Regional Anesthesia Techniques for Shoulder Surgery in High-Risk Pulmonary Patients
Abstract
1. Introduction
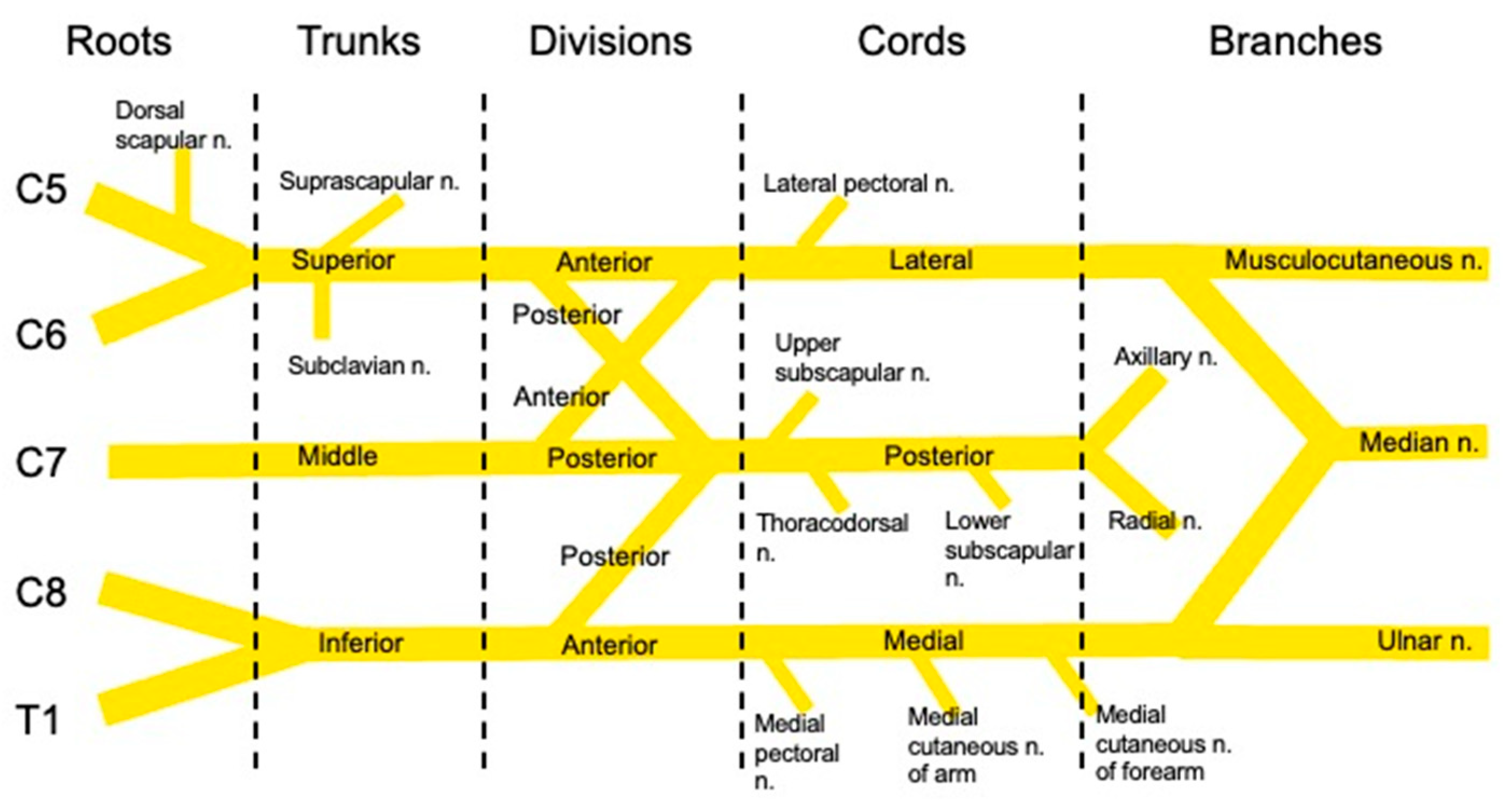
2. Shoulder Anatomy and Innervation
3. Novel Diaphragm-Sparing Regional Techniques
3.1. Superior Trunk Block
3.2. Combined Axillary and Suprascapular Nerve Block
3.3. Combined Infraclavicular and Suprascapular Nerve Block
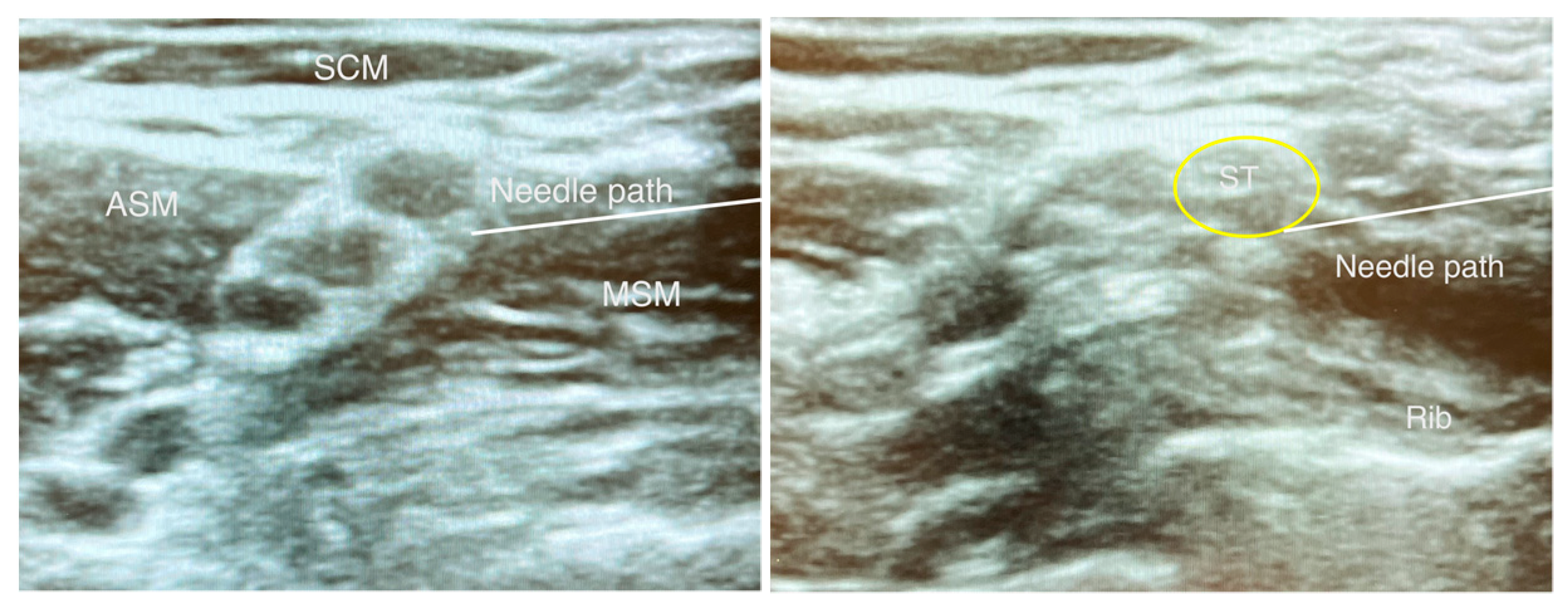
3.4. Costoclavicular
4. Postoperative Complications in Patients with Pulmonary Disease
5. General Anesthesia versus Regional Anesthesia
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selzer, A.; Sarkiss, M. Preoperative Pulmonary Evaluation. Med. Clin. N. Am. 2019, 103, 585–599. [Google Scholar] [CrossRef]
- Mannino, D.M.; Buist, S. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet 2007, 370, 765–773. [Google Scholar] [CrossRef]
- Kim, S.; Wise, B.; Zhang, Y.; Szabo, R. Increasing incidence of shoulder arthroplasty in the United States. J. Bone Jt. Surg. Am. 2011, 93, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Memtsoudis, S.G.; Kuo, C.; Ma, Y.; Edwards, A.; Mazumdar, M.; Liguori, G. Changes in anesthesia-related factors in ambulatory knee and shoulder surgery: United States 1996–2006. Reg. Anesth. Pain Med. 2011, 36, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.H.; Weber, E.C.; Schell, M.J.; Wong, A.B.; Anderson, C.T.; Barker, S.J. Factors Associated with Postoperative Pulmonary Complications in Patients with Severe Chronic Obstructive Pulmonary Disease. Anesth. Analg. 1995, 80, 276–284. [Google Scholar]
- Friere, C.; Sennes, L.; Polotsky, V. Opioids and obstructive sleep apnea. J. Clin. Sleep Med. 2022, 18, 647–652. [Google Scholar] [CrossRef]
- Kim, D.H.; Lin, Y.; Beathe, J.C.; Liu, J.; Oxendine, J.A.; Haskins, S.C.; Ho, M.C.; Wetmore, D.S.; Allen, A.A.; Wilson, L.; et al. Superior Trunk Block: A Phrenic-sparing Alternative to the Interscalene Block: A Randomized Controlled Trial. Anesthesiology 2019, 131, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Itoi, E.; An, K.N. Anatomy and biomechanics of the shoulder. Orthop. Clin. 2000, 31, 159–176. [Google Scholar] [CrossRef]
- Tran, J.; Peng, P.W.H.; Agur, A.M.R. Anatomical study of the innervation of glenohumeral and acromioclavicular joint capsules: Implications for image-guided intervention. Reg. Anesth. Pain Med. 2019, 44, 452–458. [Google Scholar] [CrossRef]
- Chan, C.W.; Peng, P.W. Suprascapular nerve block: A narrative review. Reg. Anesth. Pain Med. 2011, 36, 358–373. [Google Scholar] [CrossRef]
