Combined Brain–Heart Imaging in Takotsubo Syndrome: Towards a Holistic Patient Assessment
Abstract
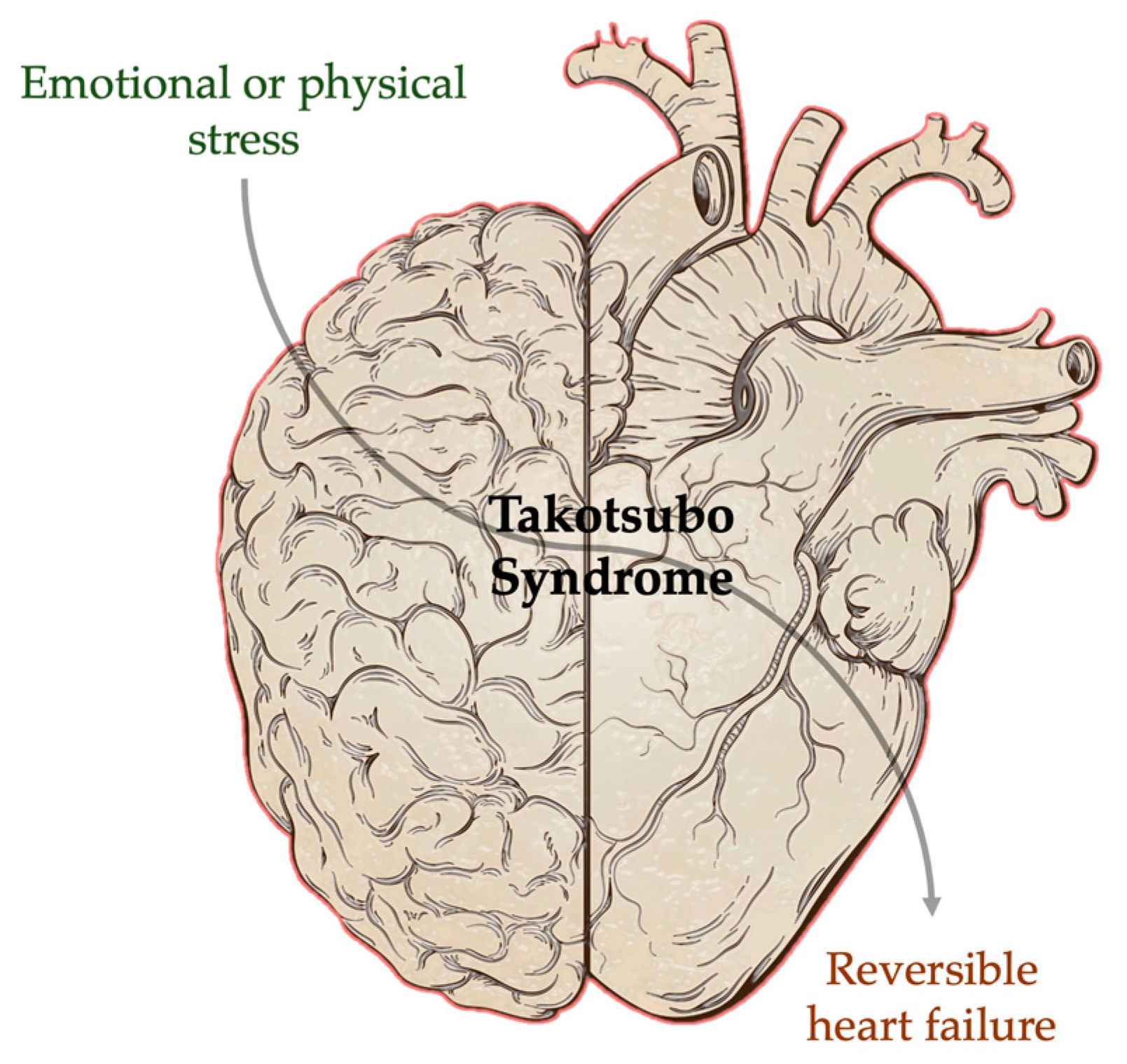
1. Introduction
2. Mechanisms of Brain–Heart Interaction in Takotsubo Syndrome
3. Imaging Brain–Heart Interactions in Takotsubo Syndrome
3.1. Molecular/Nuclear Imaging
3.1.1. Metabolic and Sympathetic Activity Imaging
3.1.2. Inflammation Imaging
3.1.3. Perfusion and Blood Flow Imaging
3.2. Magnetic Resonance Imaging (MRI)
3.2.1. Brain MRI
3.2.2. Cardiovascular Magnetic Resonance
3.3. Combined Nuclear and Magnetic Resonance Evaluation of Brain–Heart Interactions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Arbelo, E.; Barriales-Villa, R.; Basso, C.; Biagini, E.; Blom, N.A.; De Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Napp, L.C.; Cammann, V.L.; Jaguszewski, M.; Szawan, K.A.; Wischnewsky, M.; Gili, S.; Knorr, M.; Heiner, S.; Citro, R.; Bossone, E.; et al. Coexistence and outcome of coronary artery disease in takotsubo syndrome. Eur. Heart J. 2020, 41, 3255–3268. [Google Scholar] [CrossRef]
- Bybee, K.A.; Prasad, A. Stress-related cardiomyopathy syndromes. Circulation 2008, 118, 397–409. [Google Scholar] [CrossRef]
- Saito, H.; Itoh, T.; Itoh, M.; Kanaya, Y.; Suzuki, T.; Hiramori, K. Simultaneous multivessel coronary spasm causing acute myocardial infarction: A case report. Angiology 2007, 58, 112–117. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; LeDoux, J.E.; Sapolsky, R.M. The influence of stress hormones on fear circuitry. Annu. Rev. Neurosci. 2009, 32, 289–313. [Google Scholar] [CrossRef]
- Pelliccia, F.; Kaski, J.C.; Crea, F.; Camici, P.G. Pathophysiology of Takotsubo Syndrome. Circulation 2017, 135, 2426–2441. [Google Scholar] [CrossRef] [PubMed]
- Sved, A.F.; Cano, G.; Passerin, A.M.; Rabin, B.S. The locus coeruleus, Barrington’s nucleus, and neural circuits of stress. Physiol. Behav. 2002, 77, 737–742. [Google Scholar] [CrossRef]
- Francis, G.S. Modulation of peripheral sympathetic nerve transmission. J. Am. Coll. Cardiol. 1988, 12, 250–254. [Google Scholar] [CrossRef]
- Wink, J.; van Delft, R.; Notenboom, R.G.E.; Wouters, P.F.; DeRuiter, M.C.; Plevier, J.W.M.; Jongbloed, M.R.M. Human adult cardiac autonomic innervation: Controversies in anatomical knowledge and relevance for cardiac neuromodulation. Auton. Neurosci. Basic Clin. 2020, 227, 102674. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Matsumoto, Y.; Kaneta, T.; Sugimura, K.; Takahashi, J.; Fukumoto, Y.; Takahashi, S.; Shimokawa, H. Evidence for brain activation in patients with takotsubo cardiomyopathy. Circ. J. 2014, 78, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Hiestand, T.; Ghadri, J.R.; Templin, C.; Jäncke, L.; Hänggi, J. Takotsubo Syndrome—Predictable from brain imaging data. Sci. Rep. 2017, 7, 5434. [Google Scholar] [CrossRef]
