Primary-Sjögren’s-Syndrome-Related Interstitial Lung Disease: A Clinical Review Discussing Current Controversies
Abstract
1. Introduction
2. Epidemiology of pSS-ILD
2.1. What Is the Real Prevalence of ILD in Patients Diagnosed with pSS?
2.2. What Are the Common Demographic Characteristics of Patients with pSS-ILD?
3. Clinical Manifestations of pSS-Related Lung Involvement
3.1. When Should Pulmonary Disease Be Suspected in pSS Patients?
3.2. When Should pSS Be Suspected in Patients with Pulmonary Disease?
3.3. Do Patients with pSS-ILD Have Distinguishing Clinical or Serologic Features?
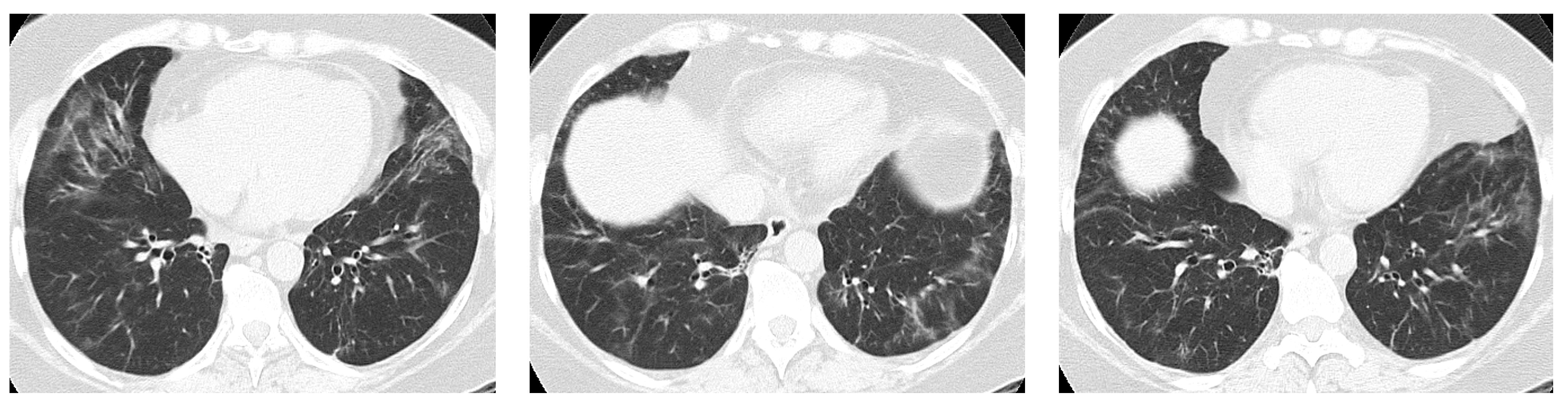
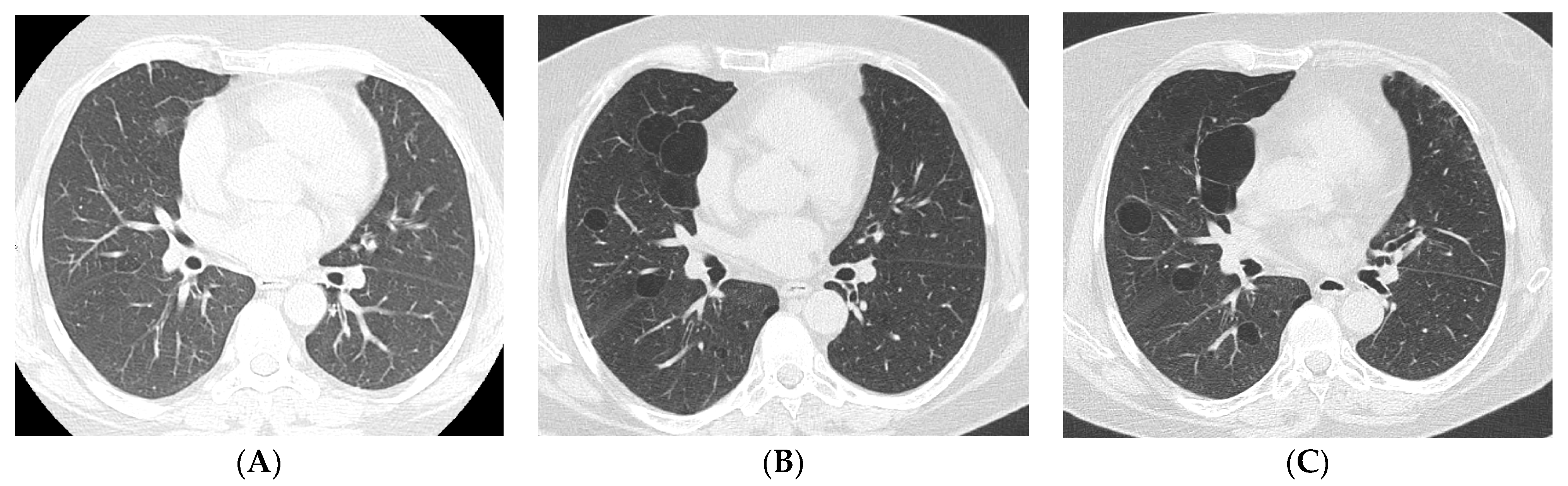
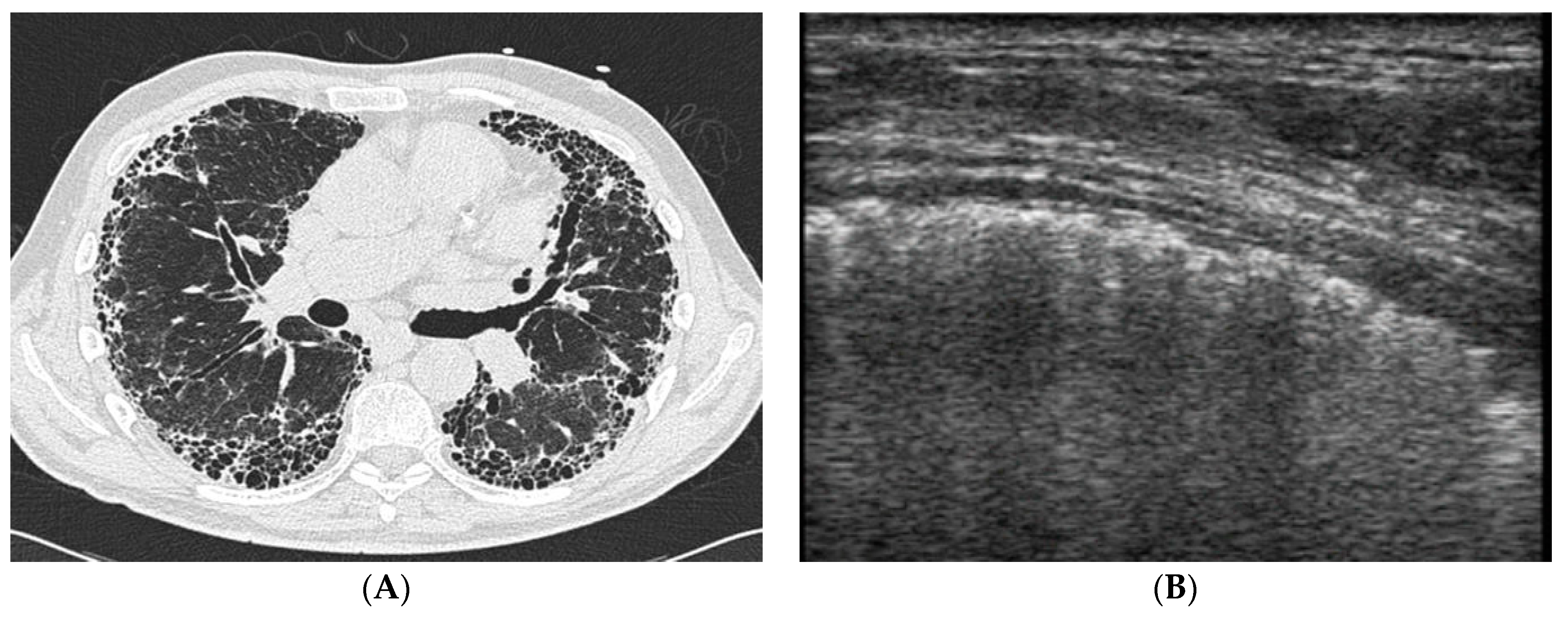
4. Imaging and Histopathology of ILD in pSS
4.1. How Does Radiologic/Histopathologic Pattern Influence the Clinical Picture and Management of pSS-ILD Patients? What Is the Role of Lung Biopsy?
4.2. Does Lung Ultrasound (LUS) Have a Defined Role in pSS-ILD Screening and Follow-Up?
5. Prognosis and Treatment of Progressive pSS-ILD
5.1. What Is the Prognosis of Patients with pSS-ILD? How Can We Identify Progressive ILD Phenotypes Deserving Pharmacologic Treatment?
5.2. What Are the Most Commonly Employed Treatment Approaches for pSS-ILD? What Therapeutic Innovations Can We Expect Based on New Insights into pSS Pathogenesis?
5.3. Is Lung Transplantation a Feasible Option for pSS-ILD Patients with Advanced Lung Disease?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mariette, X.; Criswell, L.A. Primary Sjögren’s Syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Seror, R.; Bowman, S.J.; Brito-Zeron, P.; Theander, E.; Bootsma, H.; Tzioufas, A.; Gottenberg, J.E.; Ramos-Casals, M.; Dörner, T.; Ravaud, P.; et al. EULAR Sjögren’s syndrome disease activity index [ESSDAI]: A user guide. RMD Open. 2015, 1, e000022. [Google Scholar] [CrossRef] [PubMed]
- Egashira, R.; Kondo, T.; Hirai, T.; Kamochi, N.; Yakushiji, M.; Yamasaki, F.; Irie, H. CT Findings of Thoracic Manifestations of Primary Sjögren Syndrome: Radiologic-Pathologic Correlation. Radiographics 2013, 33, 1933–1949. [Google Scholar] [CrossRef] [PubMed]
- Flament, T.; Bigot, A.; Chaigne, B.; Henique, H.; Diot, E.; Marchand-Adam, S. Pulmonary manifestations of Sjögren’s syndrome. Eur. Respir. Rev. 2016, 25, 110–123. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; Seror, R.; Bootsma, H.; Bowman, S.J.; Dörner, T.; Gottenberg, J.E.; Mariette, X.; Theander, E.; Bombardieri, S.; et al. Characterization of systemic disease in primary Sjögren’s syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology 2015, 54, 2230–2238. [Google Scholar] [CrossRef]
- He, C.; Chen, Z.; Liu, S.; Chen, H.; Zhang, F. Prevalence and risk factors of interstitial lung disease in patients with primary Sjögren’s syndrome: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2020, 23, 1009–1018. [Google Scholar] [CrossRef]
- Fauchais, A.L.; Martel, C.; Gondran, G.; Lambert, M.; Launay, D.; Jauberteau, M.O.; Hachulla, E.; Vidal, E.; Hatron, P.Y. Immunological profile in primary Sjögren syndrome: Clinical significance, prognosis and long-term evolution to other auto-immune disease. Autoimmun. Rev. 2010, 9, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Uffmann, M.; Kiener, H.P.; Bankier, A.A.; Baldt, M.M.; Zontsich, T.; Herold, C.J. Lung Manifestation in Asymptomatic Patients with Primary Sjögren Syndrome: Assessment with High Resolution CT and Pulmonary Function Tests. J. Thorac. Imaging 2001, 16, 282–289. [Google Scholar] [CrossRef]
- Franquet, T.; Giménez, A.; Monill, J.M.; Díaz, C.; Geli, C. Primary Sjögren’s syndrome and associated lung disease: CT findings in 50 patients. AJR Am. J. Roentgenol. 1997, 169, 655–658. [Google Scholar] [CrossRef]
