Case Report: “Spontaneous Descemet Membrane Detachment”
Abstract
:1. Introduction
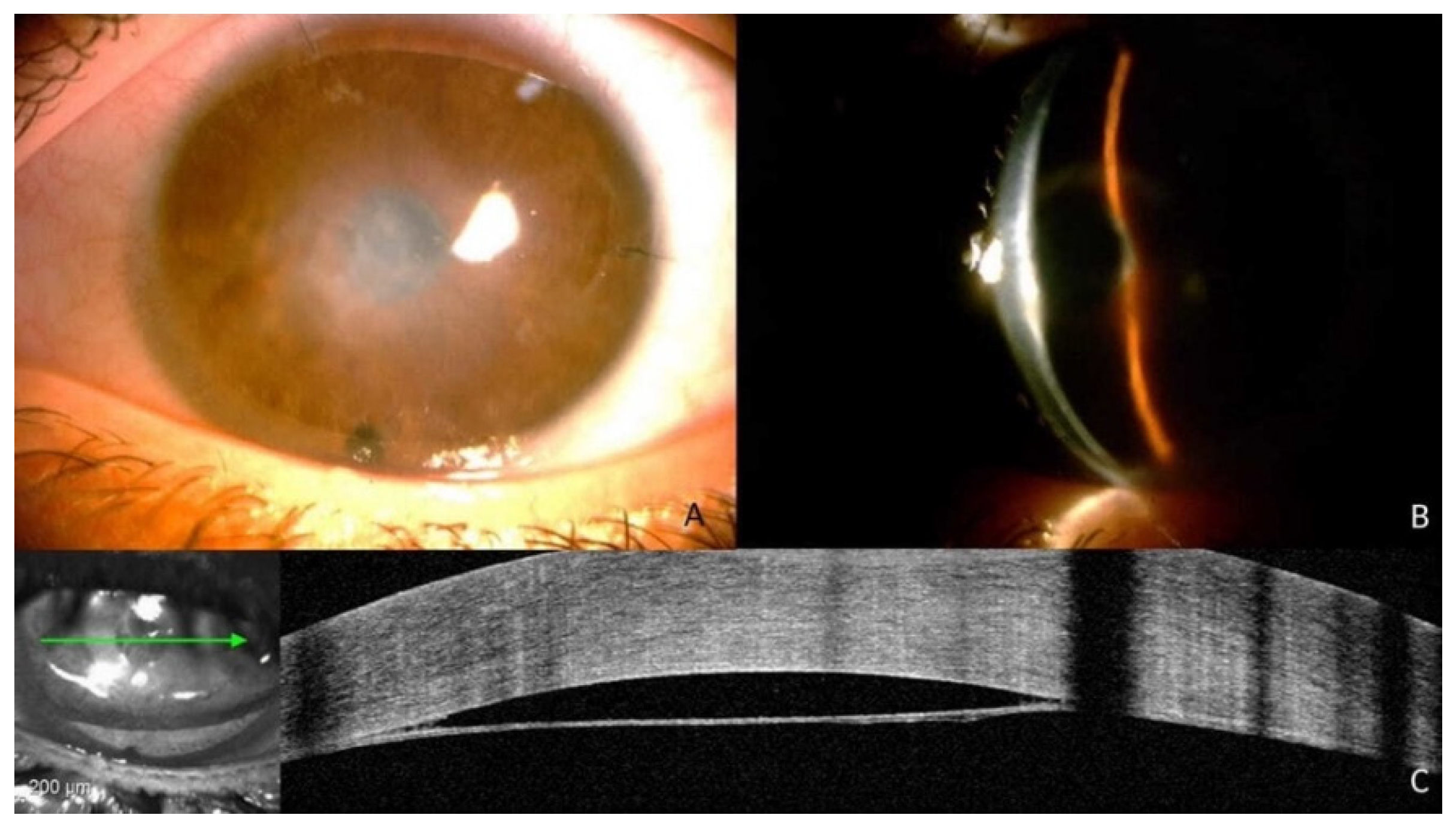
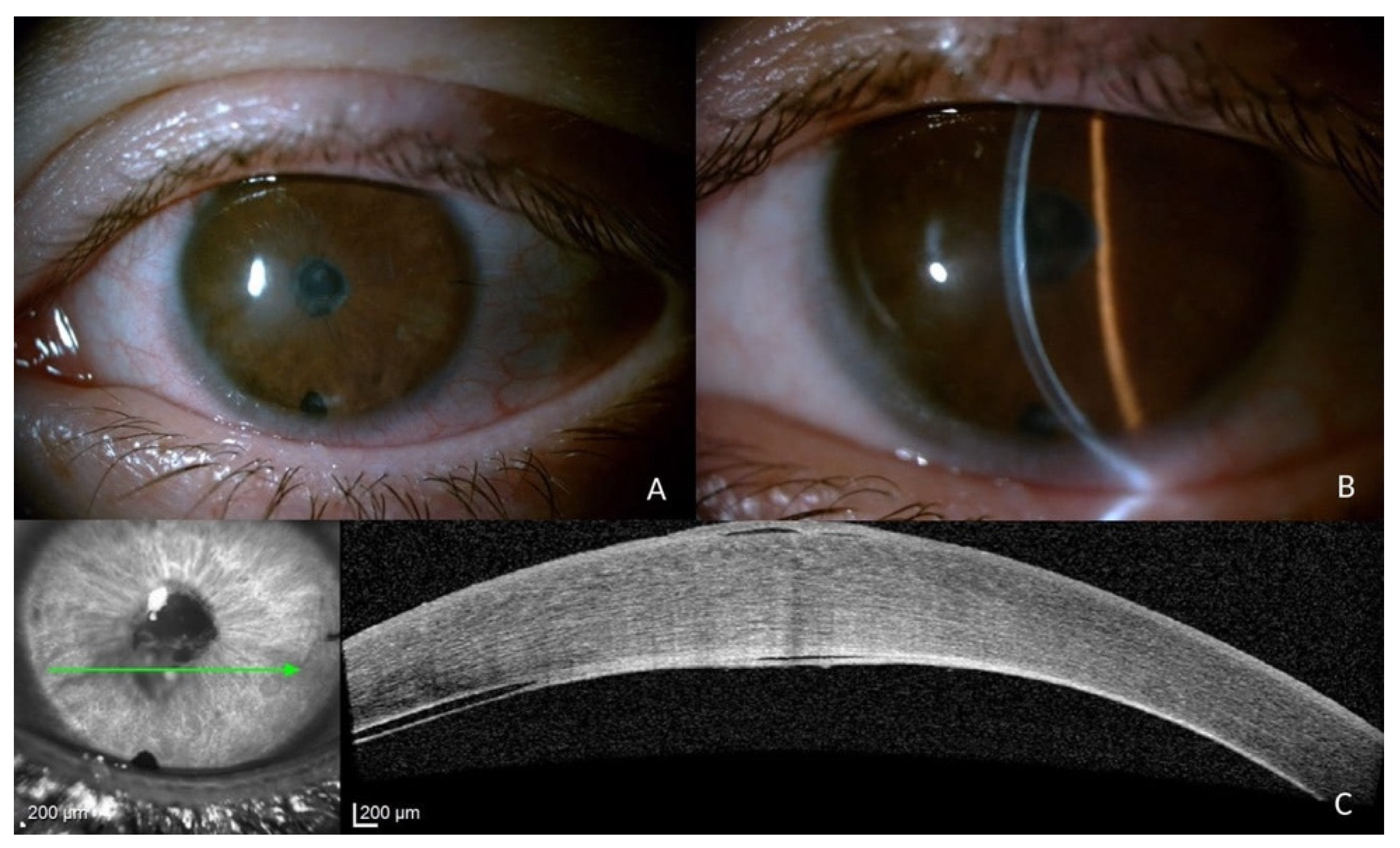
2. Case Description
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singhal, D.; Sahay, P.; Goel, S.; Asif, M.I.; Maharana, P.K.; Sharma, N. Descemet membrane detachment. Surv. Ophthalmol. 2020, 65, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Gupta, S.; Maharana, P.; Shanmugam, P.; Nagpal, R.; Vajpayee, R.B. Anterior Segment Optical Coherence Tomography-Guided Management Algorithm for Descemet Membrane Detachment after Intraocular Surgery. Cornea 2015, 34, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.W.S.; Agarwal, T.; Vajpayee, R.B.; Jhanji, V. Update on diagnosis and management of Descemet’s membrane detachment. Curr. Opin. Ophthalmol. 2013, 24, 356–361. [Google Scholar] [CrossRef]
- Monroe, W.M. Gonioscopy after cataract extraction. South Med. J. 1971, 64, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Ting, D.S.J.; Al Saadi, A.; Said, D.G. Chemical eye injury: Pathophysiology, assessment and management. Eye 2020, 34, 2001–2019. [Google Scholar] [CrossRef] [PubMed]
- Bizrah, M.; Yusuf, A.; Ahmad, S. An update on chemical eye burns. Eye 2019, 33, 1362–1377. [Google Scholar] [CrossRef]
- Najjar, D.M.; Rapuano, C.J.; Cohen, E.J. Descemet membrane detachment with hemorrhage after alkali burn to the cornea. Am. J. Ophthalmol. 2004, 137, 185–187. [Google Scholar] [CrossRef]
- Mackool, R.J.; Holtz, S.J. Descemet membrane detachment. Arch. Ophthalmol. 1977, 95, 459–463. [Google Scholar] [CrossRef]
