The Comparison between the Composition of 100% Autologous Serum and 100% Platelet-Rich Plasma Eye Drops and Their Impact on the Treatment Effectiveness of Dry Eye Disease in Primary Sjogren Syndrome
Abstract
1. Introduction
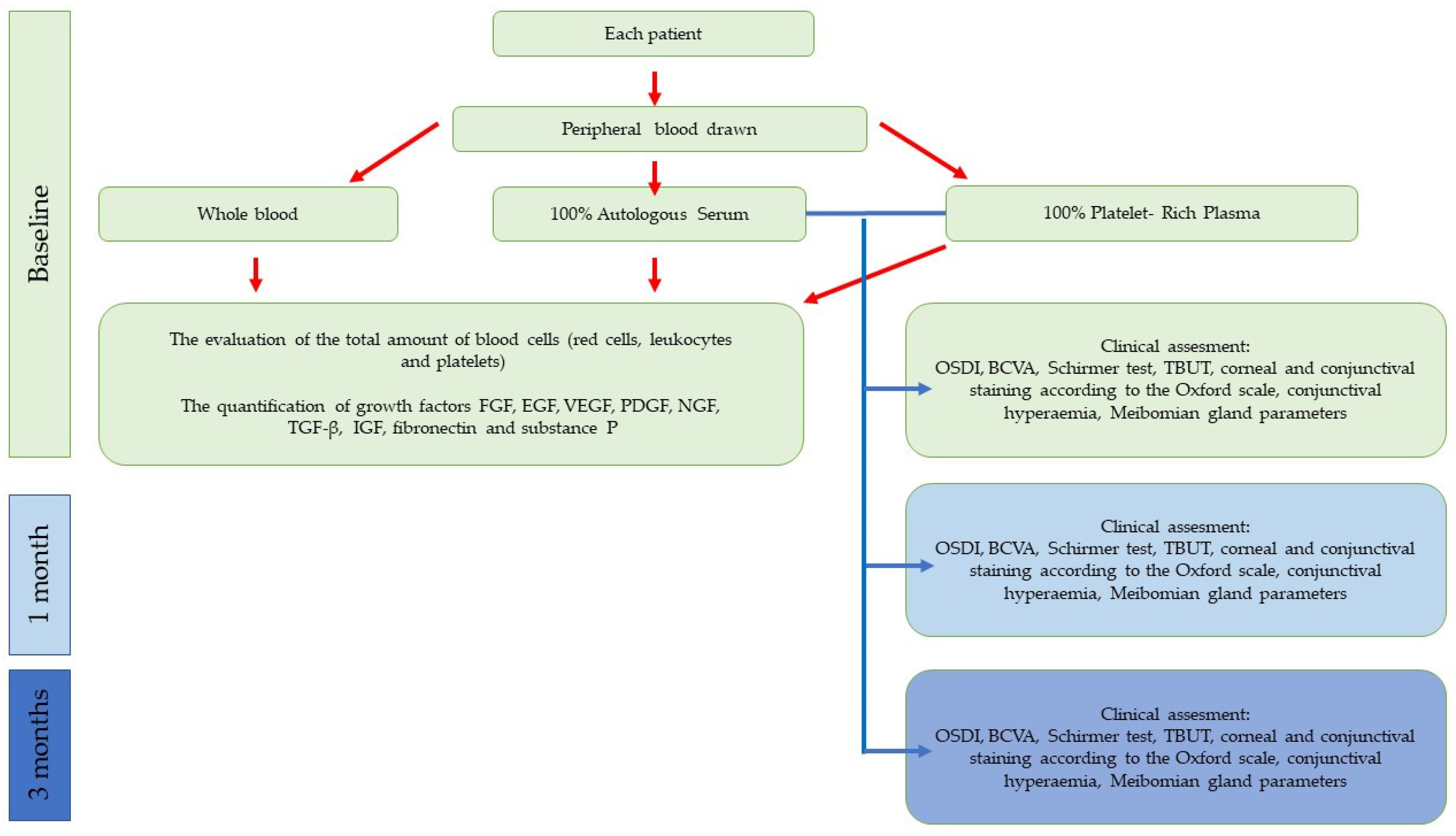
2. Materials and Methods
2.1. Participants
- Women with dry eye disease due to primary Sjogren syndrome diagnosed according to the AECG 2002 classification [17];
- age ≥ 18 years old;
- no general disease;
- the same diet habits (a balanced diet according to the patient’s opinion);
- Ocular Surface Disease Index questionnaire (OSDI ≥ 23), at least moderate DE symptoms;
- tear break-up time (TBUT < 10 s);
- Schirmer’s test (≤10 mm);
- abnormal ocular surface staining—Oxford Scale ≥ 2.
- pregnancy or breastfeeding;
- other connective tissue disorders;
- diabetes;
- acute or chronic general infection, infectious disease;
- cancer;
- blood pathology or blood-transmitted disease;
- chronic liver or kidney disease;
- anticoagulant or antiplatelet therapy;
- use of haemoderivates within 3 months before the enrolment;
- glaucoma;
- previous history of ocular trauma, ocular surgery within 6 months;
- use of contact lenses;
- punctal plug.