- Gao, H.; Zou, Y.D.; Zhang, X.W.; He, J.; Zhang, J.; Sun, Y.; Li, Z.G. Interstitial lung disease in non-sicca onset primary Sjögren’s syndrome: A large-scale case-control study. Int. J. Rheum. Dis. 2018, 21, 1423–1429. [Google Scholar] [CrossRef]
- Reina, D.; Roig Vilaseca, D.; Torrente-Segarra, V.; Cerdà, D.; Castellví, I.; Díaz Torné, C.; Moreno, M.; Narváez, J.; Ortiz, V.; Blavia, R.; et al. Sjögren’s syndrome-associated interstitial lung disease: A multicenter study. Reumatol. Clin. 2016, 12, 201–205. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Theander, E.; Baldini, C.; Seror, R.; Retamozo, S.; Quartuccio, L.; Bootsma, H.; Bowman, S.J.; Dörner, T.; Gottenberg, J.-E.; et al. Early diagnosis of primary Sjögren’s syndrome: EULAR-SS task force clinical recommendations. Expert Rev. Clin. Immunol. 2016, 12, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Sambataro, G.; Ferro, F.; Orlandi, M.; Sambataro, D.; Torrisi, S.E.; Quartuccio, L.; Vancheri, C.; Baldini, C.; Cerinic, M.M. Clinical, morphological features and prognostic factors associated with interstitial lung disease in primary Sjögren’s syndrome: A systematic review from the Italian Society of Rheumatology. Autoimmun. Rev. 2020, 19, 102447. [Google Scholar] [CrossRef] [PubMed]
- Nannini, C.; Jebakumar, A.J.; Crowson, C.S.; Ryu, J.H.; Matteson, E.L. Primary Sjogren’s syndrome 1976-2005 and associated interstitial lung disease: A population-based study of incidence and mortality. BMJ Open. 2013, 3, e003569. [Google Scholar] [CrossRef] [PubMed]
- Luppi, F.; Sebastiani, M.; Silva, M.; Sverzellati, N.; Cavazza, A.; Salvarani, C.; Manfredi, A. Interstitial lung disease in Sjögren’s syndrome: A clinical review. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S126), 291–300. [Google Scholar]
- Parambil, J.G.; Myers, J.L.; Lindell, R.M.; Matteson, E.L.; Ryu, J.H. Interstitial Lung Disease in Primary Sjögren Syndrome. Chest 2006, 130, 1489–1495. [Google Scholar] [CrossRef]
- Gupta, S.; Ferrada, M.A.; Hasni, S.A. Pulmonary Manifestations of Primary Sjögren’s Syndrome: Underlying Immunological Mechanisms, Clinical Presentation, and Management. Front. Immunol. 2019, 10, 1327. [Google Scholar] [CrossRef]
- Li, X.; Xu, B.; Ma, Y.; Li, X.; Cheng, Q.; Wang, X.; Wang, G.; Qian, L.; Wei, L. Clinical and laboratory profiles of primary Sjogren’s syndrome in a Chinese population: A retrospective analysis of 315 patients. Int. J. Rheum. Dis. 2015, 18, 439–446. [Google Scholar] [CrossRef]
- Lee, A.S.; Scofield, R.H.; Hammitt, K.M.; Gupta, N.; Thomas, D.E.; Moua, T.; Ussavarungsi, K.; Clair, E.W.S.; Meehan, R.; Dunleavy, K.; et al. Consensus Guidelines for Evaluation and Management of Pulmonary Disease in Sjögren’s. Chest 2021, 159, 683–698. [Google Scholar] [CrossRef]
- Graney, B.A.; Fischer, A. Interstitial Pneumonia with Autoimmune Features. Ann. Am. Thorac. Soc. 2019, 16, 525–533. [Google Scholar] [CrossRef]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Sambataro, G.; Sambataro, D.; Spicuzza, L.; Meloni, F.; Lorini, G.; Malatino, L.; Colaci, M.; Sebastiani, G.; Iuliano, A.; Canofari, C.; et al. Progression and prognosis of interstitial pneumonia with autoimmune features: A longitudinal, prospective, multi-centre study. Clin. Exp. Rheumatol. 2023, 41, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Sambataro, D.; Sambataro, G.; Pignataro, F.; Zanframundo, G.; Codullo, V.; Fagone, E.; Martorana, E.; Ferro, F.; Orlandi, M.; Del Papa, N. Patients with Interstitial Lung Disease Secondary to Autoimmune Diseases: How to Recognize Them? Diagnostics 2020, 10, 208. [Google Scholar] [CrossRef]
- Generali, E.; Costanzo, A.; Mainetti, C.; Selmi, C. Cutaneous and Mucosal Manifestations of Sjögren’s Syndrome. Clin. Rev. Allergy Immunol. 2017, 53, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Vivino, F.B. Sjogren’s syndrome: Clinical aspects. Clin. Immunol. 2017, 182, 48–54. [Google Scholar] [CrossRef]