- Trinh, L.; Brignole-Baudouin, F.; Labbé, A.; Raphaël, M.; Bourges, J.L.; Baudouin, C. The corneal endothelium in an endotoxin-induced uveitis model: Correlation between in vivo confocal microscopy and immunohistochemistry. Mol. Vis. 2008, 14, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Alfawaz, A.M.; Holland, G.N.; Yu, F.; Margolis, M.S.; Giaconi, J.A.; Aldave, A.J. Corneal Endothelium in Patients with Anterior Uveitis. Ophthalmology 2016, 123, 1637–1645. [Google Scholar] [CrossRef]
- Olsen, T. Changes in the corneal endothelium after acute anterior uveitis as seen with the specular microscope. Acta Ophthalmol. 1980, 58, 250–256. [Google Scholar] [CrossRef]
- De Oliveira, F.; de Oliveira Motta, A.C.; Muccioli, C. Corneal specular microscopy in infectious and noninfectious uveitis. Arq. Bras. Oftalmol. 2009, 72, 457–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mezaine, H.S. Descemet’s membrane detachment after cataract extraction surgery. Int. Ophthalmol. 2010, 30, 391–396. [Google Scholar] [CrossRef]
- Assia, E.I.; Levkovich-Verbin, H.; Blumenthal, M. Management of Descemet’s membrane detachment. J. Cataract Refract. Surg. 1995, 21, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.T.; Betz, P. Descemet membrane detachment after alkali ocular surface burn. Bull. Soc. Belg. Ophtalmol. 2010, 316, 85–86. [Google Scholar]
- Zhang, B.; Pan, F.; Yao, Y.F. Spontaneous resolution of extensive descemet membrane detachment caused by sodium cyanide injury to the eye. Cornea 2012, 31, 1344–1347. [Google Scholar] [CrossRef]
- Zhang, X.; Jhanji, V.; Chen, H. Tractional Descemet’s membrane detachment after ocular alkali burns: Case reports and review of literature. BMC Ophthalmol. 2018, 18, 256. [Google Scholar] [CrossRef] [PubMed]
- Yuen, H.K.L.; Yeung, B.Y.M.; Wong, T.H.; Wu, W.K.; Lam, D.S.C. Descemet membrane detachment caused by hydrogen peroxide injury. Cornea 2004, 23, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Nahum, Y.; Gal-Or, O.; Dadon, J.; Greenbaum, A.; Israeli, D.; Melles, G.R.J.; Bahar, I.; Livny, E. Spontaneous