- Baglien, P.; Varacallo, M. Anatomy, Shoulder and Upper Limb, Cutaneous Innervation; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kessler, J.; Schafhalter-Zoppoth, I.; Gray, A.T. An ultrasound study of the phrenic nerve in the posterior cervical triangle: Implications for the interscalene brachial plexus block. Reg. Anesth. Pain Med. 2008, 33, 545–550. [Google Scholar] [CrossRef]
- Burckett-St Laurent Chan, V.; Chin, K.J. Refining the ultrasound-guided interscalene brachial plexus block: The superior trunk approach. Can. J. Anesth. 2014, 61, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Robles, C.; Berardone, N.; Orebaugh, S. Effect of superior trunk block on diaphragm function and respiratory parameters after shoulder surgery. Reg. Anesth. Pain Med. 2022, 47, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Jeong, J.S.; Chin, K.J.; Yoo, J.C.; Lee, J.H.; Choi, S.J.; Gwak, M.S.; Hahm, T.S.; Ko, J.S. Superior Trunk Block Provides Noninferior Analgesia Compared with Interscalene Brachial Plexus Block in Arthroscopic Shoulder Surgery. Anesthesiology 2019, 131, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Han, J.U.; Lee, W.; Jeon, Y.S.; Jeong, J.; Yang, C.; Uhm, J.W.; Kim, Y. Effects of local anesthetic volume (standard versus low) on incidence of hemidiaphragmatic paralysis and analgesic quality for ultrasound-guided superior trunk block after arthroscopic shoulder surgery. Anesth. Analg 2021, 133, 1303–1310. [Google Scholar] [CrossRef]
- Price, D.J. The Shoulder Block: A New Alternative to Interscalene Brachial Plexus Blockade for the Control of Postoperative Shoulder Pain. Anaesth. Intensive Care 2007, 35, 575–581. [Google Scholar] [CrossRef]
- Siegenthaler, A.; Moriggl, B.; Mlekhusch, S.; Schliessbach, J.; Haug, M.; Curatolo, M.; Eichenberger, U. Ultrasound-guided suprascapular nerve block, description of a novel supraclavicular approach. Reg. Anesth. Pain Med. 2012, 37, 325–328. [Google Scholar] [CrossRef]
- Feigl, G.; Aichner, E.; Mattersberger, C.; Zahn, P.K.; Gonzalez, C.A.; Litz, R. Ultrasound-guided anterior approach to the axillary and intercostobrachial nerves in the axillary fossa: An anatomical investigation. Br. J. Anaesth 2018, 121, 883–889. [Google Scholar] [CrossRef]
- Dhir, S.; Sondekoppam, R.V.; Sharma, R.; Ganapathy, S.; Athwal, G.S. A comparison of combined suprascapular and axillary nerve blocks to interscalene nerve block for analgesia in arthroscopic shoulder surgery: An equivalence study. Reg. Anesth. Pain Med. 2016, 41, 564–571. [Google Scholar] [CrossRef]
- Neuts, A.; Stessel, B.; Wouters, P.F. Elective suprascapular and axillary nerve block versus interscalene plexus block for pain control after arthroscopic shoulder surgery: A noninferiority randomized parallel-controlled clinical trial. Reg. Anesth. Pain Med. 2018, 43, 738–744. [Google Scholar]
