.; Athanasiadis, A.; Stöllberger, C.; Pistner, W.; Schwab, J.; Gottwald, U.; Schoeller, R.; Gerecke, B.; Hoffmann, E.; Wegner, C.; et al. Gender Differences in the Manifestation of Tako-Tsubo Cardiomyopathy. Int. J. Cardiol. 2013, 166, 584–588. [Google Scholar] [CrossRef]
- Shimizu, M.; Nishizaki, M.; Yamawake, N.; Fujii, H.; Sakurada, H.; Isobe, M.; Hiraoka, M. J Wave and Fragmented QRS Formation during the Hyperacute Phase in Takotsubo Cardiomyopathy. Circ. J. 2014, 78, 943–949. [Google Scholar] [CrossRef]
- Namgung, J. Electrocardiographic Findings in Takotsubo Cardiomyopathy: ECG Evolution and Its Difference from the ECG of Acute Coronary Syndrome. Clin. Med. Insights Cardiol. 2014, 8, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Sclarovsky, S.; Nikus, K. The Electrocardiographic Paradox of Tako-Tsubo Cardiomyopathy-Comparison with Acute Ischemic Syndromes and Consideration of Molecular Biology and Electrophysiology to Understand the Electrical-Mechanical Mismatching. J. Electrocardiol. 2010, 43, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Gaibazzi, N.; Ugo, F.; Vignali, L.; Zoni, A.; Reverberi, C.; Gherli, T. Tako-Tsubo Cardiomyopathy with Coronary Artery Stenosis: A Case-Series Challenging the Original Definition. Int. J. Cardiol. 2009, 133, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical Ballooning Syndrome (Tako-Tsubo or Stress Cardiomyopathy): A Mimic of Acute Myocardial Infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Haghi, D.; Roehm, S.; Hamm, K.; Harder, N.; Suselbeck, T.; Borggrefe, M.; Papavassiliu, T. Takotsubo Cardiomyopathy Is Not Due to Plaque Rupture: An Intravascular Ultrasound Study. Clin. Cardiol. 2010, 33, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Lennon, R.J.; Prasad, A. Regional Wall Motion Abnormality in Apical Ballooning Syndrome (Takotsubo/Stress Cardiomyopathy): Importance of Biplane Left Ventriculography for Differentiating from Spontaneously Aborted Anterior Myocardial Infarction. Int. J. Cardiovasc. Imaging 2012, 28, 687–694. [Google Scholar] [CrossRef]
- Napp, L.C.; Ghadri, J.R.; Bauersachs, J.; Templin, C. Acute Coronary Syndrome or Takotsubo Cardiomyopathy: The Suspect May Not Always Be the Culprit. Int. J. Cardiol. 2015, 187, 116–119. [Google Scholar] [CrossRef]
- Desmet, W.; Bennett, J.; Ferdinande, B.; de Cock, D.; Adriaenssens, T.; Coosemans, M.; Sinnaeve, P.; Kayaert, P.; Dubois, C. The Apical Nipple Sign: A Useful Tool for Discriminating between Anterior Infarction and Transient Left Ventricular Ballooning Syndrome. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 264–267. [Google Scholar] [CrossRef]
- de Backer, O.; Debonnaire, P.; Gevaert, S.; Missault, L.; Gheeraert, P.; Muyldermans, L. Prevalence, Associated Factors and Management Implications of Left Ventricular Outflow Tract Obstruction in Takotsubo Cardiomyopathy: A Two-Year, Two-Center Experience. BMC Cardiovasc. Disord. 2014, 14, 147. [Google Scholar] [CrossRef]
- Gianni, M.; Dentali, F.; Grandi, A.M.; Sumner, G.; Hiralal, R.; Lonn, E. Apical Ballooning Syndrome or Takotsubo Cardiomyopathy: A Systematic Review. Eur. Heart J. 2006, 27, 1523–1529. [Google Scholar] [CrossRef]
- Dewachter, P.; Tanase, C.; Levesque, E.; Nicaise-Roland, P.; Chollet-Martin, S.; Mouton-Faivre, C.; Benhamou, D. Apical Ballooning Syndrome Following Perioperative Anaphylaxis Is Likely Related to High Doses of Epinephrine. J. Anesth. 2011, 25, 282–285. [Google Scholar] [CrossRef]
