Prognostic Significance of Baseline Blood Glucose Levels and Glucose Variability in Severe Acute Kidney Injury: A Secondary Analysis from the RENAL Study
Abstract
:1. Background
2. Methods
2.1. Blood Glucose Levels
2.2. Glycemic Variability
2.3. Study Outcomes
2.4. Statistical Analysis
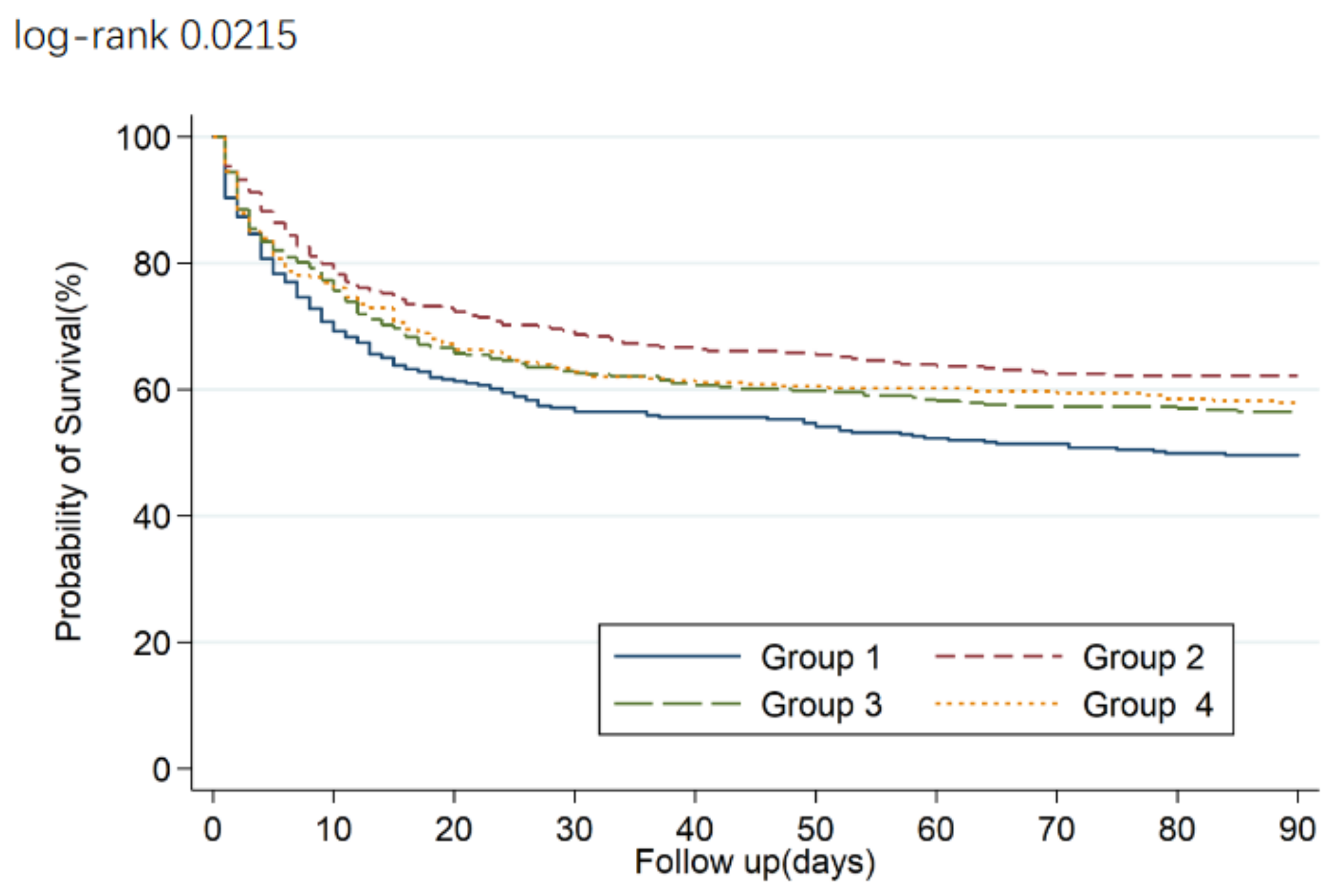
3. Results
Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoste, E.A.J.; Kellum, J.A. Acute kidney injury: Epidemiology and diagnostic criteria. Curr. Opin. Crit. Care 2006, 12, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Kellum, J.A.; Schmidt, C.; Van Aken, H.; Wempe, C.; Pavenstädt, H.; Boanta, A.; Gerß, J.; Meersch, M. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA 2016, 315, 2190–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heung, M.; Steffick, D.E.; Zivin, K.; Gillespie, B.W.; Banerjee, T.; Hsu, C.-Y.; Powe, N.R.; Pavkov, M.E.; Williams, D.E.; Saran, R.; et al. Acute Kidney Injury Recovery Pattern and Subsequent Risk of CKD: An Analysis of Veterans Health Administration Data. Am. J. Kidney Dis. 2016, 67, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Rossignol, P. Cardiovascular Consequences of Acute Kidney Injury. N. Engl. J. Med. 2020, 382, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, A.; Abdel-Rahman, E.M.; Liu, K.D.; Goldstein, S.L.; Agarwal, A.; Okusa, M.D.; Cerda, J.; AKI!NOW Steering Committee. Recovery after Critical Illness and Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2021, 16, 1601–1609. [Google Scholar] [CrossRef]
- Siegelaar, S.E.; Hermanides, J.; Oudemans-van Straaten, H.M.; van der Voort, P.H.J.; Bosman, R.J.; Zandstra, D.F.; DeVries, J.H. Mean Glucose during ICU Admission Is Related to Mortality by a U-Shaped Curve in Surgical and Medical Patients: A Retrospective Cohort Study. Crit. Care 2010, 14, R224. [Google Scholar] [CrossRef] [Green Version]
- van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive Insulin Therapy in Critically Ill Patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef]
- NICE-SUGAR Study Investigators; Finfer, S.; Chittock, D.R.; Su, S.Y.-S.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P.; et al. Intensive versus Conventional Glucose Control in Critically Ill Patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef] [Green Version]
- Meyfroidt, G.; Keenan, D.M.; Wang, X.; Wouters, P.J.; Veldhuis, J.D.; Van den Berghe, G. Dynamic characteristics of blood glucose time series during the course of critical illness: Effects of intensive insulin therapy and relative association with mortality. Crit. Care Med. 2010, 38, 1021–1029. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Bellomo, R.; Jacka, M.J.; Egi, M.; Hart, G.K.; George, C. The impact of early hypoglycemia and blood glucose variability on outcome in critical illness. Crit. Care 2009, 13, R91. [Google Scholar] [CrossRef]
