Abstract
The effects of celecoxib on a broad spectrum of mood disorders and on inflammatory parameters have not yet been comprehensively evaluated. The aim of this study was to systematically summarize the available knowledge on this topic. Data from both preclinical and clinical studies were analyzed, considering the efficacy and safety of celecoxib in the treatment of mood disorders, as well as the correlation of inflammatory parameters with the effect of celecoxib treatment. Forty-four studies were included. We found evidence supporting the antidepressant efficacy of celecoxib in a dose of 400 mg/day used for 6 weeks as an add-on treatment in major depression (SMD = −1.12 [95%Cl: −1.71,−0.52], p = 0.0002) and mania (SMD = −0.82 [95% CI:−1.62,−0.01], p = 0.05). The antidepressant efficacy of celecoxib in the above dosage used as sole treatment was also confirmed in depressed patients with somatic comorbidity (SMD = −1.35 [95% CI:−1.95,−0.75], p < 0.0001). We found no conclusive evidence for the effectiveness of celecoxib in bipolar depression. Celecoxib at a dose of 400 mg/d used for up to 12 weeks appeared to be a safe treatment in patients with mood disorders. Although an association between celecoxib response and inflammatory parameters has been found in preclinical studies, this has not been confirmed in clinical trials. Further studies are needed to evaluate the efficacy of celecoxib in bipolar depression, as well as long-term studies evaluating the safety and efficacy of celecoxib in recurrent mood disorders, studies involving treatment-resistant populations, and assessing the association of celecoxib treatment with inflammatory markers.
1. Introduction
Major depressive disorder (MDD) and bipolar disorder (BD) are mood disorders that impair the quality of life and shorten life expectancy [1]. Studies report that MDD and BD affect, respectively, 246 and over 39 million people globally [2,3].
Due to a high rate of treatment resistance, novel therapies are being considered [4,5,6,7,8,9,10]. Nevertheless, insufficient knowledge of the etiology is a limiting factor in obtaining a satisfactory treatment. Numerous correlations and mechanisms have been studied to understand the pathophysiology of mood disorders [11,12,13]. Of particular note is the hypothesis that includes the role of inflammatory background in mood disorders. It comes from studies of comorbidity between mood disorders and chronic inflammatory disorders, neuroimaging, levels of pro-inflammatory and anti-inflammatory biomarkers, as well as post-mortem brain studies [14,15,16,17,18,19,20,21,22,23,24,25].
Regarding inflammatory biomarkers, patients with MDD had significantly higher mean levels of IL-1β, IL-3, IL-6, IL-10, IL-12, IL-18, the soluble IL-2 receptor (sIL-2R), and tumor necrosis factor α (TNFα) compared to healthy controls [26,27,28]. Patients with BD also exhibited increased concentrations of CRP, IL-4, IL-10, and decreased concentrations of brain-derived neurotrophic factor (BDNF) [27,29,30].
Among anti-inflammatory agents, cyclooxygenase-2 inhibitors are a potential treatment for mood disorders [31,32,33,34]. A representative of this class of drugs is celecoxib, which belongs to the non-steroidal anti-inflammatory drugs (NSAIDs) [35]. NSAIDs inhibit the enzyme cyclooxygenase (COX), which converts arachidonic acid into prostanoids via two distinct isozymes, COX-1 and COX-2. COX-1 helps maintain gastrointestinal mucosa lining. COX-2 plays a role in inflammation; it synthesizes prostaglandin E2 (PGE2), which acts as a stimulator of indoleamine-2,3-dioxygenase (IDO) and mediates inflammatory response [35,36]. Thereby, selective cyclooxygenase-2 inhibitors provide anti-inflammatory effects without causing stomach irritation. Moreover, according to research, celecoxib has a better cardiovascular safety profile in comparison with ibuprofen or naproxen [37,38].
In recent years, several reviews and meta-analyses have been published on the use of celecoxib for mood disorders. These mainly concerned depression [34,39,40], with one study focused on mania [41]. The Kittur et al. (2022) study, a scoping review, analyzed celecoxib in patients with MDD and bipolar depression. Since conflicting results were found, the authors suggested the need to stratify patients according to the inflammation status and clinical presentation [39]. On the other hand, a meta-analysis by Wang et al. (2022) showed that celecoxib was effective in treating major depression in the course of both bipolar and unipolar disorder [40]. As in the Kittur et al. (2022) study, it was found that the type of depression was a possible source of variation in efficacy results. Since in this study the inflammatory markers were not evaluated, they recommended that future meta-analyses should take into account depression type along with inflammation markers. Finally, in a recent systematic review of meta-analyses of anti-inflammatory agents (including celecoxib) in MDD, it was stated that no clear-cut recommendations can be made due to the heterogeneity of patient populations. The authors emphasized the need to identify anti-inflammatory biomarkers in given populations of patients with depression for more tailored therapy [34]. As regards the use of celecoxib for affective conditions other than depression, we identified only one systematic review and meta-analysis highlighting its potential efficacy in mania [41].
Given the conclusions of the reviews and meta-analyses cited above, as well as the postulated inflammatory background in a wide range of mood disorders, a combined analysis and comparison is relevant. We decided to bring together in a single paper the broadest possible spectrum of mood disorders, including different types of depression and different affective episodes with a simultaneous attempt to identify inflammatory markers in given types of depression and in given affective episodes. Thus, this review analyzed the effect of celecoxib’s efficacy and safety according to diagnosis, including major depression, bipolar depression and depressive symptoms in somatic disorders, and including all affective episodes (both mania and depression). We also aimed to investigate the association of celecoxib treatment with inflammatory markers in a given type of depression and affective state. In addition, we also pooled preclinical studies in models of depression and mania with clinical reports to make suggestions for future research. To the best of our knowledge, the effects of celecoxib on major depression, bipolar depression and mania, depressive symptoms in somatic disorders, and inflammatory parameters in preclinical and clinical studies have not yet been evaluated in such a comprehensive manner.
2. Materials and Methods
This systematic review was conducted according to the PRISMA statement (Preferred Reporting Items For Systematic Review and Meta-Analysis), based on previously prepared, unregistered protocol [42]. Two reviewers conducted each stage throughout the review process independently. Any disagreements between investigators were resolved via discussion and the opinion of a senior researcher to achieve a consensus.
2.1. Eligibility Criteria
Each relevant publication was evaluated using the PICO model (Table 1). Articles that met predefined criteria presented were included and categorized as preclinical and clinical (observational or interventional studies).