2.2. Clinical Examination
2.2.1. Ocular Surface Disease Index Score (OSDI)
2.2.2. Best Corrected Visual Acuity (BCVA)
2.2.3. Schirmer Test I without Anaesthesia
2.2.4. Oral Schirmer Test
2.2.5. Tear Break-Up Time (TBUT)
2.2.6. Ocular Surface Fluorescein Staining—Oxford Scale
2.2.7. Conjunctival Hyperaemia
2.2.8. Meibomian Gland Parameters
2.2.9. Adverse Events
2.3. Autologous Serum and Platelet-Rich Plasma Preparation
2.3.1. AS Preparation
2.3.2. PRP Preparation
2.4. Haematological Analysis
2.5. Quantification of Growth Factors, Fibronectin, and Substance P
2.6. Statistical Analysis
2.6.1. Sample Size Calculation
2.6.2. Statistical Analysis
3. Results
3.1. Patients
3.2. Growth Factor Level
3.3. Clinical Status
3.3.1. OSDI
3.3.2. BCVA
3.3.3. Schirmer Test
3.3.4. TBUT
3.3.5. Ocular Surface Staining
3.3.6. Conjunctival Hyperaemia
3.3.7. Meibomian Gland Parameters
3.3.8. Safety
3.4. Relationship between GFs and Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhan, Q.; Zhang, J.; Lin, Y.; Chen, W.; Fan, X.; Zhang, D. Pathogenesis and treatment of Sjogren’s syndrome: Review and update. Front. Immunol. 2023, 14, 1127417. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K.J.; Buchanan, W.W.; Wohl, M.J.; Bunim, J.J. Sjoegren’s syndrome. A clinical, pathological, and serological study of sixty-two cases. Medicine 1965, 44, 187–231. [Google Scholar] [CrossRef] [PubMed]
- Perella, C.; Steenackers, M.; Robbins, B.; Stone, L.; Gervais, R.; Schmidt, T.; Goswami, P. Patient Experience of Sjögren’s Disease and its Multifaceted Impact on Patients’ Lives. Rheumatol. Ther. 2023. ahead of print. [Google Scholar] [CrossRef]
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjögren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510, Erratum in Ocul. Surf. 2019, 17, 842. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Criswell, L.A. Primary Sjögren’s Syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef]
- Pucker, A.D.; Ng, S.M.; Nichols, J.J. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst. Rev. 2016, 2016, CD009729. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]
- Metheetrairut, C.; Ngowyutagon, P.; Tunganuntarat, A.; Khowawisetsut, L.; Kittisares, K.; Prabhasawat, P. Comparison of epitheliotrophic factors in platelet-rich plasma versus autologous serum and their treatment efficacy in dry eye disease. Sci. Rep. 2022, 12, 8906. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Munaron, L.; Petrillo, S.; Erovigni, F.; Carossa, S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets 2016, 27, 467–471. [Google Scholar] [CrossRef]
- Franchini, M.; Cruciani, M.; Mengoli, C.; Marano, G.; Capuzzo, E.; Pati, I.; Masiello, F.; Veropalumbo, E.; Pupella, S.; Vaglio, S.; et al. Serum eye drops for the treatment of ocular surface diseases: A systematic review and meta-analysis. Blood Transfus. 2019, 17, 200–209. [Google Scholar] [CrossRef]
- Pan, Q.; Angelina, A.; Marrone, M.; Stark, W.J.; Akpek, E.K. Autologous serum eye drops for dry eye. Cochrane Database Syst. Rev. 2017, 2017, CD009327. [Google Scholar] [CrossRef] [PubMed]
- Ralph, R.A.; Doane, M.G.; Dohlman, C.H. Clinical Experience with a Mobile Ocular Perfusion Pump. Arch. Ophthalmol. 1975, 93, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.I.; Chan, R.; Michelson, J.B.; Belmont, J.B.; Michelson, P.E. Beneficial Effect of Artificial Tears Made with Autologous Serum in Patients with Keratoconjunctivitis Sicca. Arthritis Rheum. 1984, 27, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, A.P.; Mejia, H.A.; Wright, V.J. Application of Platelet-Rich Plasma to Enhance Tissue Repair. Oper. Tech. Orthop. 2010, 20, 98–105. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Ski. Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and Validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Wróbel-Dudzińska, D.; Kubik-Komar, A.; Rykwa, D.; Kosior-Jarecka, E.; Żarnowski, T.; Chałas, R. The use of Schirmer strips to measure salivary and lacrimal flow in non-Sjögren patients. Clin. Oral Investig. 2021, 25, 4107–4114. [Google Scholar] [CrossRef]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of Corneal and Conjunctival Staining in the Context of Other Dry Eye Tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef]