- Hiestand, T.; Hänggi, J.; Klein, C.; Topka, M.S.; Jaguszewski, M.; Ghadri, J.R.; Lüscher, T.F.; Jäncke, L.; Templin, C. Takotsubo Syndrome Associated with Structural Brain Alterations of the Limbic System. J. Am. Coll. Cardiol. 2018, 71, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Hänggi, J.; Klein, C.; Topka, M.S.; Hiestand, T.; Levinson, R.A.; Jurisic, S.; Lüscher, T.F.; Ghadri, J.R.; Jäncke, L. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur. Heart J. 2019, 40, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Radfar, A.; Abohashem, S.; Osborne, M.T.; Wang, Y.; Dar, T.; Hassan, M.Z.O.; Ghoneem, A.; Naddaf, N.; Patrich, T.; Abbasi, T.; et al. Stress-Associated neurobiological activity associates with the risk for and timing of subsequent Takotsubo syndrome. Eur. Heart J. 2021, 42, 1898–1908. [Google Scholar] [CrossRef]
- Markousis-mavrogenis, G.; Mitsikostas, D.D.; Koutsogeorgopoulou, L.; Dimitroulas, T.; Katsifis, G.; Argyriou, P.; Apostolou, D.; Velitsista, S.; Vartela, V.; Manolopoulou, D.; et al. Combined brain-heart magnetic resonance imaging in autoimmune rheumatic disease patients with cardiac symptoms: Hypothesis generating insights from a cross-sectional study. J. Clin. Med. 2020, 9, 447. [Google Scholar] [CrossRef]
- Wollenweber, T.; Roentgen, P.; Schäfer, A.; Schatka, I.; Zwadlo, C.; Brunkhorst, T.; Berding, G.; Bauersachs, J.; Bengel, F.M. Characterizing the inflammatory tissue response to acute myocardial infarction by clinical multimodality noninvasive imaging. Circ. Cardiovasc. Imaging 2014, 7, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Citro, R.; Okura, H.; Ghadri, J.R.; Izumi, C.; Meimoun, P.; Izumo, M.; Dawson, D.; Kaji, S.; Eitel, I.; Kagiyama, N.; et al. Multimodality imaging in takotsubo syndrome: A joint consensus document of the European Association of Cardiovascular Imaging (EACVI) and the Japanese Society of Echocardiography (JSE). J. Echocardiogr. 2020, 18, 199–224. [Google Scholar] [CrossRef]
- Matsuo, S.; Nakajima, K.; Kinuya, S.; Yamagishi, M. Diagnostic utility of 123I-BMIPP imaging in patients with Takotsubo cardiomyopathy. J. Cardiol. 2014, 64, 49–56. [Google Scholar] [CrossRef]
- Bengel, F.M.; Ross, T.L. Emerging imaging targets for infiltrative cardiomyopathy: Inflammation and fibrosis. J. Nucl. Cardiol. 2019, 26, 208–216. [Google Scholar] [CrossRef]
- Bengel, F.M.; Hermanns, N.; Thackeray, J.T. Radionuclide Imaging of the Molecular Mechanisms Linking Heart and Brain in Ischemic Syndromes. Circ. Cardiovasc. Imaging 2020, 13, E011303. [Google Scholar] [CrossRef] [PubMed]
- Ora, M.; Gambhir, S. Myocardial perfusion imaging: A brief review of nuclear and nonnuclear techniques and comparative evaluation of recent advances. Indian J. Nucl. Med. 2019, 34, 263–270. [Google Scholar]
- Khan, H.; Gamble, D.T.; Rudd, A.; Mezincescu, A.M.; Abbas, H.; Noman, A.; Stewart, A.; Horgan, G.; Krishnadas, R.; Williams, C.; et al. Structural and Functional Brain Changes in Acute Takotsubo Syndrome. JACC Heart Fail. 2023, 11, 307–317. [Google Scholar] [CrossRef]