Table 1.
PICO framework for a different design of studies.
The included criteria were as follows: (1) preclinical, observational, or interventional study of any designs; (2) study on the effect of celecoxib on mood disorders or affective symptoms or for behavioral testing in an animal model of mood disorders; (3) participants over 18 years of age and under 65 years of age (applies to clinical studies); (4) published in English. The excluded criteria were as follows: (1) not conforming with PICO; (2) not an original article; (3) not in English; (4) full text was not available; (5) not published.
2.2. Data Acquisition and Search Strategy
We searched PubMed, Scopus, and Web of Science for studies published from inception to November 2022. We selected only databases that were accessible to reviewers through the institution. The search string used was (“bipolar disorder” or “bipolar depression” or “mania” or “hypomania” or “mixed episode” or “major depression” or “mood disorders”) and (“celecoxib” or “celebrex” or “4-(5-(4-methylphenyl)-3-(trifluoro methyl)-1H-pyrazol-1-yl) benzenesulfonamide”). Full search strategy for each database and registry are presented in Supplementary Material File S1. Follow-up citations were also scanned for relevant articles. After removing duplicates and reviewing titles and abstracts, the full text of all qualified studies were obtained to access the eligibility criteria.
2.3. Data Extraction
Data related to the effects of celecoxib on mood disorders were extracted independently using a tailored form. The form included: authors, year of publication, country, study design, sample and control size, duration, characteristics of the research and control group (sex, mean age, diagnosis, treatment), dose of celecoxib, and outcomes (impact on affective symptoms/behavioral tests, adverse effects, inflammatory markers).
2.4. Quality Assessment
Risk of bias of clinical trials was conducted in accordance with the Cochrane Collaboration guidelines [43] with RoB2 and ROBINS-I [44,45]. The Robvis tool was used for visualization [46]. A detailed description of the risk assessment is included in Supplementary Material File S1.
2.5. Synthesis and Analysis
Search results from Mendeley Desktop (version 1.19.8) have been transferred to Review Manager (RevMan5 version 5.4; Cochrane Collaboration). Continuous outcomes were pooled as standardized mean difference (SMD). Whenever the heterogeneity I2 test was below 75% the results were pooled. A fixed-effects model was used for the analysis. Studies with a risk of bias judged as “high” were excluded from the analysis. A subgroup analysis of treatment-resistant patients (TRD) was also planned. A detailed description of the synthesis and analysis is included in Supplementary Material File S1.
3. Results
3.1. Study Selections
A total of 1640 papers were identified through the search strategy. After the removal of duplicates and exclusion based on titles or abstracts, 98 articles were screened in more detail for eligibility. Subsequently, another 54 were excluded, which resulted in the 44 publications used in this systematic review. This process is described in the PRISMA flowchart (Figure 1).