- Fischer, A.; Swigris, J.J.; du Bois, R.M.; Groshong, S.D.; Cool, C.D.; Sahin, H.; Lynch, D.A.; Gillis, J.Z.; Cohen, M.D.; Meehan, R.T.; et al. Minor Salivary Gland Biopsy To Detect Primary Sjögren Syndrome in Patients With Interstitial Lung Disease. Chest 2009, 136, 1072–1078. [Google Scholar] [CrossRef]
- La Rocca, G.; Ferro, F.; Bulleri, A.; Fulvio, G.; Fonzetti, S.; Donati, V.; Romei, C.; Mosca, M.; Baldini, C. Glandular involvement in primary Sjögren’s syndrome patients with interstitial lung disease-onset and sicca-onset, a single centre cross-sectional study. Clin. Exp. Rheumatol. 2022, 40, 2344–2349. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, J.; Guo, X.; Li, Y.; Xu, Y.; Fu, Q.; Lu, Y.; Zheng, Y. A retrospective analysis of distinguishing features of chest HRCT and clinical manifestation in primary Sjögren’s syndrome-related interstitial lung disease in a Chinese population. Clin. Rheumatol. 2018, 37, 2981–2988. [Google Scholar] [CrossRef]
- Buvry, C.; Cassagnes, L.; Tekath, M.; Artigues, M.; Pereira, B.; Rieu, V.; Le Guenno, G.; Tournadre, A.; Ruivard, M.; Grobost, V. Anti-Ro52 antibodies are a risk factor for interstitial lung disease in primary Sjögren syndrome. Respir. Med. 2020, 163, 105895. [Google Scholar] [CrossRef]
- Roca, F.; Dominique, S.; Schmidt, J.; Smail, A.; Duhaut, P.; Lévesque, H.; Marie, I. Interstitial lung disease in primary Sjögren’s syndrome. Autoimmun. Rev. 2017, 16, 48–54. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Z.; Qiu, M.; Ye, Q. Risk factors for primary Sjögren syndrome-associated interstitial lung disease. J. Thorac. Dis. 2018, 10, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, X.W.; He, J.; Zhang, J.; An, Y.; Sun, Y.; Jia, R.L.; Li, S.G.; Zhang, L.J.; Li, Z.G. Prevalence, risk factors, and prognosis of interstitial lung disease in a large cohort of Chinese primary Sjögren syndrome patients: A case-control study. Medicine 2018, 97, e11003. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, G.; Francesco, F.; Fulvio, G.; Fonzetti, S.; Navarro García, I.; Elefante, E.; Romei, C.; Mosca, M.; Baldini, C. Serology Driven Pulmonary Phenotype Characterization of Sjögren Syndrome-associated Interstitial Lung Disease: A Monocentric Cohort Study. Arthritis Rheumatol. 2022, 74 (Suppl. S9), 2022. [Google Scholar]
- Dong, X.; Gao, Y.-L.; Lu, Y.; Zheng, Y. Characteristics of primary Sjögren’s syndrome related lymphocytic interstitial pneumonia. Clin. Rheumatol. 2021, 40, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.H.; Liu, H.R.; Xu, W.B.; Feng, R.E.; Zhang, Z.H.; Tian, X.L.; Zhu, Y.J. Pulmonary manifestations of Sjögren’s syndrome. Respiration 2009, 78, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Kakugawa, T.; Sakamoto, N.; Ishimoto, H.; Shimizu, T.; Nakamura, H.; Nawata, A.; Ito, C.; Sato, S.; Hanaka, T.; Oda, K.; et al. Lymphocytic focus score is positively related to airway and interstitial lung diseases in primary Sjögren’s syndrome. Respir Med. 2018, 137, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bonella, F.; Costabel, U. Biomarkers in Connective Tissue Disease-Associated Interstitial Lung Disease. Semin. Respir. Crit. Care Med. 2014, 35, 181–200. [Google Scholar] [CrossRef]
- Oguz, E.O.; Kucuksahin, O.; Turgay, M.; Yildizgoren, M.T.; Ates, A.; Demir, N.; Kumbasar, O.O.; Kinikli, G.; Duzgun, N. Association of serum KL-6 levels with interstitial lung disease in patients with connective tissue disease: A cross-sectional study. Clin. Rheumatol. 2016, 35, 663–666. [Google Scholar] [CrossRef]