- Krinsley, J.S. Glycemic Variability and Mortality in Critically Ill Patients: The Impact of Diabetes. J. Diabetes Sci. Technol. 2009, 3, 1292–1301. [Google Scholar] [CrossRef] [Green Version]
- Krinsley, J.S.; Grover, A. Severe hypoglycemia in critically ill patients: Risk factors and outcomes. Crit. Care Med. 2007, 35, 2262–2267. [Google Scholar] [CrossRef]
- Meynaar, I.A.; Eslami, S.; Abu-Hanna, A.; van der Voort, P.; de Lange, D.W.; de Keizer, N. Blood glucose amplitude variability as predictor for mortality in surgical and medical intensive care unit patients: A multicenter cohort study. J. Crit. Care 2012, 27, 119–124. [Google Scholar] [CrossRef]
- Aldawood, A. Outcome and prognostic factors of critically ill patients with acute renal failure requiring continuous renal replacement therapy. Saudi J. Kidney Dis. Transpl. 2010, 21, 1106–1110. [Google Scholar]
- RENAL Replacement Therapy Study Investigators; Bellomo, R.; Cass, A.; Cole, L.; Finfer, S.; Gallagher, M.; Lo, S.; McArthur, C.; McGuinness, S.; Myburgh, J.; et al. Intensity of Continuous Renal-Replacement Therapy in Critically Ill Patients. N. Engl. J. Med. 2009, 361, 1627–1638. [Google Scholar] [CrossRef] [Green Version]
- Eslami, S.; Taherzadeh, Z.; Schultz, M.J.; Abu-Hanna, A. Glucose Variability Measures and Their Effect on Mortality: A Systematic Review. Intensive Care Med. 2011, 37, 583–593. [Google Scholar] [CrossRef] [Green Version]
- Dela Cruz, C.M.; Pineda, L.; Rogelio, G.; Alano, F. Clinical Profile and Factors Affecting Mortality in Acute Renal Failure. Ren. Fail. 1992, 14, 161–168. [Google Scholar] [CrossRef]
- Sezer, M.T.; Demir, M.; Gungor, G.; Senol, A. Predictors of Mortality in Patients with Acute Renal Failure. Acta Med. 2006, 49, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Haap, M.; Heller, E.; Thamer, C.; Tschritter, O.; Stefan, N.; Fritsche, A. Association of Serum Phosphate Levels with Glucose Tolerance, Insulin Sensitivity and Insulin Secretion in Non-Diabetic Subjects. Eur. J. Clin. Nutr. 2006, 60, 734–739. [Google Scholar] [CrossRef] [Green Version]