- Baudouin, C.; Barton, K.; Cucherat, M.; Traverso, C. The Measurement of Bulbar Hyperemia: Challenges and Pitfalls. Eur. J. Ophthalmol. 2015, 25, 273–279. [Google Scholar] [CrossRef]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The International Workshop on Meibomian Gland Dysfunction: Executive Summary. Investig. Opthalmology Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hartwig, D.; Harloff, S.; Herminghaus, P.; Wedel, T.; Geerling, G. An optimised protocol for the production of autologous serum eyedrops. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 243, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Poon, A.C.; Geerling, G.; Dart, J.K.G.; Fraenkel, G.E.; Daniels, J.T. Autologous serum eyedrops for dry eyes and epithelial defects: Clinical and in vitro toxicity studies. Br. J. Ophthalmol. 2001, 85, 1188–1197. [Google Scholar] [CrossRef]
- Tahmaz, V.; Gehlsen, U.; Sauerbier, L.; Holtick, U.; Engel, L.; Radojska, S.; Petrescu-Jipa, V.-M.; Scheid, C.; Hallek, M.; Gathof, B.; et al. Treatment of severe chronic ocular graft-versus-host disease using 100% autologous serum eye drops from a sealed manufacturing system: A retrospective cohort study. Br. J. Ophthalmol. 2017, 101, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Botella, A.J.; Peiró, J.M.; Márques, K.; Cambero, N.M.; Otaolaurruchi, J.S. ffectiveness of 100% autologous serum drops in ocular surface disorders. Farm. Hosp. 2011, 35, 8–13. [Google Scholar] [CrossRef]
- Akyol-Salman, I. Effects of Autologous Serum Eye Drops on Corneal Wound Healing after Superficial Keratectomy in Rabbits. Cornea 2006, 25, 1178–1181. [Google Scholar] [CrossRef]
- Cho, Y.K.; Huang, W.; Kim, G.Y.; Lim, B.S. Comparison of Autologous Serum Eye Drops with Different Diluents. Curr. Eye Res. 2013, 38, 9–17. [Google Scholar] [CrossRef]
- Freire, V.; Andollo, N.; Etxebarria, J.; Moya, R.H.; Durán, J.A.; Morales, M.C. Corneal Wound Healing Promoted by 3 Blood Derivatives. Cornea 2014, 33, 614–620. [Google Scholar] [CrossRef]
- Jongkhajornpong, P.; Anothaisintawee, T.M.; Lekhanont, K.; Numthavaj, P.M.; McKay, G.; Attia, J.M.; Thakkinstian, A. Short-term Efficacy and Safety of Biological Tear Substitutes and Topical Secretagogues for Dry Eye Disease: A Systematic Review and Network Meta-analysis. Cornea 2022, 41, 1137–1149. [Google Scholar] [CrossRef]
- Urzua, C.A.; Vasquez, D.H.; Huidobro, A.; Hernandez, H.; Alfaro, J. Randomized Double-Blind Clinical Trial of Autologous Serum versus Artificial Tears in Dry Eye Syndrome. Curr. Eye Res. 2012, 37, 684–688. [Google Scholar] [CrossRef]
- Kojima, T.; Ishida, R.; Dogru, M.; Goto, E.; Matsumoto, Y.; Kaido, M.; Tsubota, K. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: A prospective randomized case-control study. Am. J. Ophthalmol. 2005, 139, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Celebi, A.R.C.; Ulusoy, C.; Mirza, G.E. The efficacy of autologous serum eye drops for severe dry eye syndrome: A randomized double-blind crossover study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 619–626. [Google Scholar] [CrossRef]