- Jee, A.S.; Sahhar, J.; Youssef, P.; Bleasel, J.; Adelstein, S.; Nguyen, M.; Corte, T.J. Review: Serum biomarkers in idiopathic pulmonary fibrosis and systemic sclerosis associated interstitial lung disease—Frontiers and horizons. Pharmacol. Ther. 2019, 202, 40–52. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Zheng, S.; Lin, J.; Hu, S.; Zhuang, J.; Lin, Q.; Xie, X.; Zheng, K.; Zhang, W.; et al. The role of lung ultrasound B-lines and serum KL-6 in the screening and follow-up of rheumatoid arthritis patients for an identification of interstitial lung disease: Review of the literature, proposal for a preliminary algorithm, and clinical application to cases. Arthritis Res. Ther. 2021, 23, 212. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, E.Y.; Ha, Y.-J.; Kang, E.H.; Lee, Y.J.; Song, Y.W. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Thromb. Haemost. 2019, 21, 58. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, Y.; Fujisawa, T.; Kono, M.; Nakamura, H.; Yokomura, K.; Koshimizu, N.; Toyoshima, M.; Imokawa, S.; Sumikawa, H.; Johkoh, T.; et al. Prognostic factors for primary Sjögren’s syndrome-associated interstitial lung diseases. Respir Med. 2019, 159, 105811. [Google Scholar] [CrossRef]
- Shi, L.; Han, X.-L.; Guo, H.-X.; Wang, J.; Tang, Y.-P.; Gao, C.; Li, X.-F. Increases in tumor markers are associated with primary Sjögren’s syndrome-associated interstitial lung disease. Ther. Adv. Chronic Dis. 2020, 11, 2040622320944802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yuan, F.; Xu, L.; Sun, W.; Liu, L.; Xue, J. Characteristics of patients with primary Sjögren’s syndrome associated interstitial lung disease and relevant features of disease progression. Clin. Rheumatol. 2020, 39, 1561–1568. [Google Scholar] [CrossRef]
- Enomoto, Y.; Takemura, T.; Hagiwara, E.; Iwasawa, T.; Fukuda, Y.; Yanagawa, N.; Sakai, F.; Baba, T.; Nagaoka, S.; Ogura, T. Prognostic Factors in Interstitial Lung Disease Associated with Primary Sjögren’s Syndrome: A Retrospective Analysis of 33 Pathologically–Proven Cases. PLoS ONE 2013, 8, e73774. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Nagai, S.; Kitaichi, M.; Nicholson, A.G.; Johkoh, T.; Noma, S.; Kim, D.S.; Handa, T.; Izumi, T.; Mishima, M. Pulmonary manifestations of primary Sjogren’s syndrome: A clinical, radiologic, and pathologic study. Am. J. Respir. Crit. Care Med. 2005, 171, 632–638. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, D.S.; Park, I.N.; Jang, S.J.; Kitaichi, M.; Nicholson, A.G.; Colby, T.V. Prognosis of fibrotic interstitial pneumonia: Idiopathic versus collagen vascular disease-related subtypes. Am. J. Respir. Crit. Care Med. 2007, 175, 705–711. [Google Scholar] [CrossRef]
- Singh, N.; Varghese, J.; England, B.R.; Solomon, J.J.; Michaud, K.; Mikuls, T.R.; Healy, H.S.; Kimpston, E.M.; Schweizer, M.L. Impact of the pattern of interstitial lung disease on mortality in rheumatoid arthritis: A systematic literature review and meta-analysis. Semin. Arthritis Rheum. 2019, 49, 358–365. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Song, J.W.; Jang, S.J.; Lee, C.K.; Kim, M.Y.; Lee, H.-K.; Jegal, Y.; Kim, D.S. Comparison between cryptogenic organizing pneumonia and connective tissue disease-related organizing pneumonia. Rheumatology 2011, 50, 932–938. [Google Scholar] [CrossRef]
- Park, I.N.; Jegal, Y.; Kim, D.S.; Do, K.-H.; Yoo, B.; Shim, T.S.; Lim, C.-M.; Lee, S.D.; Koh, Y.; Kim, W.S.; et al. Clinical course and lung function change of idiopathic nonspecific interstitial pneumonia. Eur. Respir. J. 2009, 33, 68–76. [Google Scholar] [CrossRef]
- Shao, T.; Shi, X.; Yang, S.; Zhang, W.; Li, X.; Shu, J.; Alqalyoobi, S.; Zeki, A.A.; Leung, P.S.; Shuai, Z. Interstitial Lung Disease in Connective Tissue Disease: A Common Lesion With Heterogeneous Mechanisms and Treatment Considerations. Front. Immunol. 2021, 12, 684699. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Anstrom, K.J.; King, T.E.; Lasky, J.A.; Martinez, F.J.; Idiopathic Pulmonary Fibrosis Clinical Research Network. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 2012, 366, 1968–1977. [Google Scholar] [PubMed]