- Meimoun, P.; Passos, P.; Benali, T.; Boulanger, J.; Elmkies, F.; Zemir, H.; Clerc, J.; Luycx-Bore, A. Assessment of Left Ventricular Twist Mechanics in Tako-Tsubo Cardiomyopathy by Two-Dimensional Speckle-Tracking Echocardiography. Eur. J. Echocardiogr. 2011, 12, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, S.; Awad, A.; Nam, D.K.; Hoskins, M.H.; Samuels, O.; Higginson, J.; Clements, S.D. Cardiomyopathy with Inverted Tako-Tsubo Pattern in the Setting of Subarachnoid Hemorrhage: A Series of Four Cases. Neurocrit. Care 2013, 18, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Y.-S. Clinical Features and Outcome of Epinephrine-Induced Takotsubo Syndrome: Analysis of 33 Published Cases. Cardiovasc. Revasc. Med. 2016, 17, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Amin, A.; Setayesh, A.; Pouraliakbar, H.; Mozaffari, K.; Maleki, M. Pheochromocytoma-Induced Reverse Tako-Tsubo with Rapid Recovery of Left Ventricular Function. Cardiol. J. 2012, 19, 527–531. [Google Scholar] [CrossRef]
- Komamura, K. Takotsubo Cardiomyopathy: Pathophysiology, Diagnosis and Treatment. World J. Cardiol. 2014, 6, 602. [Google Scholar] [CrossRef]
- Isogai, T.; Matsui, H.; Tanaka, H.; Fushimi, K.; Yasunaga, H. Early β-Blocker Use and in-Hospital Mortality in Patients with Takotsubo Cardiomyopathy. Heart 2016, 102, 1029–1035. [Google Scholar] [CrossRef]
- Takashi, U.; Ken, K.; Takuzo, H.; Katsuhiro, Y.; Yoshihiro, T.; Ichiro, N. Emotional Stress Induces Transient Left Ventricular Hypocontraction in the Rat Via Activation of Cardiac Adrenoceptors A Possible Animal Model of ‘Tako-Tsubo’ Cardiomyopathy. Circ. J. 2002, 66, 712–713. [Google Scholar] [CrossRef]
- Santoro, F.; Ieva, R.; Ferraretti, A.; Ienco, V.; Carpagnano, G.; Lodispoto, M.; di Biase, L.; di Biase, M.; Brunetti, N.D. Safety and Feasibility of Levosimendan Administration in Takotsubo Cardiomyopathy: A Case Series. Cardiovasc. Ther. 2013, 31, e133–e137. [Google Scholar] [CrossRef]
- Boyd, B.; Solh, T. Takotsubo Cardiomyopathy: Review of Broken Heart Syndrome. J. Am. Acad. PAs 2020, 33, 24–29. [Google Scholar] [CrossRef]
- Ong, G.J.; Nguyen, T.H.; Stansborough, J.; Surikow, S.; Mahadavan, G.; Worthley, M.; Horowitz, J. The N-AcetylCysteine and RAMipril in Takotsubo Syndrome Trial (NACRAM): Rationale and Design of a Randomised Controlled Trial of Sequential N-Acetylcysteine and Ramipril for the Management of Takotsubo Syndrome. Contemp. Clin. Trials 2020, 90, 105894. [Google Scholar] [CrossRef]
- Nayeri, A.; Rafla-Yuan, E.; Krishnan, S.; Ziaeian, B.; Cadeiras, M.; McPherson, J.A.; Wells, Q.S. Psychiatric Illness in Takotsubo (Stress) Cardiomyopathy: A Review. Psychosomatics 2018, 59, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Bybee, K.A.; Kara, T.; Prasad, A.; Lerman, A.; Barsness, G.W.; Scott Wright, R.; Rihal, C.S. Systematic Review: Transient Left Ventricular Apical Ballooning: A Syndrome That Mimics ST-Segment Elevation Myocardial Infarction. Ann. Intern. Med. 2004, 141, 858–865. [Google Scholar] [CrossRef]
- Citro, R.; Rigo, F.; D’Andrea, A.; Ciampi, Q.; Parodi, G.; Provenza, G.; Piccolo, R.; Mirra, M.; Zito, C.; Giudice, R.; et al. Echocardiographic Correlates of Acute Heart Failure, Cardiogenic Shock, and in-Hospital Mortality in Tako-Tsubo Cardiomyopathy. JACC Cardiovasc. Imaging 2014, 7, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Athanasiadis, A.; Schwab, J.; Pistner, W.; Gottwald, U.; Schoeller, R.; Toepel, W.; Winter, K.D.; Stellbrink, C.; Müller-Honold, T.; et al. Complications in the Clinical Course of Tako-Tsubo Cardiomyopathy. Int. J. Cardiol. 2014, 176, 199–205. [Google Scholar] [CrossRef]
- Pant, S.; Deshmukh, A.; Mehta, K.; Badheka, A.O.; Tuliani, T.; Patel, N.J.; Dabhadkar, K.; Prasad, A.; Paydak, H. Burden of Arrhythmias in Patients with Takotsubo Cardiomyopathy (Apical Ballooning Syndrome). Int. J. Cardiol. 2013, 170, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Stiermaier, T.; Santoro, F.; Eitel, C.; Graf, T.; Möller, C.; Tarantino, N.; Guastafierro, F.; di Biase, M.; Thiele, H.; Brunetti, N.D.; et al. Prevalence and Prognostic Relevance of Atrial Fibrillation in Patients with Takotsubo Syndrome. Int. J. Cardiol. 2017, 245, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Madias, C.; Fitzgibbons, T.P.; Alsheikh-Ali, A.A.; Bouchard, J.L.; Kalsmith, B.; Garlitski, A.C.; Tighe, D.A.; Estes, N.A.M.; Aurigemma, G.P.; Link, M.S. Acquired Long QT Syndrome from Stress Cardiomyopathy Is Associated with Ventricular Arrhythmias and Torsades de Pointes. Heart Rhythm. 2011, 8, 555–561. [Google Scholar] [CrossRef] [PubMed]