- Wang, L.; Cao, K.; Wei, Z.; Baudouin, C.; Labbé, A.; Liang, Q. Autologous Serum Eye Drops versus Artificial Tear Drops for Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ophthalmic Res. 2020, 63, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Chung, S.-H.; Jeon, S.; Kwok, S.-K.; Park, S.-H.; Kim, M.-S. Comparison of Clinical Efficacies of Autologous Serum Eye Drops in Patients with Primary and Secondary Sjögren Syndrome. Cornea 2014, 33, 663–667. [Google Scholar] [CrossRef]
- Jirsova, K.; Brejchova, K.; Krabcova, I.; Filipec, M.; Al Fakih, A.; Palos, M.; Vesela, V.; Rybičková, I. The Application of Autologous Serum Eye Drops in Severe Dry Eye Patients; Subjective and Objective Parameters before and after Treatment. Curr. Eye Res. 2014, 39, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; Rodriguez, A.E.; Ferreira-Oliveira, R.; Wróbel-Dudzińska, D.; Abdelghany, A.A. Treatment of Dry Eye Disease with Autologous Platelet-Rich Plasma: A Prospective, Interventional, Non-Randomized Study. Ophthalmol. Ther. 2017, 6, 285–293. [Google Scholar] [CrossRef]
- García-Conca, V.; Abad-Collado, M.; Hueso-Abancens, J.R.; Mengual-Verdú, E.; Piñero, D.P.; Aguirre-Balsalobre, F.; Molina, J.C. Efficacy and safety of treatment of hyposecretory dry eye with platelet-rich plasma. Acta Ophthalmol. 2019, 97, e170–e178. [Google Scholar] [CrossRef]
- Agrawal, R.; Rawat, P.; Bhaisare, V.; Walia, S.; Kori, N.; Gupta, R. Autologous platelet-rich plasma eye drop versus artificial tear eye drop for symptomatic dry eye disease: A prospective comparative interventional study. Indian J. Ophthalmol. 2022, 70, 1549. [Google Scholar] [CrossRef]
- Murtaza, F.; Toameh, D.; Chiu, H.H.; Tam, E.S.; Somani, S. Autologous Platelet-Rich Plasma Drops for Evaporative Dry Eye Disease from Meibomian Gland Dysfunction: A Pilot Study. Clin. Ophthalmol. 2022, 16, 2199–2208. [Google Scholar] [CrossRef]
- Avila, M.Y.; Igua, A.M.; Mora, A.M. Randomised, prospective clinical trial of platelet-rich plasma injection in the management of severe dry eye. Br. J. Ophthalmol. 2018, 103, 648–653. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Allam, I.Y.; Shaheen, M.S.; Lazreg, S.; Doheim, M.F. Lacrimal gland injection of platelet rich plasma for treatment of severe dry eye: A comparative clinical study. BMC Ophthalmol. 2022, 22, 343. [Google Scholar] [CrossRef] [PubMed]
- Drew, V.J.; Tseng, C.-L.; Seghatchian, J.; Burnouf, T. Reflections on Dry Eye Syndrome Treatment: Therapeutic Role of Blood Products. Front. Med. 2018, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Shin, Y.-T.; Kim, H.K. Effect of autologous platelet-rich plasma on persistent corneal epithelial defect after infectious keratitis. Jpn. J. Ophthalmol. 2012, 56, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Fernández, V.; Claros, S.; Alcoholado, C.; Cifuentes, M.; Merayo-Lloves, J.; Andrades, J.A.; Becerra, J. Regenerative Therapies in Dry Eye Disease: From Growth Factors to Cell Therapy. Int. J. Mol. Sci. 2017, 18, 2264. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, B.; Jiang, T.; Xiong, C.; Xue, Q.; Li, H.; Fang, H.; Zhang, G.; Ngiam, M. bFGF alleviated dry eye disease by modulating inflammatory response and promoting healing process both in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 2021, 62, 993. [Google Scholar]
- Xiao, X.; He, H.; Lin, Z.; Luo, P.; He, H.; Zhou, T.; Zhou, Y.; Liu, Z. Therapeutic Effects of Epidermal Growth Factor on Benzalkonium Chloride–Induced Dry Eye in a Mouse Model. Investig. Ophthalmol. Vis. Sci. 2012, 53, 191–197. [Google Scholar] [CrossRef]
- Ripa, M.; Jabbehdari, S.; Yazdanpanah, G.; Lukacs, E.; Karcher, B.; Iqbal, O.; Bouchard, C. The Role of Multisystem Disease in Composition of Autologous Serum tears and ocular surface symptom improvement. Ocul. Surf. 2020, 18, 499–504. [Google Scholar] [CrossRef]