- Schutte, E.; Lambers Heerspink, H.J.; Lutgers, H.L.; Bakker, S.J.L.; Vart, P.; Wolffenbuttel, B.H.R.; Umanath, K.; Lewis, J.B.; de Zeeuw, D.; Gansevoort, R.T. Serum Bicarbonate and Kidney Disease Progression and Cardiovascular Outcome in Patients with Diabetic Nephropathy: A Post Hoc Analysis of the RENAAL (Reduction of End Points in Non-Insulin-Dependent Diabetes with the Angiotensin II Antagonist Losartan) Study and IDNT (Irbesartan Diabetic Nephropathy Trial). Am. J. Kidney Dis. 2015, 66, 450–458. [Google Scholar] [CrossRef]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute Renal Failure in Critically Ill Patients: A Multinational, Multicenter Study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Ympa, Y.P.; Sakr, Y.; Reinhart, K.; Vincent, J.-L. Has Mortality from Acute Renal Failure Decreased? A Systematic Review of the Literature. Am. J. Med. 2005, 118, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Egi, M.; George, C.; Bellomo, R. Australia New Zealand Intensive Care Society Database Management Committee Early Blood Glucose Control and Mortality in Critically Ill Patients in Australia. Crit. Care Med. 2009, 37, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.M.; Heung, M.; Vilay, A.M.; Eyler, R.F.; Patel, C.; Mueller, B.A. In Vitro Glucose Kinetics during Continuous Renal Replacement Therapy: Implications for Caloric Balance in Critically Ill Patients. Int. J. Artif. Organs 2013, 36, 861–868. [Google Scholar] [CrossRef]
- Kim, Y.; Rajan, K.B.; Sims, S.A.; Wroblewski, K.E.; Reutrakul, S. Impact of Glycemic Variability and Hypoglycemia on Adverse Hospital Outcomes in Non-Critically Ill Patients. Diabetes Res. Clin. Pract. 2014, 103, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Donati, A.; Damiani, E.; Domizi, R.; Botticelli, L.; Castagnani, R.; Gabbanelli, V.; Nataloni, S.; Carsetti, A.; Scorcella, C.; Adrario, E.; et al. Glycaemic Variability, Infections and Mortality in a Medical-Surgical Intensive Care Unit. Crit. Care Resusc. 2014, 16, 13–23. [Google Scholar] [PubMed]
- Sim, M.A.; Liu, W.; Ng, R.R.G.; Ti, L.K.; Chew, S.T.H. Wider Perioperative Glycemic Fluctuations Increase Risk of Postoperative Acute Kidney Injury: A Prospective Cohort Study. Medicine 2015, 94, e1953. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Hermanides, J.; Vriesendorp, T.M.; Bosman, R.J.; Zandstra, D.F.; Hoekstra, J.B.; Devries, J.H. Glucose Variability Is Associated with Intensive Care Unit Mortality. Crit. Care Med. 2010, 38, 838–842. [Google Scholar] [CrossRef]
- Ying, C.; Zhou, X.; Chang, Z.; Ling, H.; Cheng, X.; Li, W. Blood Glucose Fluctuation Accelerates Renal Injury Involved to Inhibit the AKT Signaling Pathway in Diabetic Rats. Endocrine 2016, 53, 81–96. [Google Scholar] [CrossRef]
- Chang, C.-M.; Hsieh, C.-J.; Huang, J.-C.; Huang, I.-C. Acute and Chronic Fluctuations in Blood Glucose Levels Can Increase Oxidative Stress in Type 2 Diabetes Mellitus. Acta Diabetol. 2012, 49 (Suppl. S1), S171–S177. [Google Scholar] [CrossRef]