- Lodhi, T.; Hughes, G.; Stanel, S.; Chaudhuri, N.; Hayton, C. Transbronchial Lung Cryobiopsy in Idiopathic Pulmonary Fibrosis: A State of the Art Review. Adv. Ther. 2019, 36, 2193–2204. [Google Scholar] [CrossRef] [PubMed]
- Ravaglia, C.; Bonifazi, M.; Wells, A.U.; Tomassetti, S.; Gurioli, C.; Piciucchi, S.; Dubini, A.; Tantalocco, P.; Sanna, S.; Negri, E.; et al. Safety and Diagnostic Yield of Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Diseases: A Comparative Study versus Video-Assisted Thoracoscopic Lung Biopsy and a Systematic Review of the Literature. Respiration 2016, 91, 215–227. [Google Scholar] [CrossRef]
- Tomassetti, S.; Colby, T.V.; Wells, A.U.; Poletti, V.; Costabel, U.; Matucci-Cerinic, M. Bronchoalveolar lavage and lung biopsy in connective tissue diseases, to do or not to do? Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211059605. [Google Scholar] [CrossRef]
- Lichtenstein, D.A. Lung ultrasound in the critically ill. Ann. Intensive Care 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Picano, E.; Frassi, F.; Agricola, E.; Gligorova, S.; Gargani, L.; Mottola, G. Ultrasound Lung Comets: A Clinically Useful Sign of Extravascular Lung Water. J. Am. Soc. Echocardiogr. 2006, 19, 356–363. [Google Scholar] [CrossRef]
- Yue Lee, F.C.; Jenssen, C.; Dietrich, C.F. A common misunderstanding in lung ultrasound: The comet tail artefact. Med. Ultrason. 2018, 20, 379–384. [Google Scholar] [CrossRef]
- Gutierrez, M.; Tardella, M.; Rodriguez, L.; Mendoza, J.; Clavijo-Cornejo, D.; García, A.; Bertolazzi, C. Ultrasound as a potential tool for the assessment of interstitial lung disease in rheumatic patients. Where are we now? La Radiol. Med. 2019, 124, 989–999. [Google Scholar] [CrossRef]
- Reissig, A.; Kroegel, C. Transthoracic sonography of diffuse parenchymal lung disease: The role of comet tail artifacts. J. Ultrasound. Med. 2003, 22, 173–180. [Google Scholar] [CrossRef]
- Gargani, L.; Doveri, M.; D’Errico, L.; Frassi, F.; Bazzichi, M.L.; Delle Sedie, A.; Scali, M.C.; Monti, S.; Mondillo, S.; Bombardieri, S.; et al. Ultrasound lung comets in systemic sclerosis: A chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology 2009, 48, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Fanelli, F.R.; Lucci, S.; Barilaro, G.; Quarta, S.; Barbano, B.; Giovannetti, A.; Amoroso, A.; Rosato, E. Lung ultrasound in systemic sclerosis: Correlation with high-resolution computed tomography, pulmonary function tests and clinical variables of disease. Intern. Emerg. Med. 2016, 11, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Barskova, T.; Gargani, L.; Guiducci, S.; Randone, S.B.; Bruni, C.; Carnesecchi, G.; Conforti, M.L.; Porta, F.; Pignone, A.; Caramella, D.; et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann. Rheum. Dis. 2013, 72, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Salaffi, F.; Carotti, M.; Tardella, M.; Pineda, C.; Bertolazzi, C.; Bichisecchi, E.; Filippucci, E.; Grassi, W. Utility of a simplified ultrasound assessment to assess interstitial pulmonary fibrosis in connective tissue disorders—Preliminary results. Arthritis Res. Ther. 2011, 13, R134. [Google Scholar] [CrossRef]
- Tardella, M.; Gutierrez, M.; Salaffi, F.; Carotti, M.; Ariani, A.; Bertolazzi, C.; Filippucci, E.; Grassi, W. Ultrasound in the Assessment of Pulmonary Fibrosis in Connective Tissue Disorders: Correlation with High-Resolution Computed Tomography. J. Rheumatol. 2012, 39, 1641–1647. [Google Scholar] [CrossRef]
- Vasco, P.G.; Cardenal, G.D.L.; Garrido, I.M.; Pinilla, J.M.L.; Rodríguez, G.F.; Mateo, J.J.N.; Ruiz, D.C. Assessment of interstitial lung disease in Sjögren’s syndrome by lung ultrasound: A pilot study of correlation with high-resolution chest tomography. Intern. Emerg. Med. 2017, 12, 327–331. [Google Scholar] [CrossRef]