Figure 1.
Flowchart showing an overview of the study selection process.
3.2. Description of Studies
The included studies were published between 2006 and 2021. Among identified studies, 19 were preclinical, 17 were interventional (16 randomized controlled trials, and 1 open-label study), and 8 were secondary analyses of RCTs. All preclinical studies involved rodents and used models of depression or mania. Clinical studies were published in the population of adults 18–65, two studies included patients up to 70 years old [47,48], and one up to 75 years old [49]. Study duration ranged from 6 to 12 weeks, with a mean of 6.5 weeks. The studies were conducted in the following countries: Iran (12), USA (8), Brazil (4), Germany (4), China (3), Canada (2), India (2), Italy (2), Netherlands (2), Australia (1), Denmark (1), Pakistan (1), Portugal (1), and Russia (1).
3.3. Quality Assesment
The interventional randomized controlled studies and secondary analysis of RCT with relevant outcomes were ranked according to the RoB2 tool. Eight of the twenty-four included studies were rated as ‘low risk of bias’, the other twelve as ‘some concerns’, and the remaining four as ‘high risk’. A non-randomized interventional study was assessed according to the ROBINS-I tool and ranked as ‘some concerns’. Risk of bias for all studies are presented in Figure 2, Figure 3 and Figure 4.

Figure 2.
Risk of bias for interventional randomized studies with RoB2 tool [47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70].

Figure 3.
Risk of bias summary for interventional randomized studies with RoB2 tool.