- Rodbard, D. Clinical Interpretation of Indices of Quality of Glycemic Control and Glycemic Variability. Postgrad. Med. 2011, 123, 107–118. [Google Scholar] [CrossRef]
| Characteristic | Group 1 Glucose < 5.8 (n = 339) | Group 2 5.8 ≤ Glucose < 7.2 (n = 344) | Group 3 7.2 ≤ Glucose < 9.1 (n = 362) | Group 4 Glucose ≥ 9.1 (n = 360) | p Value |
|---|---|---|---|---|---|
| Age (years) | 63.7 ± 15.6 | 63.7 ± 15.0 | 64.1 ± 14.5 | 66.3 ± 14.1 | 0.06 |
| Male sex–no (%) | 190 (56.1) | 216 (62.8) | 255 (70.4) | 241 (66.9) | 0.0006 |
| Time in ICU before randomization (hour) | 41.5 ± 102.5 | 58.7 ± 145.9 | 66.7 ± 140.6 | 41.3 ± 78.0 | 0.008 |
| Mechanical ventilation–no. (%) | 239 (70.5) | 251 (73.0) | 275 (76.0) | 287 (79.7) | 0.03 |
| Severe sepsis–no. (%) | 188 (55.6) | 169 (49.1) | 182 (50.3) | 163 (45.3) | 0.06 |
| APACHE III score | 106.3 ± 25.7 | 98.2 ± 25.0 | 99.4 ± 26.2 | 107.0 ± 24.7 | <0.0001 |
| SOFA score | 10.7 ± 3.0 | 10.1 ± 2.9 | 10.5 ± 2.7 | 10.2 ± 2.5 | 0.03 |
| Weight (kg) | 78.7 ± 12.7 | 81.2 ± 12.7 | 81.7 ± 13.0 | 80.8 ± 12.9 | 0.01 |
| Albumin (g/L) | 24.9 ± 7.1 | 26.7 ± 7.2 | 25.5 ± 6.8 | 26.8 ± 7.1 | 0.0004 |
| Creatinine (ummol/L) | 341.9 ± 218.4 | 336.8 ± 197.5 | 330.1 ± 197.0 | 323.3 ± 215.0 | 0.66 |
| Potassium (mmol/L) | 4.9 ± 0.9 | 4.8 ± 0.8 | 4.8 ± 0.9 | 4.9 ± 1.0 | 0.11 |
| Phosphate (mmol/L) | 2.05 ± 0.81 | 2.07 ± 0.83 | 1.93 ± 0.80 | 2.02 ± 0.89 | 0.12 |
| Bicarbonate (mmol/L) | 17.4 ± 6.0 | 19.1 ± 5.9 | 19.3 ± 5.6 | 17.7 ± 5.6 | <0.0001 |
| No. of days in ICU | 12.8 ± 16.7 | 11.6 ± 13.3 | 12.6 ± 14.9 | 10.9 ± 12.2 | 0.30 |
| No. of days in hospital | 25.6 ± 26.2 | 26.8 ± 24.5 | 26.8 ± 25.8 | 24.4 ± 24.4 | 0.55 |
| Outcomes | Fourth of Glucose | Event, n (%) | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|---|
| Category | n | HR (95% CI) | p-Value | P Trend | HR (95% CI) | p-Value | P Trend | ||
| Death in 90 days | <5.8 | 339 | 175 (51.7) | 1.49 (1.19–1.86) | 0.0006 | 0.20 | 1.48 (1.17–1.88) | 0.001 | 0.02 |
| 5.8–7.2 | 344 | 133 (38.7) | 1 | ||||||
| 7.2–9.1 | 362 | 161 (44.5) | 1.21 (0.96–1.52) | 0.11 | 1.22 (0.96–1.55) | 0.11 | |||
| >9.1 | 359 | 158 (44.0) | 1.21 (0.96–1.53) | 0.10 | 1.03 (0.81–1.32) | 0.79 | |||
| Continuous Glucose CV (%) | Quartile of CV (%) | P Trend for Quartile | ||||
|---|---|---|---|---|---|---|
| Q1 <16 | Q2 16–22 | Q3 22–30 | Q4 >30 | |||
| n | 1340 | 356 | 311 | 337 | 337 | |
| Median of Glucose CV | 22 | 12 | 19 | 26 | 37 | |
| Outcomes | ||||||
| No. of outcomes | 568 | 139 | 103 | 157 | 169 | |
| Crude | 1.02 (1.01–1.02) | 1 | 0.78 (0.61–1.01) | 1.14 (0.91–1.44) | 1.40 (1.12–1.75) | 0.0002 |
| Model 1 | 1.02 (1.01–1.02) | 1 | 0.79 (0.61–1.03) | 1.14 (0.90–1.43) | 1.37 (1.09–1.72) | 0.0007 |