- Ferro, F.; Bulleri, A.; Elefante, E.; Tripoli, A.; Mosca, M.; Baldini, C. AB0510 Lung Ultrasound of Pleural Irregularities in Sub-clinical Primary Sjögren’s Syndrome-Lung Involvement: A Single Centre Experience. Ann. Rheum. Dis. 2019, 78 (Suppl. S2), 1718. [Google Scholar] [CrossRef]
- Ferro, F.; Sedie, A.D. The use of ultrasound for assessing interstitial lung involvement in connective tissue diseases. Ann. Rheum. Dis. 2018, 36 (Suppl. S114), 165–170. [Google Scholar]
- Yazisiz, V.; Göçer, M.; Erbasan, F.; Uçar, İ.; Aslan, B.; Oygen, Ş.; Gökalp Gök, E.; Terzioğlu, M.E. Survival analysis of patients with Sjögren’s syndrome in Turkey: A tertiary hospital-based study. Clin. Rheumatol. 2020, 39, 233–241. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Zhang, X.-Y.; Xie, L.; Zhang, X.-W.; Zhong, Y.-C.; Zhang, J.; Hou, Y.-K.; Li, Z.-G. Characteristics and mortality in primary Sjögren syndrome–related interstitial lung disease. Medicine 2021, 100, e26777. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Zhou, W.; Guo, J.; He, M.; Li, P.; Gao, J.; Gu, Z.; Dong, C. Associated factors with interstitial lung disease and health-related quality of life in Chinese patients with primary Sjögren’s syndrome. Clin. Rheumatol. 2020, 39, 483–489. [Google Scholar] [CrossRef] [PubMed]
- He, S.H.; He, Y.J.; Guo, K.J.; Liang, X.; Li, S.S.; Li, T.F. Risk factors for progression of interstitial lung disease in Sjögren’s syndrome: A single-centered, retrospective study. Clin. Rheumatol. 2022, 41, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; Bombardieri, S.; Bootsma, H.; De Vita, S.; Dörner, T.; Fisher, B.A.; Gottenberg, J.-E.; Hernandez-Molina, G.; Kocher, A.; et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann. Rheum. Dis. 2020, 79, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Schmidkonz, C. Perspective on Fibroblast Activation Protein-Specific PET/CT in Fibrotic Interstitial Lung Diseases: Imaging Fibrosis-A New Paradigm for Molecular Imaging? J. Nucl. Med. 2022, 63, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, Y.; Nakamura, Y.; Colby, T.V.; Inui, N.; Suda, T. Pirfenidone for primary Sjögren’s syndrome-related fibrotic interstitial pneumonia. Sarcoidosis Vasc. Diffus. Lung Dis. 2017, 34, 91–96. [Google Scholar]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, D.; Wang, L.; Wang, S.; Roden, A.C.; Zhao, H.; Li, X.; Prakash, Y.S.; Matteson, E.L.; Tschumperlin, D.J.; et al. Profibrotic effect of IL-17A and elevated IL-17RA in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated lung disease support a direct role for IL-17A/IL-17RA in human fibrotic interstitial lung disease. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L487–L497. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Lorusso, L.; Ingravallo, G.; Tamma, R.; Ribatti, D.; Lisi, S. The TGF-β1 Signaling Pathway as an Attractive Target in the Fibrosis Pathogenesis of Sjögren’s Syndrome. Mediat. Inflamm. 2018, 2018, 1965935. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Lorusso, L.; Tamma, R.; Ingravallo, G.; Ribatti, D.; Lisi, S. Interleukin-17 and -22 synergy linking inflammation and EMT-dependent fibrosis in Sjögren’s syndrome. Clin. Exp. Immunol. 2019, 198, 261–272. [Google Scholar] [CrossRef]
- Mi, S.; Li, Z.; Yang, H.Z.; Liu, H.; Wang, J.P.; Ma, Y.G.; Wang, X.X.; Liu, H.Z.; Sun, W.; Hu, Z.W. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J. Immunol. 2011, 187, 3003–3014. [Google Scholar] [CrossRef]
