Impact of Chronic Kidney Disease on Corneal Neuroimmune Features in Type 2 Diabetes
Abstract
:1. Introduction
2. Methods
2.1. Study Participants
2.2. Indicators of Metabolism and Kidney Function
2.3. Corneal Confocal Microscopy
2.4. Peripheral Neuropathy Assessment
2.5. Data Analysis
3. Results
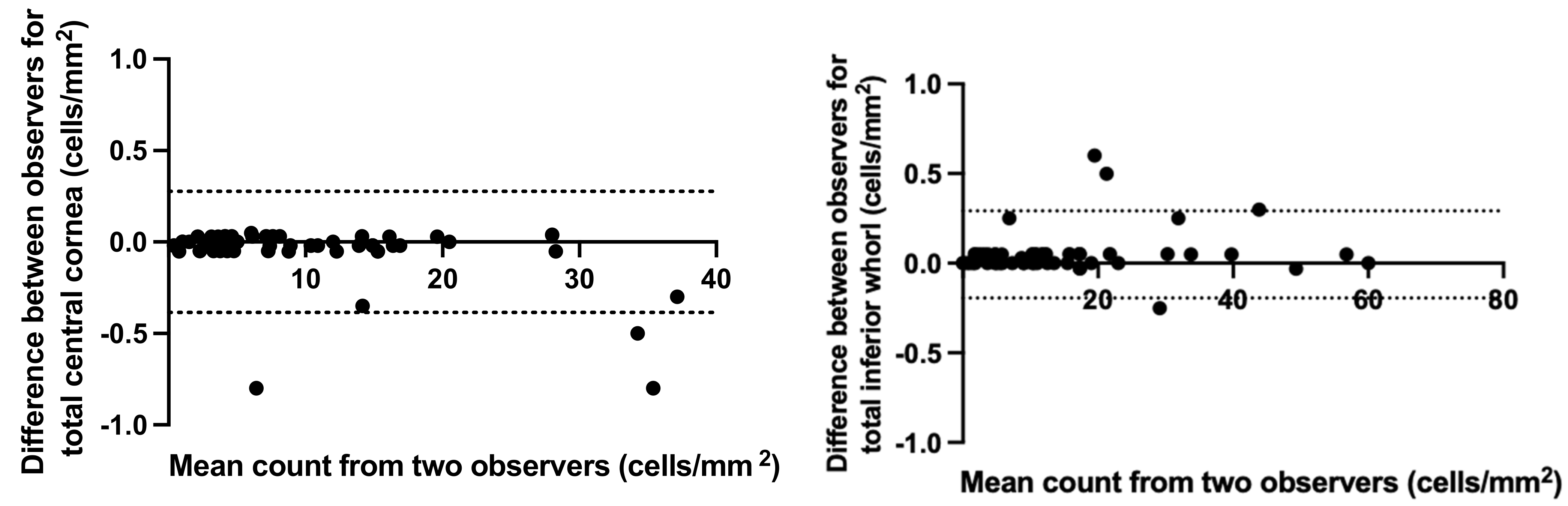
3.1. Concordance between Masked Independent Observers
3.2. Participant Demographics, Metabolic Indicators and Measures of Ocular Surface Discomfort
3.3. Corneal Nerve Parameters
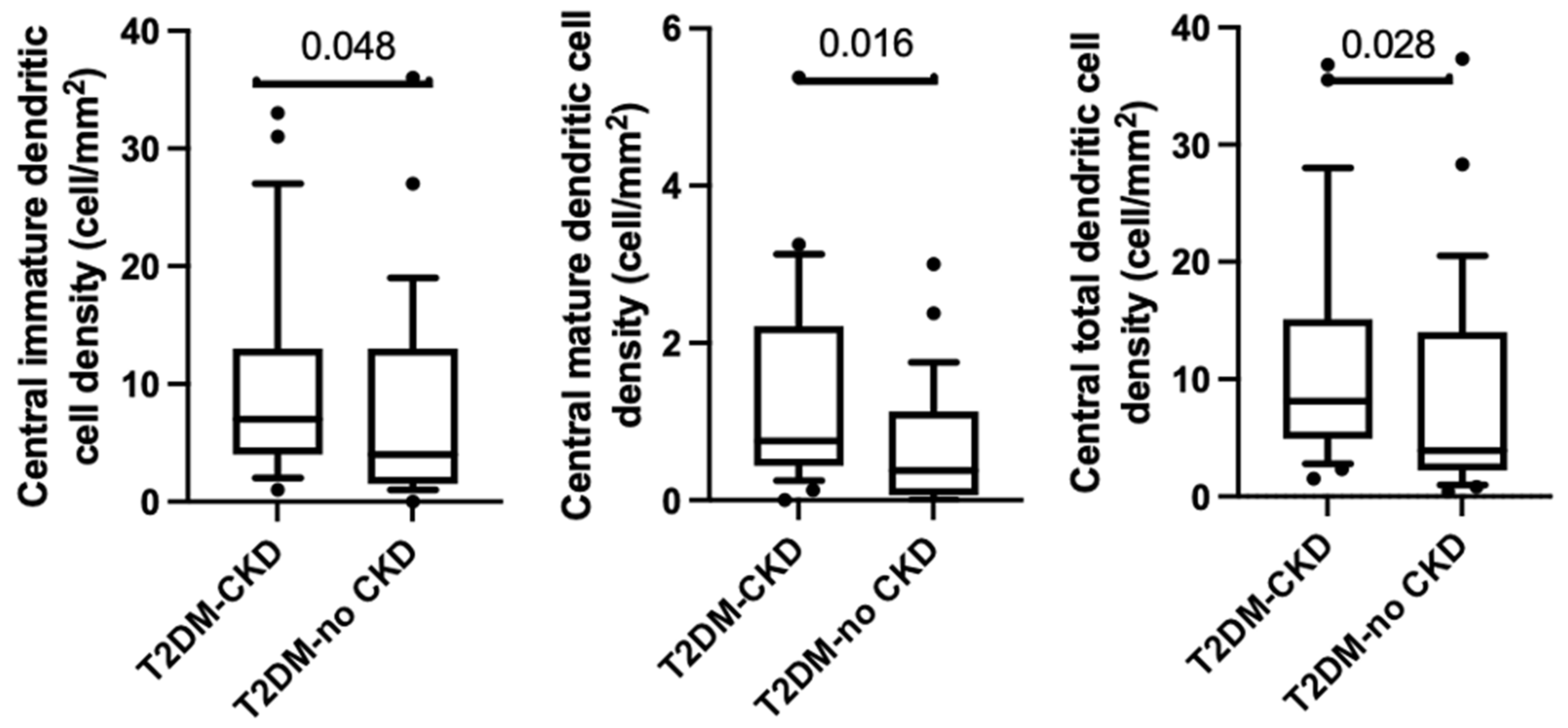
3.4. Corneal Dendritic Cell Density
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tummanapalli, S.S.; Issar, T.; Yan, A.; Kwai, N.; Poynten, A.M.; Krishnan, A.V.; Willcox, M.D.P.; Markoulli, M. Corneal nerve fiber loss in diabetes with chronic kidney disease. Ocul. Surf. 2020, 18, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Lagali, N.S.; Badian, R.A.; Liu, X.; Feldreich, T.R.; Arnlov, J.; Utheim, T.P.; Dahlin, L.B.; Rolandsson, O. Dendritic cell maturation in the corneal epithelium with onset of type 2 diabetes is associated with tumor necrosis factor receptor superfamily member 9. Sci. Rep. 2018, 8, 14248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, M.; Boulton, A.J.; Efron, N.; Malik, R.A. Increased Langerhan cell density and corneal nerve damage in diabetic patients: Role of immune mechanisms in human diabetic neuropathy. Cont. Lens Anterior Eye 2011, 34, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Lisowska-Myjak, B. Uremic toxins and their effects on multiple organ systems. Nephron Clin. Pract. 2014, 128, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [Green Version]
- Lasagni Vitar, R.M.; Rama, P.; Ferrari, G. The two-faced effects of nerves and neuropeptides in corneal diseases. Prog. Retin. Eye Res. 2022, 86, 100974. [Google Scholar] [CrossRef]
- Kim, J.; Markoulli, M. Automatic analysis of corneal nerves imaged using in vivo confocal microscopy. Clin. Exp. Optom. 2018, 101, 147–161. [Google Scholar] [CrossRef] [Green Version]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; de Francisco, A.L.; de Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Dabbah, M.A.; Graham, J.; Petropoulos, I.N.; Tavakoli, M.; Malik, R.A. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med. Image Anal. 2011, 15, 738–747. [Google Scholar] [CrossRef]