| Model 2 | 1.02 (1.01–1.03) | 1 | 0.72 (0.55–0.94) | 1.05 (0.83–1.33) | 1.26 (0.99–1.60) | 0.007 |
| Model 3 | 1.02 (1.02–1.03) | 1 | 0.72 (0.55–0.94) | 1.10 (0.86–1.40) | 1.37 (1.08–1.75) | 0.0008 |
| Continuous Glucose std (mmol/L) | Quartile of STD (%) | P Trend for Quartile | ||||
|---|---|---|---|---|---|---|
|
Q1 <1.03 |
Q2 1.03–1.52 |
Q3 1.52–2.21 |
Q4 >2.21 | |||
| n | 1340 | 331 | 332 | 342 | 335 | |
| Median of Glucose std | 1.52 | 0.77 | 1.26 | 1.82 | 2.79 | |
| Outcomes | ||||||
| No. of outcomes | 568 | 136 | 125 | 149 | 158 | |
| Crude | 1.10 (1.03–1.17) | 1 | 0.84 (0.66–1.08) | 0.99 (0.79–1.26) | 1.17 (0.93–1.47) | 0.08 |
| Model 1 | 1.09 (1.03–1.17) | 1 | 0.85 (0.67–1.09) | 0.98 (0.78–1.24) | 1.14 (0.90–1.43) | 0.15 |
| Model 2 | 1.27 (1.16–1.38) | 1 | 0.73 (0.56–0.94) | 0.86 (0.68–1.10) | 1.01 (0.80–1.29) | 0.54 |
| Model 3 | 1.29 (1.17–1.42) | 1 | 0.76 (0.58–0.98) | 0.97 (0.76–1.25) | 1.33 (1.02–1.74) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Lin, J.; Gallagher, M.; Bellomo, R.; Wang, X.; Jardine, M.; Duan, M.; Wang, A., on behalf of the Renal Study Investigators and the Anzics Clinical Trials Group. Prognostic Significance of Baseline Blood Glucose Levels and Glucose Variability in Severe Acute Kidney Injury: A Secondary Analysis from the RENAL Study. J. Clin. Med. 2023, 12, 15. https://doi.org/10.3390/jcm12010015
Xie Y, Lin J, Gallagher M, Bellomo R, Wang X, Jardine M, Duan M, Wang A on behalf of the Renal Study Investigators and the Anzics Clinical Trials Group. Prognostic Significance of Baseline Blood Glucose Levels and Glucose Variability in Severe Acute Kidney Injury: A Secondary Analysis from the RENAL Study. Journal of Clinical Medicine. 2023; 12(1):15. https://doi.org/10.3390/jcm12010015
Chicago/Turabian StyleXie, Ying, Jin Lin, Martin Gallagher, Rinaldo Bellomo, Xia Wang, Meg Jardine, Meili Duan, and Amanda Wang on behalf of the Renal Study Investigators and the Anzics Clinical Trials Group. 2023. "Prognostic Significance of Baseline Blood Glucose Levels and Glucose Variability in Severe Acute Kidney Injury: A Secondary Analysis from the RENAL Study" Journal of Clinical Medicine 12, no. 1: 15. https://doi.org/10.3390/jcm12010015
APA StyleXie, Y., Lin, J., Gallagher, M., Bellomo, R., Wang, X., Jardine, M., Duan, M., & Wang, A., on behalf of the Renal Study Investigators and the Anzics Clinical Trials Group. (2023). Prognostic Significance of Baseline Blood Glucose Levels and Glucose Variability in Severe Acute Kidney Injury: A Secondary Analysis from the RENAL Study. Journal of Clinical Medicine, 12(1), 15. https://doi.org/10.3390/jcm12010015