Descemet Membrane Detachment after Penetrating Keratoplasty-Clinical Presentation and Outcome of Air/Gas Descemetopexy. Cornea 2020, 39, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Gorski, M.; Shih, C.; Savoie, B.; Udell, I. Spontaneous Descemet Membrane Detachment 20 Years after Penetrating Keratoplasty for Keratoconus. Cornea 2016, 35, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Kit, V.; Kriman, J.; Vasquez-Perez, A.; Muthusamy, K.; Thaung, C.; Tuft, S. Descemet Membrane Detachment after Penetrating Keratoplasty for Keratoconus. Cornea 2020, 39, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hassanaly, S.; Hyde, R.A.; Brown, J.; Yoon, D.; Yu, C.Q. Late detachment of Descemet’s membrane after penetrating keratoplasty for pellucid marginal degeneration. Am. J. Ophthalmol. Case Rep. 2019, 13, 151–153. [Google Scholar] [CrossRef]
- Lewis, R.A.; von Wolff, K.; Tetz, M.; Koerber, N.; Kearney, J.R.; Shingleton, B.J.; Samuelson, T.W. Canaloplasty: Circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: Two-year interim clinical study results. J. Cataract Refract. Surg. 2009, 35, 814–824. [Google Scholar] [CrossRef]
- Palmiero, P.M.; Aktas, Z.; Lee, O.; Tello, C.; Sbeity, Z. Bilateral Descemet membrane detachment after canaloplasty. J. Cataract Refract. Surg. 2010, 36, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.T.L.; Lai, J.S.M.; Lam, D.S.C. Descemet membrane detachment after sequential argon-neodymium: YAG laser peripheral iridotomy. Am. J. Ophthalmol. 2002, 134, 621–622. [Google Scholar] [CrossRef]
- Rosetta, P.; Legrottaglie, E.F.; Vinciguerra, R.; Vinciguerra, P. Late Onset Descemet Membrane Detachment after Radial Keratotomy Resolved with Medical Therapy. Case Rep. Ophthalmol. Med. 2017, 2017, 5804965. [Google Scholar] [CrossRef] [Green Version]