- Misra, S.; Craig, J.P.; McGhee, C.N.; Patel, D.V. Interocular comparison by in vivo confocal microscopy of the 2-dimensional architecture of the normal human corneal subbasal nerve plexus. Cornea 2012, 31, 1376–1380. [Google Scholar] [CrossRef]
- Patel, D.V.; McGhee, C.N. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4485–4488. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.V.; McGhee, C.N.J. In vivo laser scanning confocal microscopy confirms that the human corneal sub-basal nerve plexus is a highly dynamic structure. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3409–3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petropoulos, I.N.; Ferdousi, M.; Marshall, A.; Alam, U.; Ponirakis, G.; Azmi, S.; Fadavi, H.; Efron, N.; Tavakoli, M.; Malik, R.A. The Inferior Whorl For Detecting Diabetic Peripheral Neuropathy Using Corneal Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2498–2504. [Google Scholar] [CrossRef] [Green Version]
- Vagenas, D.; Pritchard, N.; Edwards, K.; Shahidi, A.M.; Sampson, G.P.; Russell, A.W.; Malik, R.A.; Efron, N. Optimal image sample size for corneal nerve morphometry. Optom. Vis. Sci. 2012, 89, 812–817. [Google Scholar] [CrossRef]
- Chiang, J.C.B.; Goldstein, D.; Tavakoli, A.; Trinh, T.; Klisser, J.; Lewis, C.R.; Friedlander, M.; Naduvilath, T.J.; Au, K.; Park, S.B.; et al. Corneal dendritic cells and the subbasal nerve plexus following neurotoxic treatment with oxaliplatin or paclitaxel. Sci. Rep. 2021, 11, 22884. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Nubile, M.; Lanzini, M.; Carpineto, P.; Ciancaglini, M.; Pannellini, T.; di Nicola, M.; Dua, H.S. Epithelial dendritic cell distribution in normal and inflamed human cornea: In vivo confocal microscopy study. Am. J. Ophthalmol. 2006, 142, 736–744. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petropoulos, I.N.; Ponirakis, G.; Ferdousi, M.; Azmi, S.; Kalteniece, A.; Khan, A.; Gad, H.; Bashir, B.; Marshall, A.; Boulton, A.J.M.; et al. Corneal Confocal Microscopy: A Biomarker for Diabetic Peripheral Neuropathy. Clin. Ther. 2021, 43, 1457–1475. [Google Scholar] [CrossRef]