- Tran, D.Q.; Layera, S.; Bravo, D.; Cristi-Sanchéz, I.; Bermudéz, L.; Aliste, J. Diaphragm-sparing nerve blocks for shoulder surgery, revisited. Reg. Anesth. Pain Med. 2020, 45, 73–78. [Google Scholar] [CrossRef]
- Panchamia, J.K.; Olsen, D.A.; Sanchez-Sotelo, J.; Amundson, A.W. Combined selective nerve blockade and local infiltration analgesia in a total shoulder arthroplasty patient with chronic pain and severe restrictive lung disease: A case report. Case Rep. 2017, 9, 360–363. [Google Scholar] [CrossRef]
- Vorster, W.; Lange, C.P.E.; Briet, R.J.P. The sensory branch distribution of the suprascapular nerve: An anatomic study. J. Shoulder Elb. Surg. 2008, 17, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J. Restricted infraclavicular distribution of the local anesthetic solution after infraclavicular brachial plexus block. Reg. Anesth. Pain Med. 2003, 28, 33–36. [Google Scholar] [CrossRef]
- Aliste, J.; Bravo, D.; Finlayson, R.J.; Tran, D.Q. A randomized comparison between interscalene and combined infraclavicular-suprascapular blocks for arthroscopic shoulder surgery. Can. J. Anesth. J. Can. D’anesthésie 2018, 65, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Gianesello, L.; Pavoni, V.; Burzio, I.; Boccaccini, A. Respiratory effect of interscalene brachial plexus block vs combined infraclavicular plexus block with suprascapular nerve block for arthroscopic shoulder surgery. J. Clin. Anesth. 2018, 44, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Klaastad, Ø.; Ytrebo, L.M. A combination of of infraclavicular and suprascapular nerve blocks for total shoulder arthroplasty: A case series. Acta Anaesthesiol. Scand 2021, 65, 674–680. [Google Scholar] [CrossRef]
- Petrar, S.D.; Seltenrich, M.E.; Head, S.J.; Schwarz, S.K.W. Hemidiaphragmatic paralysis following ultrasound-guided supraclavicular versus infraclavicular brachial plexus blockade: A randomized clinical trial. Reg. Anesth. Pain Med. 2015, 40, 133–138. [Google Scholar] [CrossRef]
- Karmakar, M.K.; Sala-Blanch, X.; Songthamwat, B.; Tsui, B.C.H. Benefits of the costoclavicular space for ultrasound-guided infraclavicular brachial plexus block: Description of a costoclavicular approach. Reg. Anesth. Pain Med. 2015, 40, 287–288. [Google Scholar] [CrossRef]
- Aliste, J.; Bravo, D.; Layera, S. Randomized comparison between interscalene and costoclavicular blocks for arthroscopic shoulder surgery. Reg. Anesth. Pain Med. 2019, 44, 472–477. [Google Scholar] [CrossRef]