- Dichtl, W.; Tuovinen, N.; Barbieri, F.; Adukauskaite, A.; Senoner, T.; Rubatscher, A.; Hintringer, F.; Siedentopf, C.; Bauer, A.; Gizewski, E.R.; et al. Functional neuroimaging in the acute phase of Takotsubo syndrome: Volumetric and functional changes of the right insular cortex. Clin. Res. Cardiol. 2020, 109, 1107–1113. [Google Scholar] [CrossRef]
- Steiger, R.; Tuovinen, N.; Adukauskaite, A.; Senoner, T.; Spitaler, P.; Bilgeri, V.; Dabkowska-Mika, A.; Siedentopf, C.; Bauer, A.; Gizewski, E.R.; et al. Limbic Responses to Aversive Visual Stimuli during the Acute and Recovery Phase of Takotsubo Syndrome. J. Clin. Med. 2022, 11, 4891. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Crimì, F.; Vernuccio, F.; Cabrelle, G.; Lupi, A.; Zanon, C.; Gambato, S.; Perazzolo, A.; Quaia, E. Medical Radiology: Current Progress. Diagnostics 2023, 13, 2439. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.I.; Kallifatidis, A.; Kourtidou, S.; Lama, N.; Christidi, A.; Detorakis, E.; Chatzantonis, G.; Vrachliotis, T.; Karamitsos, T.; Kouskouras, K.; et al. Cardiovascular magnetic resonance for the evaluation of patients with cardiovascular disease: An overview of current indications, limitations, and procedures. Hell. J. Cardiol. 2023, 70, 53–64. [Google Scholar] [CrossRef]
- Rischpler, C.; Nekolla, S.G.; Heusch, G.; Umutlu, L.; Rassaf, T.; Heusch, P.; Herrmann, K.; Nensa, F. Cardiac PET/MRI—An update. Eur. J. Hybrid Imaging 2019, 3, 2. [Google Scholar] [CrossRef]
- Pyatigorskaya, N.; Habert, M.O.; Rozenblum, L. Contribution of PET-MRI in brain diseases in clinical practice. Curr. Opin. Neurol. 2020, 33, 430–438. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markousis-Mavrogenis, G.; Pepe, A.; Bacopoulou, F.; Lupi, A.; Quaia, E.; Chrousos, G.P.; Mavrogeni, S.I. Combined Brain–Heart Imaging in Takotsubo Syndrome: Towards a Holistic Patient Assessment. J. Clin. Med. 2024, 13, 2991. https://doi.org/10.3390/jcm13102991
Markousis-Mavrogenis G, Pepe A, Bacopoulou F, Lupi A, Quaia E, Chrousos GP, Mavrogeni SI. Combined Brain–Heart Imaging in Takotsubo Syndrome: Towards a Holistic Patient Assessment. Journal of Clinical Medicine. 2024; 13(10):2991. https://doi.org/10.3390/jcm13102991
Chicago/Turabian StyleMarkousis-Mavrogenis, George, Alessia Pepe, Flora Bacopoulou, Amalia Lupi, Emilio Quaia, George P. Chrousos, and Sophie I. Mavrogeni. 2024. "Combined Brain–Heart Imaging in Takotsubo Syndrome: Towards a Holistic Patient Assessment" Journal of Clinical Medicine 13, no. 10: 2991. https://doi.org/10.3390/jcm13102991
APA StyleMarkousis-Mavrogenis, G., Pepe, A., Bacopoulou, F., Lupi, A., Quaia, E., Chrousos, G. P., & Mavrogeni, S. I. (2024). Combined Brain–Heart Imaging in Takotsubo Syndrome: Towards a Holistic Patient Assessment. Journal of Clinical Medicine, 13(10), 2991. https://doi.org/10.3390/jcm13102991