- Alunno, A.; Carubbi, F.; Bartoloni, E.; Bistoni, O.; Caterbi, S.; Cipriani, P.; Giacomelli, R.; Gerli, R. Unmasking the pathogenic role of IL-17 axis in primary Sjögren’s syndrome: A new era for therapeutic targeting? Autoimmun. Rev. 2014, 13, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Rafael-Vidal, C.; Pérez, N.; Altabás, I.; Garcia, S.; Pego-Reigosa, J.M. Blocking IL-17: A Promising Strategy in the Treatment of Systemic Rheumatic Diseases. Int. J. Mol. Sci. 2020, 21, 7100. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, Y.; Takemura, T.; Hagiwara, E.; Iwasawa, T.; Okudela, K.; Yanagawa, N.; Baba, T.; Sakai, F.; Fukuda, Y.; Nagaoka, S.; et al. Features of usual interstitial pneumonia in patients with primary Sjögren’s syndrome compared with idiopathic pulmonary fibrosis. Respir. Investig. 2014, 52, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.A.; Szanto, A.; Ng, W.F.; Bombardieri, M.; Posch, M.G.; Papas, A.S.; Farag, A.M.; Daikeler, T.; Bannert, B.; Kyburz, D.; et al. Assessment of the anti-CD40 antibody iscalimab in patients with primary Sjögren’s syndrome: A multicentre, randomised, double-blind, placebo-controlled, proof-of-concept study. Lancet Rheumatol. 2020, 2, e142–e152. [Google Scholar] [CrossRef]
- Manfrè, V.; Chatzis, L.G.; Cafaro, G.; Fonzetti, S.; Calvacchi, S.; Fulvio, G.; Navarro Garcia, I.C.; La Rocca, G.; Ferro, F.; Perricone, C.; et al. Sjögren’s syndrome: One year in review 2022. Clin. Exp. Rheumatol. 2022, 40, 2211–2224. [Google Scholar] [CrossRef]
- Montero, P.; Milara, J.; Roger, I.; Cortijo, J. Role of JAK/STAT in Interstitial Lung Diseases; Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2021, 22, 6211. [Google Scholar] [CrossRef]
- Nombel, A.; Fabien, N.; Coutant, F. Dermatomyositis With Anti-MDA5 Antibodies: Bioclinical Features, Pathogenesis and Emerging Therapies. Front. Immunol. 2021, 12, 773352. [Google Scholar] [CrossRef]
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; Kartman, C.E.; Krishnan, V.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Alatorre-Alexander, J.; de Cassia Pellegrini, R.; et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 [COV-BARRIER]: A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 1407–1418. [Google Scholar] [CrossRef]
- Ferro, F.; Elefante, E.; Italiano, N.; Moretti, M.; La Rocca, G.; Mozzo, R.; De Simone, L.; Baldini, C.; Mosca, M. Pos1242 Baricitinib and Pulse Steroids Combined Treatment in Severe COVID-19 Pneumonia: Preliminary Data from a Rheumatologic Experience in Intensive Care Unit. Ann. Rheum. Dis. 2022, 81 (Suppl. S1), 955. [Google Scholar] [CrossRef]
- La Rocca, G.; Ferro, F.; Baldini, C.; Libra, A.; Sambataro, D.; Colaci, M.; Malatino, L.; Palmucci, S.; Vancheri, C.; Sambataro, G. Targeting intracellular pathways in idiopathic inflammatory myopathies: A narrative review. Front. Med. 2023, 10, 1158768. [Google Scholar] [CrossRef]
- Price, E.; Bombardieri, M.; Kivitz, A.; Matzkies, F.; Gurtovaya, O.; Pechonkina, A.; Jiang, W.; Downie, B.; Mathur, A.; Mozaffarian, A.; et al. Safety and Efficacy of Filgotinib, Lanraplenib, and Tirabrutinib in Sjögren’s Syndrome: Randomised, Phase 2, Double-Blind, Placebo-Controlled Study. Rheumatology 2022, 61, 4797–4808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, S.; Zhang, Z.; Liu, Y.; Mi, L.; Xu, K. Lung transplantation: A viable option for connective tissue disease. Arthritis Care Res. 2023. [Google Scholar] [CrossRef]
- Shah, R.J.; Boin, F. Lung Transplantation in Patients With Systemic Sclerosis. Curr. Rheumatol. Rep. 2017, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Natalini, J.G.; Diamond, J.M.; Porteous, M.K.; Lederer, D.J.; Wille, K.M.; Weinacker, A.B.; Orens, J.B.; Shah, P.D.; Lama, V.N.; McDyer, J.F.; et al. Risk of primary graft dysfunction following lung transplantation in selected adults with connective tissue disease-associated interstitial lung disease. J. Heart Lung Transplant. 2021, 40, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Lian, Q.; Chen, A.; Xu, X.; Zhang, J.; Luo, Q.; Huang, D.; Chen, R.; He, J. Outcomes after lung transplantation among Chinese patients with connective tissue disease-associated interstitial lung disease and pulmonary hypertension: A retrospective cohort study. Clin. Exp. Rheumatol. 2022, 40, 1666–1673. [Google Scholar] [CrossRef]