- El-Battrawy, I.; Cammann, V.L.; Kato, K.; Szawan, K.A.; di Vece, D.; Rossi, A.; Wischnewsky, M.; Hermes-Laufer, J.; Gili, S.; Citro, R.; et al. Impact of Atrial Fibrillation on Outcome in Takotsubo Syndrome: Data from the International Takotsubo Registry. J. Am. Heart Assoc. 2021, 10, e014059. [Google Scholar] [CrossRef]
- Prasitlumkum, N.; Kittipibul, V.; Limpruttidham, N.; Rattanawong, P.; Chongsathidkiet, P.; Boondarikpornpant, T. The Presence of Atrial Fibrillation in Takotsubo Cardiomyopathy Is Predictive of Mortality: Systematic Review and Meta-analysis. Ann. Noninvasive Electrocardiol. 2019, 24, e12566. [Google Scholar] [CrossRef]
- Parodi, G.; del Pace, S.; Salvadori, C.; Carrabba, N.; Olivotto, I.; Gensini, G.F. Left Ventricular Apical Ballooning Syndrome as a Novel Cause of Acute Mitral Regurgitation. J. Am. Coll. Cardiol. 2007, 50, 647–649. [Google Scholar] [CrossRef]
- Heckle, M.R.; McCoy, C.W.; Akinseye, O.A.; Khouzam, R.N. Stress-Induced Thrombus: Prevalence of Thromboembolic Events and the Role of Anticoagulation in Takotsubo Cardiomyopathy. Ann. Transl. Med. 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Mayer, T.; Salanitri, J.; Salinger, M.H. Cardiac MRI Documented Left Ventricular Thrombus Complicating Acute Takotsubo Syndrome: An Uncommon Dilemma. Int. J. Cardiovasc. Imaging 2007, 23, 591–593. [Google Scholar] [CrossRef] [PubMed]
- del Buono, M.G.; O’Quinn, M.P.; Garcia, P.; Gerszten, E.; Roberts, C.; Moeller, F.G.; Abbate, A. Cardiac Arrest Due to Ventricular Fibrillation in a 23-Year-Old Woman with Broken Heart Syndrome. Cardiovasc. Pathol. 2017, 30, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaushik, S.; Nautiyal, A.; Choudhary, S.K.; Kayastha, B.L.; Mostow, N.; Lazar, J.M. Cardiac Rupture in Takotsubo Cardiomyopathy: A Systematic Review. Clin. Cardiol. 2011, 34, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Kato, K.; Cammann, V.L.; Gili, S.; Jurisic, S.; di Vece, D.; Candreva, A.; Ding, K.J.; Micek, J.; Szawan, K.A.; et al. Long-Term Prognosis of Patients with Takotsubo Syndrome. J. Am. Coll. Cardiol. 2018, 72, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; El-Sayed, A.M.; Salka, S. In-Hospital Mortality among Patients with Takotsubo Cardiomyopathy: A Study of the National Inpatient Sample 2008 to 2009. Am. Heart J. 2012, 164, 215–221. [Google Scholar] [CrossRef]
- Singh, K.; Carson, K.; Shah, R.; Sawhney, G.; Singh, B.; Parsaik, A.; Gilutz, H.; Usmani, Z.; Horowitz, J. Meta-Analysis of Clinical Correlates of Acute Mortality in Takotsubo Cardiomyopathy. Am. J. Cardiol. 2014, 113, 1420–1428. [Google Scholar] [CrossRef]
- Nomura, S.; Komuro, I. Precision Medicine for Heart Failure Based on Molecular Mechanisms: The 2019 ISHR Research Achievement Award Lecture. J. Mol. Cell Cardiol. 2021, 152, 29–39. [Google Scholar] [CrossRef]
- Moinuddin, A.; Sethi, Y.; Goel, A.; Uniyal, N. Predicting Long-Term Ablation Targets for Ventricular Arrhythmia; the Evolution with Computational Cardiology—Correspondence. Int. J. Surg. 2022, 108, 106987. [Google Scholar] [CrossRef]



| Disease | Clinical Presentation | Electrocardiography | Echocardiography | Coronary Angiography | Cardiac Magnetic Resonance | Biomarkers |
|---|---|---|---|---|---|---|