- Perkins, B.A.; Lovblom, L.E.; Lewis, E.J.H.; Bril, V.; Ferdousi, M.; Orszag, A.; Edwards, K.; Pritchard, N.; Russell, A.; Dehghani, C.; et al. Corneal Confocal Microscopy Predicts the Development of Diabetic Neuropathy: A Longitudinal Diagnostic Multinational Consortium Study. Diabetes Care 2021, 44, 2107–2114. [Google Scholar] [CrossRef]
- Cornblath, D.R.; Chaudhry, V.; Carter, K.; Lee, D.; Seysedadr, M.; Miernicki, M.; Joh, T. Total neuropathy score: Validation and reliability study. Neurology 1999, 53, 1660–1664. [Google Scholar] [CrossRef]
- Issar, T.; Arnold, R.; Kwai, N.C.G.; Pussell, B.A.; Endre, Z.H.; Poynten, A.M.; Kiernan, M.C.; Krishnan, A.V. The utility of the Total Neuropathy Score as an instrument to assess neuropathy severity in chronic kidney disease: A validation study. Clin. Neurophysiol. 2018, 129, 889–894. [Google Scholar] [CrossRef]
- Dehghani, C.; Pritchard, N.; Edwards, K.; Vagenas, D.; Russell, A.W.; Malik, R.A.; Efron, N. Natural history of corneal nerve morphology in mild neuropathy associated with type 1 diabetes: Development of a potential measure of diabetic peripheral neuropathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7982–7990. [Google Scholar] [CrossRef] [Green Version]
- D’Onofrio, L.; Kalteniece, A.; Ferdousi, M.; Azmi, S.; Petropoulos, I.N.; Ponirakis, G.; Alam, U.; Asghar, O.; Marshall, A.; Boulton, A.J.M.; et al. Small Nerve Fiber Damage and Langerhans Cells in Type 1 and Type 2 Diabetes and LADA Measured by Corneal Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2021, 62, 5. [Google Scholar] [CrossRef]
- Whelchel, A.E.; Nicholas, S.E.; Ma, J.X.; Karamichos, D. Nerve influence on the metabolism of type I and type II diabetic corneal stroma: An in vitro study. Sci. Rep. 2021, 11, 13627. [Google Scholar] [CrossRef]
- Pritchard, N.; Edwards, K.; Russell, A.W.; Perkins, B.A.; Malik, R.A.; Efron, N. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care 2015, 38, 671–675. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Z.; Zhang, Y.; Wang, H.; Liu, X.; Zhang, S.; Liu, X.; Fan, D. Corneal sub-basal whorl-like nerve plexus: A landmark for early and follow-up evaluation in transthyretin familial amyloid polyneuropathy. Eur. J. Neurol. 2021, 28, 630–638. [Google Scholar] [CrossRef]