- Courtwright, A.M.; El-Chemaly, S.; Dellaripa, P.F.; Goldberg, H.J. Survival and outcomes after lung transplantation for non-scleroderma connective tissue–related interstitial lung disease. J. Heart Lung Transplant. 2017, 36, 763–769. [Google Scholar] [CrossRef]
- Cao, Y.; Du, H.; Liu, J.; Qu, H.; Ding, Y.; Shi, G.; Xie, Q.; Sun, X.; Chen, J.; Ye, S.; et al. Double lung transplantation for Sjögren’s syndrome-related interstitial lung disease: A case report and review of literature. Ann. Transl. Med. 2020, 8, 888. [Google Scholar] [CrossRef]


| Authors, Year | No. of ILD Patients | Features Associated with ILD Presence in pSS | ||
|---|---|---|---|---|
| Demographic | Clinical | Laboratoristic | ||
| Shi et al., 2020 [43] | 168 | - | Higher ESSDAI | Ca 15.3 and CEA levels |
| Zhang et al., 2020 [44] | 85 | Older age, disease duration | Fever, xerophtalmia, xerostomia | WBC, CRP, and IgG levels |
| Buvry et al., 2020 [29] | 31 | - | - | Anti-Ro52 |
| Gao et al., 2018 [32] | 165 | Older age | - | RF and CRP |
| Dong et al., 2018 [28] | 206 | Older age, smoking | Dry cough, clubbing, weight loss | Anti-Ro52, higher LDH and ESR levels, and lower albumin/globulin ratio |
| Wang et al., 2018 [31] | 158 | Male sex, older age, smoking | - | ANA |
| Roca et al., 2017 [30] | 21 | Older age | Raynaud’s phenomenon, esophageal involvement | - |
| Li et al., 2015 [18] | 66 | - | - | Anti-SSA and low C3 levels |
| Studies | Risk Factors | Outcome Assessed | Prognosis |
|---|---|---|---|
| He et al., 2021 [72] | LDH, non-sicca onset, low FVC * | ILD progression * | Progression of ILD in 38.6% of cases |
| Gao et al., 2021 [70] | TLCO/VA, MEF25, PaO2 | Mortality | 10-year survival rate of 81.7% |
| Zhang et al., 2020 [44] | ESR, UIP pattern | ILD progression at 6 months † | Progression of ILD in 20.4% of cases |
| Kamiya et al., 2019 [42] | Age, KL-6, low FVC | Mortality | 5-year survival rate of 89.8% 10-year survival rate of 79% |
| Enomoto et al., 2013 [45] | PaCO2, extension of reticulations on HRCT, severity of fibroblastic foci | Mortality | 5-year survival rate of 87.3% |
| Ito et al., 2005 [46] | PaO2, absence of microscopic honeycombing | Survival at 5 years | 5-year survival rate of 84% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Rocca, G.; Ferro, F.; Sambataro, G.; Elefante, E.; Fonzetti, S.; Fulvio, G.; Navarro, I.C.; Mosca, M.; Baldini, C. Primary-Sjögren’s-Syndrome-Related Interstitial Lung Disease: A Clinical Review Discussing Current Controversies. J. Clin. Med. 2023, 12, 3428. https://doi.org/10.3390/jcm12103428
La Rocca G, Ferro F, Sambataro G, Elefante E, Fonzetti S, Fulvio G, Navarro IC, Mosca M, Baldini C. Primary-Sjögren’s-Syndrome-Related Interstitial Lung Disease: A Clinical Review Discussing Current Controversies. Journal of Clinical Medicine. 2023; 12(10):3428. https://doi.org/10.3390/jcm12103428
Chicago/Turabian StyleLa Rocca, Gaetano, Francesco Ferro, Gianluca Sambataro, Elena Elefante, Silvia Fonzetti, Giovanni Fulvio, Inmaculada C. Navarro, Marta Mosca, and Chiara Baldini. 2023. "Primary-Sjögren’s-Syndrome-Related Interstitial Lung Disease: A Clinical Review Discussing Current Controversies" Journal of Clinical Medicine 12, no. 10: 3428. https://doi.org/10.3390/jcm12103428
APA StyleLa Rocca, G., Ferro, F., Sambataro, G., Elefante, E., Fonzetti, S., Fulvio, G., Navarro, I. C., Mosca, M., & Baldini, C. (2023). Primary-Sjögren’s-Syndrome-Related Interstitial Lung Disease: A Clinical Review Discussing Current Controversies. Journal of Clinical Medicine, 12(10), 3428. https://doi.org/10.3390/jcm12103428