Figure 4.
Risk of bias for interventional non-randomized study with ROBINS-I tool [71].
3.4. Preclinical Studies
A total of 19 in vivo studies were identified (Supplementary Material File S2).
3.4.1. Preclinical Studies—Effect of Celecoxib on Depression and Mania-Like Symptoms in Animal Models
In all preclinical studies (17/17, 100%), regardless of the depression model, antidepressant effect of celecoxib was reported. Most included reports have pointed to the antidepressant effect of celecoxib in monotherapy [72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87]. Interestingly, two studies indicated that celecoxib might enhance the antidepressant effects of fluoxetine and bupropion [75,88]. In nine studies, celecoxib alone or with co-administration of antidepressants improved behavioral despair in forced swimming test (FST) [74,75,76,80,81,82,84,85,86] and in four studies in tail suspension test (TST) [75,76,78,83]. In eight studies, a positive effect on anhedonia as measured by the sucrose preference test (SPT) was found [73,76,77,81,82,84,85,86]. Four studies showed reduced anxiety and increased locomotor activity in open field test (OFT) [72,73,74,87] and one in evaluated plus maze (EPM) [76]. However, some studies produced ambiguous results. One study showed that celecoxib was effective for only five minutes and then did not show any further effect [72]. According to Alboni’s study, celecoxib partially restored stress-induced escape deficits when combined with fluoxetine. However, full reversal of the deficit was not achieved [88].
The models used to assess the influence of celecoxib on anxiety or depressive symptoms were as follows: stress-induced depressive-like behavior [73,77,78,81,84,86,88], induced by inflammation [74,79,82,85], diet-induced [76], different disease models [75,80,83], and bulbectomy [72,87].
Studies have used a dose in the range of 2–50 mg/kg/day, with most studies examining the effects of a dose of 15–30 mg/kg/day. The duration of celecoxib administration ranged from a single dose to 5 weeks, with an average of 17 days.
We identified two studies that evaluated the effects of celecoxib on mania-like symptoms in an animal model [89,90]. Both were conducted by the same research group, using a d-AMPH-induced mania model and administering 20 mg/kg/day celecoxib p.o. for 7 days. In both studies, celecoxib and low-dose lithium co-administered successfully abrogated the d-AMPH effect in open field test (OFT). Separate drug administration did not produce this effect.
3.4.2. Preclinical Studies—Safety of Celecoxib in Rodents
No adverse effects related to celecoxib administration were reported in the preclinical studies analyzed. There was also no association of drug dose, route of administration, or time of administration with adverse effects.
3.4.3. Preclinical Studies—Effect of Celecoxib on Inflammatory Markers
The effect of celecoxib on central or peripheral inflammatory markers in depression models was studied in eight papers. As a result of celecoxib administration, elevated central brain levels of PGE2 observed in depression models were decreased [73,81,87]. The treatment normalized brain levels of IL-1β [72,75,81,83], TNFα [72,81], and IFNγ [81] which were higher in depression models. Further, the levels of IL-10 were lower in the hypothalamus and higher in the prefrontal cortex in a group of rodents taking celecoxib [72]. In serum, celecoxib reduced IL-1 β and PGE2 concentration and blocked the elevation of corticosterone levels in one study [87], but no effect on peripheral blood cytokines was observed in another [72]. One study evaluating the effect of celecoxib administration on BDNF concentrations failed to find an association [75]. Celecoxib has also been found to attenuate reduction of NGF expression in the hippocampus [87] and affect neuroinflammation by inhibiting microglia activation [81,83,84].
Only one study evaluated changes in immune parameters in a mania model. Co-administration of celecoxib and lithium (24 mg/kg/day) reversed increased IL-4 in the frontal cortex, TNFα in the striatum, and IL-10 in the serum [89].
3.5. Clinical Studies
We identified 25 reports which concerned 17 trials; 16 of them were randomized controlled trials and 1 open-label clinical trial. Eight papers were secondary analyses including primary trials. Ten studies focused on the patient population with depression (Table 2), and 12 studies focused on bipolar disorder (n = 9—bipolar depression, n = 3—mania) (Table 3 and Table 4). Three studies were aimed at affective symptoms in somatic disorders (Table 5).

Table 2.
Clinical studies on celecoxib for major depression (n = 10).

Table 3.
Clinical studies on celecoxib for bipolar depression (n = 9).

Table 4.
Clinical studies on celecoxib for mania (n = 3).