- Luo, Q.; Yang, C.; Wei, W. Effects of costoclavicular block versus interscalene block in patients undergoing arthroscopic shoulder surgery under monitored anaesthesia care: A randomized, prospective, non-inferiority study. Korean J. Anesthesiol. 2023. [Google Scholar] [CrossRef]
- Jo, Y.; Oh, C.; Lee, W.Y. Randomised comparison between superior trunk and costoclavicular blocks for arthroscopic shoulder surgery: A noninferiority study. Eur. J. Anaesthesiol. 2022, 39, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Hausman, M.S.; Jewell, E.S.; Engoren, M. Regional versus general anesthesia in surgical patients with chronic obstructive pulmonary disease: Does avoiding general anesthesia reduce the risk of postoperative complications? Anesth. Analg. 2015, 120, 1405–1412. [Google Scholar] [CrossRef]
- Yakubek, G.A.; Curtis, G.L.; Sodhi, N.; Faour, M.; Klika, A.K.; Mont, M.A.; Barsoum, W.K.; Higuera, C.A. Chronic Obstructive Pulmonary Disease Is Associated with Short-Term Complications Following Total Hip Arthroplasty. J. Arthroplast. 2018, 33, 1926–1929. [Google Scholar] [CrossRef]
- Finkel, K.J.; Searleman, A.C.; Tymkew, H.; Tanaka, C.Y.; Saager, L.; Safer-Zadeh, E.; Bottros, M.; Selvidge, J.A.; Jacobsohn, E.; Pulley, D.; et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009, 10, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Vakharia, R.M.; Cohen-Levy, W.B.; Vakharia, A.M.; Donnally, C.J.; Law, T.Y.; Roche, M.W. Sleep Apnea Increases Ninety-Day Complications and Cost Following Primary Total Joint Arthroplasty. J. Arthroplast. 2019, 34, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.A.; Palmer, J.R.; Madden, M.O.; Cohen-Levy, W.; Vakharia, R.M.; Roche, M.W. Perioperative complications in patients with sleep apnea following primary total shoulder arthroplasty: An analysis of 33,366 patients. J. Orthop. 2019, 16, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Gargano, F.; Migliorelli, S.; Strumia, A.; Carassiti, M.; Agrò, F.E. Evolution of Anesthetic Techniques for Shoulder Surgery: A Narrative Review. Osteology 2022, 2, 52–61. [Google Scholar] [CrossRef]
- Musso, D.; Flohr-Madsen, S.; Meknas, K.; Wilsgaard, T.; Ytrebø, L.M.; Klaastad, Ø. A novel combination of peripheral nerve blocks for arthroscopic shoulder surgery. Acta Anaesthesiol. Scand. 2017, 61, 1192–1202. [Google Scholar] [CrossRef]
- Marhofer, P.; Harkanyi, A.; Hopkins, P.M. Regional anesthesia for shoulder surgery. Minerva Anestesiol. 2022, 88, 629–634. [Google Scholar] [CrossRef]
- Lehmann, L.J.; Loosen, G.; Weiss, C.; Schmittner, M.D. Interscalene plexus block versus general anaesthesia for shoulder surgery: A randomized controlled study. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Winnie, A.P.; Ramamurthy, S.; Durrani, Z.; Radonjic, R. Interscalene cervical plexus block: A single injection technic. Anesth. Analg. 1975, 54, 370–375. [Google Scholar] [CrossRef]