- Tummanapalli, S.S.; Issar, T.; Kwai, N.; Pisarcikova, J.; Poynten, A.M.; Krishnan, A.V.; Willcox, M.D.P.; Markoulli, M. A Comparative Study on the Diagnostic Utility of Corneal Confocal Microscopy and Tear Neuromediator Levels in Diabetic Peripheral Neuropathy. Curr. Eye Res. 2020, 45, 921–930. [Google Scholar] [CrossRef]
- Chiang, J.C.B.; Khou, V.; Tavakoli, A.; Park, S.B.; Goldstein, D.; Krishnan, A.V.; Markoulli, M. Reproducibility and Reliability of Subbasal Corneal Nerve Parameters of the Inferior Whorl in the Neurotoxic and Healthy Cornea. Cornea 2022, 41, 1487–1494. [Google Scholar] [CrossRef]
- Mobeen, R.; Stapleton, F.; Chao, C.; Madigan, M.C.; Briggs, N.; Golebiowski, B. Corneal epithelial dendritic cell density in the healthy human cornea: A meta-analysis of in-vivo confocal microscopy data. Ocul. Surf. 2019, 17, 753–762. [Google Scholar] [CrossRef]
- Akhlaq, A.; Colon, C.; Cavalcanti, B.M.; Aggarwal, S.; Qazi, Y.; Cruzat, A.; Jersey, C.; Critser, D.B.; Watts, A.; Beyer, J.; et al. Density and distribution of dendritiform cells in the peripheral cornea of healthy subjects using in vivo confocal microscopy. Ocul. Surf. 2022, 26, 157–165. [Google Scholar] [CrossRef]
- Colorado, L.H.; Edwards, K.; Chinnery, H.R.; Bazan, H.E. In vivo immune cell dynamics in the human cornea. Exp Eye Res 2020, 199, 108168. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.; Rama, P.; Olzi, D.; Lambiase, A. Neurotrophic keratitis. Eye 2003, 17, 989–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Zong, R.R.; Zhu, F.F.; Han, W.; Wang, Y.X.; Wang, G.L.; Wang, Y.Z.; Mao, Y.B.; Guan, T.J.; Liu, Z.G.; Xue, Y.H.; et al. Tear dynamics testing and quantitative proteomics analysis in patients with chronic renal failure. J. Proteom. 2021, 248, 104351. [Google Scholar] [CrossRef]
- Kal, O.; Ulusoy, M.O.; Kal, A.; Tanriaski, G.; Cezairlioglu, S. Evaluation of Dry Eye Using Anterior Segment Optical Coherence Tomography in Patients With End-Stage Renal Disease Undergoing Hemodialysis. Ther. Apher. Dial. 2018, 22, 104–108. [Google Scholar] [CrossRef]