- Gorovoy, M.S.; Gorovoy, I.R.; Ullman, S.; Gorovoy, J.B. Descemet stripping automated endothelial keratoplasty for spontaneous descemet membrane detachment in a patient with osteogenesis imperfecta. Cornea 2012, 31, 832–835. [Google Scholar] [CrossRef]
- Polat, N.; Ulucan, P.B. Nontraumatic Descemet Membrane Detachment with Tear in Osteogenesis Imperfecta. Ophthalmol. Ther. 2015, 4, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaurasia, S.; Ramappa, M.; Rao, H.L. Descemet Membrane Detachment in a Child With Anterior Megalophthalmos Managed Using Intracameral Perflouropropane (C3F8) Gas Injection. Cornea 2015, 34, 1516–1518. [Google Scholar] [CrossRef]
- Felipe, A.F.; Rapuano, C.J.; Nottage, J.M.; Abazari, A. Descemet membrane detachment among siblings: Role of anatomic and familial predisposition. Cornea 2012, 31, 836–840. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moramarco, A.; Iannetta, D.; Cimino, L.; Romano, V.; Gardini, L.; Fontana, L. Case Report: “Spontaneous Descemet Membrane Detachment”. J. Clin. Med. 2023, 12, 330. https://doi.org/10.3390/jcm12010330
Moramarco A, Iannetta D, Cimino L, Romano V, Gardini L, Fontana L. Case Report: “Spontaneous Descemet Membrane Detachment”. Journal of Clinical Medicine. 2023; 12(1):330. https://doi.org/10.3390/jcm12010330
Chicago/Turabian StyleMoramarco, Antonio, Danilo Iannetta, Luca Cimino, Vito Romano, Lorenzo Gardini, and Luigi Fontana. 2023. "Case Report: “Spontaneous Descemet Membrane Detachment”" Journal of Clinical Medicine 12, no. 1: 330. https://doi.org/10.3390/jcm12010330
APA StyleMoramarco, A., Iannetta, D., Cimino, L., Romano, V., Gardini, L., & Fontana, L. (2023). Case Report: “Spontaneous Descemet Membrane Detachment”. Journal of Clinical Medicine, 12(1), 330. https://doi.org/10.3390/jcm12010330