- Moustaka, A.; Sarridou, D.; Konstantopoulos, K.; Gatos, N.; Mela, A. Interscaline brachal plexus block versus general anesthesia for shoulder surgery. Reg. Anesth. Pain Med. 2012, 1, 255–261. [Google Scholar]
- Soberón, J.R.; King, J.J.; Gunst, M.; Reynolds, P.S.; Urdaneta, F. Shoulder surgery using combined regional and general anesthesia versus regional anesthesia and deep sedation with a non-invasive positive pressure system: A retrospective cohort study. Trends Anaesth. Crit. Care 2021, 37, 23–29. [Google Scholar] [CrossRef]
- Orebaugh, S.; Palmeri, S.; Lin, C.; Ya Deau, J. Daring discourse: Is nerve block with sedation the safest anesthetic for beach chair position? Reg. Anesth. Pain Med. 2019, 44, 707–712. [Google Scholar] [CrossRef]
- Liu, S.S.; Gordon, M.A.; Shaw, P.M.; Wilfred, S.; Shetty, T.; YaDeau, J.T. A prospective clinical registry of ultrasound-guided regional anesthesia for ambulatory shoulder surgery. Anesth. Analg. 2010, 111, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Yadeau, J.T.; Casciano, M.; Liu, S.S.; Edmonds, C.R.; Gordon, M.; Stanton, J.; John, R.; Shaw, P.M.; Wilfred, S.E.; Stanton, M. Stroke, regional anesthesia in the sitting position, and hypotension: A review of 4169 ambulatory surgery patients. Reg. Anesth. Pain Med. 2011, 36, 430–435. [Google Scholar] [CrossRef]
- YaDeau, J.T.; Kahn, R.L.; Lin, Y.; Goytizolo, E.A.; Gordon, M.A.; Gadulov, Y.; Garvin, S.; Fields, K.; Goon, A.; Armendi, I.; et al. Cerebral Oxygenation in the Sitting Position Is Not Compromised During Spontaneous or Positive-Pressure Ventilation. HSS J. 2019, 15, 167–175. [Google Scholar] [CrossRef]
- YaDeau, J.T.; Liu, S.S.; Bang, H.; Shaw, P.M.; Wilfred, S.E.; Shetty, T.; Gordon, M. Cerebral oximetry desaturation during shoulder surgery perforMed. in a sitting position under regional anesthesia. Can. J. Anesth. 2011, 58, 986–992. [Google Scholar] [CrossRef]
- Lagier, D.; Zeng, C.; Fernandez-Bustamante, A.; Vidal Melo, M.F. Perioperative Pulmonary Atelectasis: Part II. Clinical Implications. Anesthesiology 2022, 136, 206–236. [Google Scholar] [CrossRef]
- Rehder, K.; Sessler, A.D.; Marsh, H.M. General anesthesia in the lung. Am. Rev. Respir. Dis. 1975, 112, 541–563. [Google Scholar] [PubMed]