- Aktas, S.; Sagdik, H.M.; Aktas, H.; Gulcan, E.; Tetikoglu, M.; Cosgun, S.; Caliskan, S.; Ozcura, F. Tear function in patients with chronic renal failure undergoing hemodialysis. Ren. Fail. 2015, 37, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.W.; Yoon, M.H.; Lee, S.W.; Chin, H.S. Effect of hemodialysis (HD) on intraocular pressure, ocular surface, and macular change in patients with chronic renal failure. Effect of hemodialysis on the ophthalmologic findings. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 153–162. [Google Scholar] [CrossRef]
| Parameter | T2DM-CKD | T2DM-No CKD | p-Value |
|---|---|---|---|
| Age, years | 70.6 ± 7.5 | 66.4 ± 9.2 | p = 0.06 |
| Sex, % Male | 65.5 | 79.3 | p = 0.24 |
| Body mass index, kg/m2 | 30.8 ± 7.4 | 31.8 ± 7.0 | p = 0.61 |
| Duration of diagnosis, years | 19.3 ± 9.1 | 15.1 ± 12.0 | p = 0.16 |
| HbA1c, % | 8.3 ± 1.9 | 8.8 ± 2.3 | p = 0.43 |
| Serum Urea, mg/dL | 11.6 ± 4.8 | 6.0 ± 1.9 | p < 0.01 |
| Creatinine, mg/dL | 200.3 ± 114.1 | 74.7 ± 138.2 | p < 0.01 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 35.5 ± 17.8 | 82.0 ± 10.1 | p < 0.01 |
| Urine Albumin-creatinine ratio, mg/mmol | 49.6 ± 92.1 | 3.8 ± 3.9 | p < 0.01 |
| Serum Potassium, mmol/L | 4.6 ± 0.4 | 4.2 ± 0.5 | p = 0.01 |
| Alkaline phosphatase, U/L | 78.3 ± 33.7 | 88.9 ± 26.7 | p = 0.61 |
| Gamma-glutamyl transferase, U/L | 26.8 ± 14.5 | 58.0 ± 95.1 | p = 0.20 |
| Alanine transaminase, U/L | 24.5 ± 17.1 | 32.6 ± 24.6 | p = 0.24 |
| Aspartate transaminase, U/L | 26.5 ± 13.5 | 31.3 ± 26.3 | p = 0.63 |
| Albumin, g/L | 38.1 ± 5.6 | 39.3 ± 5.6 | p = 0.32 |
| Total protein, g/L | 70.6 ± 4.8 | 69.7± 6.1 | p = 0.34 |
| Bilirubin µmol/L | 9.6 ± 5.7 | 10.2 ± 7.4 | p = 0.62 |
| Total cholesterol, mmol/l | 3.8 ± 1.0 | 3.9 ± 1.2 | p = 0.66 |
| High density lipoprotein (HDL), mmol/L | 1.1 ± 0.4 | 1.2 ± 0.4 | p = 0.44 |
| Low density Lipoprotein (LDL), mmol/L | 1.9 ± 0.9 | 1.8 ± 1.1 | p = 0.82 |
| Triglycerides, mmol/L | 2.2 ± 2.1 | 1.75 ± 1.4 | p = 0.48 |
| Total Neuropathy Score | 8.9 ± 6.8 | 6.7 ± 5.4 | p = 0.20 |
| Parameter | T2DM-CKD | T2DM-No CKD | p-Value |
|---|---|---|---|
| Corneal nerve fiber density (CNFD) (no./mm2) | 12.5 ± 6.4 | 19.6 ± 7.1 | p < 0.01 |
| Corneal nerve branch density (CNBD) (no./mm2) | 20.2 ± 20.6 | 19.6 ± 10.8 | p > 0.99 |