- Rossi, S.; Mantelli, F.; Lambiase, A.; Aloe, L. Bevacizumab eye drop treatment stimulates tear secretion in rats through changes in VEGF and NGF lacrimal gland levels. Arch. Ital. Biol. 2012, 150, 15–21. [Google Scholar]
- Cortes, M.; Esposito, G.; Sacco, R.; Gillet, V.B.; Ianni, A.; Micera, A. NGF and iNOS Changes in Tears from Video Display Terminal Workers. Curr. Eye Res. 2018, 43, 1119–1125. [Google Scholar] [CrossRef]
- Lambiase, A.; Micera, A.; Pellegrini, G.; Merlo, D.; Rama, P.; De Luca, M.; Bonini, S.; Bonini, S. In Vitro Evidence of Nerve Growth Factor Effects on Human Conjunctival Epithelial Cell Differentiation and Mucin Gene Expression. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4622–4630. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Micera, A.; Sacchetti, M.; Cortes, M.; Mantelli, F.; Bonini, S. Alterations of tear euromediators in dry eye disease. Arch. Ophthalmol. 2011, 129, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Beuerman, R.W.; Thompson, H.W.; DiLoreto, D.A. Growth Factor and Neurotrophic Factor mRNA in Human Lacrimal Gland. Cornea 1997, 16, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Saricay, L.Y.; Bayraktutar, B.N.; Lilley, J.; Mah, F.S.; Massaro-Giordano, M.; Hamrah, P. Efficacy of Recombinant Human Nerve Growth Factor in Stage 1 Neurotrophic Keratopathy. Ophthalmology 2022, 129, 1448–1450. [Google Scholar] [CrossRef] [PubMed]
- Taketani, Y.; Marmalidou, A.; Dohlman, T.H.; Singh, R.B.; Amouzegar, A.; Chauhan, S.K.; Chen, Y.; Dana, R. Restoration of Regulatory T-Cell Function in Dry Eye Disease by Antagonizing Substance P/Neurokinin-1 Receptor. Am. J. Pathol. 2020, 190, 1859–1866. [Google Scholar] [CrossRef]
- Yang, L.; Sui, W.; Li, Y.; Qi, X.; Wang, Y.; Zhou, Q.; Gao, H. Substance P Inhibits Hyperosmotic Stress-Induced Apoptosis in Corneal Epithelial Cells through the Mechanism of Akt Activation and Reactive Oxygen Species Scavenging via the Neurokinin-1 Receptor. PLoS ONE 2016, 11, e0149865. [Google Scholar] [CrossRef]
- Lee, S.-M.; Sadrai, Z.; Lee, H.S.; Stevenson, W.; Chen, Y.; Hua, J.; Katikireddy, K.R.; Dohlman, T.H.; Chauhan, S.K.; Dana, R. Increased Substance P Expression in the Ocular Surface in Murine Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3664. [Google Scholar]
- Anitua, E.; Sanchez, M.; Merayo-Lloves, J.; De La Fuente, M.; Muruzabal, F.J.; Orive, G. Plasma Rich in Growth Factors (PRGF-Endoret) Stimulates Proliferation and Migration of Primary Keratocytes and Conjunctival Fibroblasts and Inhibits and Reverts TGF- 1-Induced Myodifferentiation. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6066–6073. [Google Scholar] [CrossRef]
- Tsubota, K.; Higuchi, A. Serum Application for the Treatment of Ocular Surface Disorders. Int. Ophthalmol. Clin. 2000, 40, 113–122. [Google Scholar] [CrossRef]
- McDonnell, P.J.; Schanzlin, D.J.; Rao, N.A. Immunoglobulin Deposition in the Cornea after Application of Autologous Serum. Arch. Ophthalmol. 1988, 106, 1423–1425. [Google Scholar] [CrossRef]
- Welder, J.D.; Bakhtiari, P.; Djalilian, A.R. Limbitis Secondary to Autologous Serum Eye Drops in a Patient with Atopic Keratoconjunctivitis. Case Rep. Ophthalmol. Med. 2011, 2011, 576521. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.; de la Fuente, M.; Merayo, J.; Durán, J.; Orive, G. Plasma Rich in Growth Factors for the Treatment of Ocular Surface Diseases. Curr. Eye Res. 2016, 41, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Partal, A.; Scott, E. Low-cost protocol for the production of autologous serum eye drops by blood collection and processing centres for the treatment of ocular surface diseases. Transfus. Med. 2011, 21, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Lagnado, R.; King, A.J.; Donald, F.; Dua, H.S. A protocol for low contamination risk of autologous serum drops in the management of ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 464–465. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.R.; Opitz, A.; Böeck, M.; Geerling, G. Stability of Serum Eye Drops after Storage of 6 Months. Cornea 2012, 31, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- López-García, J.S.; García-Lozano, I.; Rivas, L.; Ramírez, N.; Méndez, M.T.; Raposo, R. Stability of Growth Factors in Autologous Serum Eyedrops after Long-Term Storage. Curr. Eye Res. 2016, 41, 292–298. [Google Scholar] [CrossRef]
- Anitua, E.M.; de la Fuente, M.M.; Muruzábal, F.; Merayo-Lloves, J.M. Short- and Long-Term Stability of Plasma Rich in Growth Factors Eye Drops. Cornea 2021, 40, 107–112. [Google Scholar] [CrossRef]
- Okumura, Y.; Inomata, T.; Fujimoto, K.; Fujio, K.; Zhu, J.; Yanagawa, A.; Shokirova, H.; Saita, Y.; Kobayashi, Y.; Nagao, M.; et al. Biological effects of stored platelet-rich plasmaeye-drops in corneal wound healing. Br. J. Ophthalmol. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
| Analysed Trait | Platelet-Rich Plasma | Autologous Serum | p-Value | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Age (years) | 62.2 | 8.5 | 62.9 | 10.9 | =0.8175 |
| BCVA (logMAR) | 0.21 | 0.23 | 0.06 | 0.07 | =0.0141 |
| OSDI score | 79.0 | 12.7 | 79.8 | 13.1 | =0.8270 |
| Classic Schirmer test | 2.9 | 2.8 | 2.9 | 2.8 | =0.9907 |
| Oral Schirmer test | 2.1 | 3.0 | 1.1 | 1.3 | =0.4635 |
| TBUT (s) | 5.4 | 1.9 | 4.7 | 1.8 | =0.2566 |
| Analysed Factor | Platelet-Rich Plasma | Autologous Serum | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Corneal fluorescein staining, Oxford scale: 2 | 6 | 27.27 | 7 | 31.82 | |
| 3 | 6 | 27.27 | 6 | 27.27 | |
| 4 | 10 | 45.45 | 9 | 40.91 | =0.7222 |
| Conjunctival fluorescein staining, Oxford scale: 2 | 8 | 36.36 | 6 | 27.27 | |
| 3 | 11 | 50 | 13 | 59.09 | |
| 4 | 2 | 9.09 | 3 | 13.64 | |
| 5 | 1 | 4.55 | 0 | 0.00 | =0.8891 |
| Conjunctival hyperaemia: 2 | 7 | 31.82 | 8 | 36.36 | |
| 3 | 12 | 54.55 | 10 | 45.45 | |
| 4 | 3 | 13.64 | 4 | 18.18 | =0.9584 |
| Quality of Meibomian gland secretion: 1 | 16 | 72.73 | 18 | 81.82 | |
| 2 | 4 | 18.18 | 4 | 18.18 | |
| 3 | 2 | 9.09 | 0 | 0.00 | =0.4020 |
| Expressibility of Meibomian glands: 1 | 16 | 72.73 | 16 | 72.73 | |
| 2 | 4 | 18.18 | 6 | 27.27 | |
| 3 | 2 | 9.09 | 0 | 0.00 | =0.8544 |
| Analyzed Factor | Study Group | Statistical Parameter | p-Value | ||||
|---|---|---|---|---|---|---|---|
| M | SD | Me | Q1–Q3 | Min.–Max. | |||
| NGF (pg/mL) | PRP | 85.22 | 23.49 | 79.54 | 65.73–92.97 | 56.77–143.21 | <0.0001 |
| AS | 8.29 | 9.06 | 4.90 | 4.18–5.96 | 3.44–34.47 | ||
| PDGF (pg/mL) | PRP | 935.38 | 434.26 | 749.48 | 633.74–1258.25 | 418.57–1816.98 | <0.0001 |
| AS | 126.66 | 54.41 | 105.19 | 95.38–171.75 | 50.73–229.94 | ||
| PDGF-AB (pg/mL) | PRP | 619.60 | 117.30 | 615.17 | 547.50–694.50 | 404.22–878.46 | <0.0001 |
| AS | 349.66 | 79.82 | 342.98 | 297.23–411.95 | 169.30–458.00 | ||
| TGF-b (pg/mL) | PRP | 726.03 | 298.95 | 777.07 | 630.22–867.81 | 91.82–1104.89 | =0.0004 |
| AS | 1031.32 | 330.23 | 943.04 | 787.18–1134.61 | 642.07–1800.80 | ||
| FGF (pg/mL) | PRP | 4.42 | 0.86 | 4.30 | 3.82–4.57 | 3.51–6.14 | <0.0001 |
| AS | 15.96 | 7.63 | 14.35 | 9.54–21.15 | 6.54–32.17 | ||
| EGF (pg/mL) | PRP | 4.98 | 0.97 | 4.85 | 4.48–5.90 | 2.98–6.22 | <0.0001 |
| AS | 39.06 | 20.18 | 34.24 | 27.72–38.63 | 19.87–98.22 | ||
| Fibronectin (pg/mL) | PRP | 929.60 | 111.50 | 918.20 | 833.53–1063.94 | 778.48–1077.68 | =0.0005 |