| Stress Cardiomyopathy | Chest pain (mostly substernal), dyspnea, syncope, tachycardia, arrhythmias (tachyarrhythmias or bradyarrhythmia), mitral regurgitation or sudden cardiac arrest. | ST-segment elevation, T-wave inversion, QTc prolongation | Hypo/ akinesia in apical, basal, midventricular, or focal regions | Evidence of obstructive CAD and acute plaque rupture are absent on angiography | Edema: Dysfunctional ventricular myocardium shows transmural edema. Cine-CMR: shows RWMAs depending on the anatomical variant DGE absent (cut-off >5 SD) | NT-proBNP and BNP are severely elevated. Troponins and CK-MB are mildly elevated. |
| Myocardial Infarction | Chest pain (usually at the center of the chest “Levine sign” and radiating to an upper extremity, particularly arms, shoulder, and lower jaw), diaphoresis, nausea/vomiting, dyspnea, malaise, arrhythmias, sudden cardiac death. | ST-segment elevation, ST-segment depression and/or T-wave inversion | RWMAs correspond to the vascular distribution of epicardial coronary arteries involved. | CAD with acute plaque rupture, thrombosis, or coronary dissection | Edema: Can be subendocardial or transmural at the locations of RWMAs. Cine-CMR: RWMAs correspond to the vascular distribution of epicardial coronary arteries involved. DGE: Affected regions show bright DGE in subendocardial or transmural patterns and correspond to vascular distribution of involved coronary arteries. | Troponin and CKMB levels markedly elevated. BNP and NT-proBNP mildly elevated |
| Myocarditis | Chest pain, dyspnea, excessive fatigue or exercise intolerance, unexplained sinus tachycardia and respiratory distress/tachypnea. May lead to acute heart failure and sudden cardiac death. Preceding upper respiratory infection or enteritis is often present. | ECG can be normal or have non-specific findings such as ST–T-wave changes (myopericarditis typically shows diffuse ST elevations) | Global systolic dysfunction (can sometimes be regional or segmental), LV dilation, changes in LV geometry, and wall motion abnormalities. The pericardium may also be involved. | Evidence of obstructive CAD and acute plaque rupture are absent on angiography | Edema: Distribution is subepicardial, lateral, or basal Cine-CMR: Generally global dysfunction except when focal edema is severe. DGE: Subepicardial, midventricular or focal “patchy” low intensity or bright DGE. Distribution does not correspond to coronary vascular patterns. | Troponin may or may not be elevated. CK-MB mildly elevated. BNP and NT-proBNP are mildly elevated. Acute phase reactants (like ESR, CRP) elevated. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sethi, Y.; Murli, H.; Kaiwan, O.; Vora, V.; Agarwal, P.; Chopra, H.; Padda, I.; Kanithi, M.; Popoviciu, M.S.; Cavalu, S. Broken Heart Syndrome: Evolving Molecular Mechanisms and Principles of Management. J. Clin. Med. 2023, 12, 125. https://doi.org/10.3390/jcm12010125
Sethi Y, Murli H, Kaiwan O, Vora V, Agarwal P, Chopra H, Padda I, Kanithi M, Popoviciu MS, Cavalu S. Broken Heart Syndrome: Evolving Molecular Mechanisms and Principles of Management. Journal of Clinical Medicine. 2023; 12(1):125. https://doi.org/10.3390/jcm12010125
Chicago/Turabian StyleSethi, Yashendra, Hamsa Murli, Oroshay Kaiwan, Vidhi Vora, Pratik Agarwal, Hitesh Chopra, Inderbir Padda, Manasa Kanithi, Mihaela Simona Popoviciu, and Simona Cavalu. 2023. "Broken Heart Syndrome: Evolving Molecular Mechanisms and Principles of Management" Journal of Clinical Medicine 12, no. 1: 125. https://doi.org/10.3390/jcm12010125
APA StyleSethi, Y., Murli, H., Kaiwan, O., Vora, V., Agarwal, P., Chopra, H., Padda, I., Kanithi, M., Popoviciu, M. S., & Cavalu, S. (2023). Broken Heart Syndrome: Evolving Molecular Mechanisms and Principles of Management. Journal of Clinical Medicine, 12(1), 125. https://doi.org/10.3390/jcm12010125