| Corneal nerve fiber length (CNFL) (mm/mm2) | 9.0 ± 3.8 | 11.6 ± 3.6 | p = 0.01 |
| Inferior whorl length (IWL) (mm/mm2) | 8.2 ± 3.6 | 8.9 ± 3.8 | p = 0.54 |
| Average corneal nerve fiber length (IWL + CNFL/2) (mm/mm2) | 8.0 ± 3.8 | 9.7 ± 3.5 | p = 0.09 |
| Parameter | T2DM-CKD | T2DM-no CKD | p-Value |
|---|---|---|---|
| Central immature dendritic cell density (cells/mm2) | 7.0 (3.8–12.8) | 3.5 (1.4–13.4) | p < 0.05 |
| Central mature dendritic cells density (cells/mm2) | 0.8 (0.4–2.2) | 0.4 (0.6–1.1) | p = 0.02 |
| Total central dendritic cells density (cells/mm2) | 10.4 (4.3–16.1) | 3.9 (2.1–21.0) | p = 0.03 |
| Inferior whorl immature dendritic cell density (cells/mm2) | 9.6 (2.8–22.8) | 10.3 (5.5 ± 13.8) | p = 0.88 |
| Inferior mature dendritic cell (cells/mm2) | 0.5 (0–1.5) | 0.0 (0.0–0.75) | p = 0.07 |
| Total whorl dendritic density (cells/mm2) | 10.7 (2.8–21.8) | 10.3 (5.3–14.5) | p = 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asiedu, K.; Markoulli, M.; Tummanapalli, S.S.; Chiang, J.C.B.; Alotaibi, S.; Wang, L.L.; Dhanapalaratnam, R.; Kwai, N.; Poynten, A.; Krishnan, A.V. Impact of Chronic Kidney Disease on Corneal Neuroimmune Features in Type 2 Diabetes. J. Clin. Med. 2023, 12, 16. https://doi.org/10.3390/jcm12010016
Asiedu K, Markoulli M, Tummanapalli SS, Chiang JCB, Alotaibi S, Wang LL, Dhanapalaratnam R, Kwai N, Poynten A, Krishnan AV. Impact of Chronic Kidney Disease on Corneal Neuroimmune Features in Type 2 Diabetes. Journal of Clinical Medicine. 2023; 12(1):16. https://doi.org/10.3390/jcm12010016
Chicago/Turabian StyleAsiedu, Kofi, Maria Markoulli, Shyam Sunder Tummanapalli, Jeremy Chung Bo Chiang, Sultan Alotaibi, Leiao Leon Wang, Roshan Dhanapalaratnam, Natalie Kwai, Ann Poynten, and Arun V. Krishnan. 2023. "Impact of Chronic Kidney Disease on Corneal Neuroimmune Features in Type 2 Diabetes" Journal of Clinical Medicine 12, no. 1: 16. https://doi.org/10.3390/jcm12010016
APA StyleAsiedu, K., Markoulli, M., Tummanapalli, S. S., Chiang, J. C. B., Alotaibi, S., Wang, L. L., Dhanapalaratnam, R., Kwai, N., Poynten, A., & Krishnan, A. V. (2023). Impact of Chronic Kidney Disease on Corneal Neuroimmune Features in Type 2 Diabetes. Journal of Clinical Medicine, 12(1), 16. https://doi.org/10.3390/jcm12010016