| AS | 823.64 | 98.49 | 780.72 | 760.12–829.82 | 703.30–1054.98 | ||
| VEGF (pg/mL) | PRP | 175.45 | 65.93 | 162.50 | 117.06–216.85 | 79.45–315.79 | <0.0001 |
| AS | 717.35 | 488.15 | 524.51 | 383.36–907.14 | 235.89–2018.68 | ||
| P substance (pg/mL) | PRP | 112.58 | 27.28 | 115.92 | 94.54–137.95 | 69.72–154.92 | =0.0125 |
| AS | 127.51 | 26.56 | 121.91 | 116.52–147.58 | 65.73–163.66 | ||
| AS | 127.51 | 26.56 | 121.91 | 116.52–147.58 | 65.73–163.66 | ||
| IGF (pg/mL) | PRP | 260.15 | 74.18 | 249.10 | 206.45–301.90 | 110.30–395.25 | =0.1627 |
| AS | 235.05 | 94.21 | 242.20 | 179.55–318.65 | 77.90–366.90 | ||
| AS | 235.05 | 94.21 | 242.20 | 179.55–318.65 | 77.90–366.90 | ||
| Analysed Trait | Study Group | Time Point | Statistical Parameter | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | Me | Q1–Q3 | Min.–Max. | |||||
| BCVA logMAR | PRP | Baseline | 0.21 | 0.23 | 0.19 | 0.05–0.40 | 0.00–1.00 | =0.0046 | =0.0063 |
| +1 month | 0.15 | 0.18 | 0.07 | 0.00–0.22 | 0.00–0.70 | ||||
| +3 months | 0.09 | 0.12 | 0.02 | 0.00–0.22 | 0.00–0.30 | ||||
| AS | Baseline | 0.06 | 0.07 | 0.05 | 0.00–0.10 | 0.00–0.22 | =0.0010 | ||
| +1 month | 0.05 | 0.05 | 0.05 | 0.00–0.10 | 0.00–0.15 | ||||
| +3 months | 0.04 | 0.04 | 0.05 | 0.00–0.10 | 0.00–0.10 | ||||
| OSDI score | PRP | Baseline | 79.00 | 12.71 | 75 | 69.40–88.80 | 54.16–97.50 | <0.0001 | =0.0031 |
| +1 month | 65.54 | 11.62 | 70 | 57.99–78.00 | 50.00–87.00 | ||||
| +3 months | 59.81 | 10.37 | 60 | 52.27–67.50 | 45.83–77.00 | ||||
| AS | Baseline | 79.85 | 13.12 | 82.55 | 72.72–91.00 | 52.50–95.00 | <0.0001 | ||
| +1 month | 70.48 | 12.13 | 71.59 | 60.10–81.25 | 49.00–87.50 | ||||
| +3 months | 70.59 | 19.20 | 70.22 | 56.26–79.00 | 47.00–120.00 | ||||
| Analysed Trait | Study Group | Time Point | Statistical Parameter | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | Me | Q1–Q3 | Min.–Max. | |||||
| Schirmer test (mm) | PRP | Baseline | 2.95 | 2.80 | 2.00 | 1–3 | 0–9 | <0.0001 | =0.1502 |
| +1 month | 4.09 | 2.62 | 3.00 | 2–5 | 1–10 | ||||
| +3 months | 5.00 | 2.86 | 4.00 | 3–7 | 0–11 | ||||
| AS | Baseline | 2.91 | 2.79 | 2.50 | 1–4 | 0–10 | =0.0002 | ||
| +1 month | 3.59 | 2.77 | 3.50 | 1–5 | 0–10 | ||||
| +3 months | 4.00 | 3.41 | 4.00 | 1–6 | 0–12 | ||||
| Oral Schirmer test (mm) | PRP | Baseline | 2.09 | 3.04 | 1 | 0–4 | 0–10 | =0.0023 | NS |
| +1 month | 2.18 | 2.99 | 1 | 0–4 | 0–10 | ||||
| +3 months | 2.23 | 3.02 | 1 | 0–4 | 0–10 | ||||
| AS | Baseline | 1.09 | 1.34 | 1 | 0–2 | 1–4 | NS | ||
| +1 month | 1.09 | 1.34 | 1 | 0–2 | 1–4 | ||||
| +3 months | 1.09 | 1.34 | 1 | 0–2 | 1–4 | ||||
| TBUT (s) | PRP | Baseline | 5.364 | 1.866 | 5 | 4–7 | 2–10 | <0.0001 | =0.1714 |
| +1 month | 5.682 | 1.783 | 6 | 4–7 | 3–9 | ||||
| +3 months | 5.954 | 2.380 | 6 | 4–7 | 1–10 | ||||
| AS | Baseline | 4.727 | 1.804 | 4 | 3–6 | 2–8 | <0.0001 | ||
| +1 month | 5.545 | 2.304 | 5 | 4–7 | 2–10 | ||||
| +3 months | 5.000 | 2.795 | 4 | 3–7 | 1–11 | ||||
| Study Group | Time Point | Grade | PRP | AS | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | Repeated Measures | Between-Group | |||
| Corneal staining, Oxford scale | Baseline | 0 | PRP <0.0001 AS <0.0001 | 0.2513 | ||||
| 1 | ||||||||
| 2 | 6 | 27.3 | 7 | 31.8 | ||||
| 3 | 6 | 27.3 | 6 | 27.3 | ||||
| 4 | 10 | 45.4 | 9 | 40.9 | ||||
| 5 | ||||||||
| +1 month | 0 | |||||||
| 1 | 2 | 9.1 | ||||||
| 2 | 10 | 45.5 | 6 | 27.3 | ||||
| 3 | 9 | 40.9 | 7 | 31.8 | ||||
| 4 | 3 | 13.6 | 7 | 31.8 | ||||
| 5 | ||||||||
| +3 months | 0 | 2 | 9.1 | |||||
| 1 | 3 | 13.6 | ||||||
| 2 | 15 | 68.2 | 11 | 50.0 | ||||
| 3 | 3 | 13.6 | 5 | 22.7 | ||||
| 4 | 1 | 4.6 | 4 | 18.2 | ||||
| 5 | ||||||||
| Conjunctical staining, Oxford scale | Baseline | 0 | PRP <0.0001 AS <0.0001 | 0.5120 | ||||