- Hickey, R.; Ramamurthy, S. The diagnosis of phrenic nerve block on chest X-ray by a double-exposure technique. Anesthesiology 1989, 70, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Ferré, F.; Pommier, M.; Laumonerie, P.; Ferrier, A.; Menut, R.; Bosch, L.; Balech, V.; Bonnevialle, N.; Minville, V. Hemidiaphragmatic paralysis following ultrasound-guided anterior vs. posterior suprascapular nerve block: A double-blind, randomised control trial. Anaesthesia 2020, 75, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Mak, P.H.K.; Irwin, M.G.; Ooi, C.G.C.; Chow, B.F.M. Incidence of diaphragmatic paralysis following supraclavicular brachial plexus block and its effect on pulmonary function. Anaesthesia 2001, 56, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Urmey, W.F.; Gloeggler, P.J. Pulmonary function changes during interscalene brachial plexus block: Effects of decreasing local anesthetic injection volume. Reg. Anesth. 1993, 18, 244–249. [Google Scholar] [CrossRef]
- Xu, W.D.; Gu, Y.D.; Liu, J.B.; Yu, C.; Zhang, C.G.; Xu, J.G. Pulmonary function after complete unilateral phrenic nerve transection. J. NeuroSurg. 2005, 103, 464–467. [Google Scholar] [CrossRef]
- Urmey, W.F.; McDonald, M. Hemidiaphragmatic paresis during interscalene brachial plexus block: Effects on pulmonary functin and chest wall mechanics. Anesth. Analg. 1992, 74, 352–357. [Google Scholar] [CrossRef] [PubMed]
| Type | Procedures Studied in RCT | Full Surgical Anesthesia? | Efficacy vs. ISB * | Incidence of HDP * |
|---|---|---|---|---|
| STB | Arthroscopic shoulder surgery | Yes | Similar | 4.8–26.7% |
| SSNB-ANB | Arthroscopic shoulder surgery | No | Inferior | No RCT data |
| ICB-SSNB | Arthroscopic shoulder surgery | Yes | Similar; slightly higher morphine consumption at 24 h | 0–3% |
| CCB | Arthroscopic shoulder surgery | Yes | Similar | 0–7.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.H.; Qiao, W.P.; McCracken, S.; Singleton, M.N.; Goman, M. Regional Anesthesia Techniques for Shoulder Surgery in High-Risk Pulmonary Patients. J. Clin. Med. 2023, 12, 3483. https://doi.org/10.3390/jcm12103483
Lee BH, Qiao WP, McCracken S, Singleton MN, Goman M. Regional Anesthesia Techniques for Shoulder Surgery in High-Risk Pulmonary Patients. Journal of Clinical Medicine. 2023; 12(10):3483. https://doi.org/10.3390/jcm12103483
Chicago/Turabian StyleLee, Bradley H., William P. Qiao, Stephen McCracken, Michael N. Singleton, and Mikhail Goman. 2023. "Regional Anesthesia Techniques for Shoulder Surgery in High-Risk Pulmonary Patients" Journal of Clinical Medicine 12, no. 10: 3483. https://doi.org/10.3390/jcm12103483
APA StyleLee, B. H., Qiao, W. P., McCracken, S., Singleton, M. N., & Goman, M. (2023). Regional Anesthesia Techniques for Shoulder Surgery in High-Risk Pulmonary Patients. Journal of Clinical Medicine, 12(10), 3483. https://doi.org/10.3390/jcm12103483