| 1 | ||||||||
| 2 | 8 | 36.36 | 6 | 27.27 | ||||
| 3 | 11 | 50 | 13 | 59.09 | ||||
| 4 | 2 | 9.09 | 3 | 13.64 | ||||
| 5 | 1 | 4.55 | 0 | 0.00 | ||||
| +1 month | 0 | |||||||
| 1 | 2 | 9.09 | ||||||
| 2 | 15 | 68.18 | 10 | 45.45 | ||||
| 3 | 6 | 27.27 | 8 | 36.36 | ||||
| 4 | 1 | 4.55 | 2 | 9.09 | ||||
| 5 | ||||||||
| +3 months | 0 | 2 | 9.09 | |||||
| 1 | 5 | 22.73 | 1 | 4.55 | ||||
| 2 | 14 | 63.64 | 10 | 45.45 | ||||
| 3 | 2 | 9.09 | 5 | 22.73 | ||||
| 4 | 1 | 4.55 | 4 | 18.18 | ||||
| 5 | ||||||||
| Conjunctival hyperaemia | Baseline | 0 | PRP <0.0001 AS <0.0001 | 0.1089 | ||||
| 1 | ||||||||
| 2 | 7 | 31.8 | 8 | 36.4 | ||||
| 3 | 12 | 54.6 | 10 | 45.4 | ||||
| 4 | 3 | 13.6 | 4 | 18.2 | ||||
| +1 month | 0 | |||||||
| 1 | 4 | 18.2 | ||||||
| 2 | 15 | 68.2 | 7 | 31.8 | ||||
| 3 | 6 | 27.3 | 9 | 40.9 | ||||
| 4 | 1 | 4.5 | 2 | 9.1 | ||||
| +3 months | 0 | |||||||
| 1 | 5 | 22.7 | 6 | 27.3 | ||||
| 2 | 17 | 72.3 | 8 | 36.4 | ||||
| 3 | 7 | 31.8 | ||||||
| 4 | 1 | 4.5 | ||||||
| Study Group | Time Point | Grade | PRP | AS | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | Repeated Measures | Between-Group | |||
| Quality of Meibomian gland secretion | Baseline | 0 | PRP NS AS NS | NS | ||||
| 1 | 16 | 72.73 | 16 | 72.73 | ||||
| 2 | 4 | 18.18 | 6 | 27.27 | ||||
| 3 | 2 | 9.09 | 6 | 27.3 | ||||
| +1 month | 0 | |||||||
| 1 | 18 | 81.82 | 16 | 72.73 | ||||
| 2 | 2 | 9.09 | 6 | 27.3 | ||||
| 3 | 2 | 9.09 | 0 | 0 | ||||
| +3 months | 0 | |||||||
| 1 | 17 | 80.95 | 16 | 72.73 | ||||
| 2 | 2 | 9.09 | 6 | 27.3 | ||||
| 3 | 2 | 9.09 | 0 | 0 | ||||
| Expressibility of Meibomian gland | Baseline | 0 | PRP NS AS NS | 0.1353 | ||||
| 1 | 16 | 72.72 | 18 | 81.82 | ||||
| 2 | 4 | 18.18 | 4 | 18.18 | ||||
| 3 | 2 | 9.09 | 0 | 0 | ||||
| +1 month | 0 | |||||||
| 1 | 18 | 81.82 | 18 | 81.82 | ||||
| 2 | 2 | 9.09 | 6 | 27.3 | ||||
| 3 | 2 | 9.09 | 0 | 0 | ||||
| +3 months | 0 | |||||||
| 1 | 18 | 81.82 | 18 | 81.82 | ||||
| 2 | 2 | 9.09 | 4 | 18.18 | ||||
| 3 | 2 | 9.09 | 0 | 0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel-Dudzińska, D.; Przekora, A.; Kazimierczak, P.; Ćwiklińska-Haszcz, A.; Kosior-Jarecka, E.; Żarnowski, T. The Comparison between the Composition of 100% Autologous Serum and 100% Platelet-Rich Plasma Eye Drops and Their Impact on the Treatment Effectiveness of Dry Eye Disease in Primary Sjogren Syndrome. J. Clin. Med. 2023, 12, 3126. https://doi.org/10.3390/jcm12093126
Wróbel-Dudzińska D, Przekora A, Kazimierczak P, Ćwiklińska-Haszcz A, Kosior-Jarecka E, Żarnowski T. The Comparison between the Composition of 100% Autologous Serum and 100% Platelet-Rich Plasma Eye Drops and Their Impact on the Treatment Effectiveness of Dry Eye Disease in Primary Sjogren Syndrome. Journal of Clinical Medicine. 2023; 12(9):3126. https://doi.org/10.3390/jcm12093126
Chicago/Turabian StyleWróbel-Dudzińska, Dominika, Agata Przekora, Paulina Kazimierczak, Agnieszka Ćwiklińska-Haszcz, Ewa Kosior-Jarecka, and Tomasz Żarnowski. 2023. "The Comparison between the Composition of 100% Autologous Serum and 100% Platelet-Rich Plasma Eye Drops and Their Impact on the Treatment Effectiveness of Dry Eye Disease in Primary Sjogren Syndrome" Journal of Clinical Medicine 12, no. 9: 3126. https://doi.org/10.3390/jcm12093126
APA StyleWróbel-Dudzińska, D., Przekora, A., Kazimierczak, P., Ćwiklińska-Haszcz, A., Kosior-Jarecka, E., & Żarnowski, T. (2023). The Comparison between the Composition of 100% Autologous Serum and 100% Platelet-Rich Plasma Eye Drops and Their Impact on the Treatment Effectiveness of Dry Eye Disease in Primary Sjogren Syndrome. Journal of Clinical Medicine, 12(9), 3126. https://doi.org/10.3390/jcm12093126