Table 5.
Clinical studies on celecoxib for depression in course of somatic diseases (n = 3).
3.5.1. Clinical Studies—Effectiveness of Celecoxib in Major Depression
Eight studies evaluated the efficacy of additional celecoxib therapy. Seven of them were double-blind randomized controlled trials and one was open-label trial. The duration of studies varied from 6 to 8 weeks and the dose of celecoxib ranged from 200 to 400 mg/daily. Four of them (4/7, 57%) showed positive effects of celecoxib in clinical symptoms after the intervention at any checkpoint [50,51,52,53], but only three at the endpoint [50,51,52]. The main treatments in these studies were SSRI [50,51,53] or NRI [52]. In three studies, improvement was not observed [49,54,55]. Celecoxib was used as an add-on treatment in these studies along with sertraline [55], vortioxetine [54], or ECT [49].
Only studies assessed as ‘low risk of bias’ or ‘some concerns’ were included in the meta-analysis [50,51,52,54]. Three studies were excluded due to the assessment as being of high risk of bias [49,53,55] (Figure 2).
The heterogeneity of studies evaluating the effect of celecoxib on major depression was substantial (I2 = 81%, Chi2 = 15.96, df = 3, Tau2 = 0.37, p < 0.001). The standardized mean difference (SMD) was −0.85 [95% CI: −1.52, −0.18]. Test for overall effect: Z = 2.5 (p = 0.01). However, we found discrepancies reflected in the considerable heterogeneity of analyzed studies. Specifically, we identified one significant outlier [54]. This study included mainly patients with TRD. Therefore, we performed a sensitivity analysis excluding the above study. It resulted in a substantial decrease in heterogeneity (I2 = 57%, Chi2 = 4.64, df = 2, Tau2 = 0.16, p = 0.10). SMD was −1.12 [−1.71, −0.52], (p = 0.0002) (Figure 5).
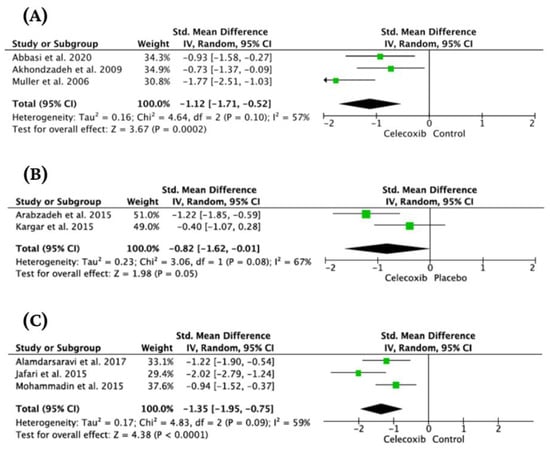
Figure 5.
Forest plots for the effect of celecoxib as an added-on treatment in major depression (A) [50,51,52], mania (B) [59,60], and sole treatment of depressed patients with somatic comorbidity (C) [47,69,70].
Visual evaluation of all funnel plots showed a symmetrical distribution, thus indicating the absence of publication bias.
As only one study with a TRD patient population was identified, the planned subgroup analysis could not be performed.
3.5.2. Clinical Studies—Effectiveness of Celecoxib in Bipolar Disorder
Effectiveness of Celecoxib in Bipolar Depression
According to one of the three interventional studies (1/3, 33%), a significantly greater improvement in depressive symptoms was noted in the celecoxib group compared to placebo [56]. However, in one of two negative studies, celecoxib was superior to placebo in the assessment after 1 week of treatment, when the analysis included only the subjects who completed the full 6-week trial [57]. At the end of treatment (after 6 weeks), there were no statistically significant differences between the two groups. In turn, a study by Husain et al. found no advantage of celecoxib over placebo in any of the interim assessments nor at the end of treatment (after 12 weeks) [58].
The duration of studies varied from 6 to 12 weeks and the dose of celecoxib ranged from 200 to 400 mg/daily. Various mood stabilizers, antipsychotics, antidepressants, and benzodiazepines were used as the main treatments in two studies [57,58]; in one study, escitalopram was administered [56]. A meta-analysis and subgroup analysis were abandoned because raw data were not available in one study [56], and high risk of quality assessment was in another [57].
Effectiveness of Celecoxib in Mania
Celecoxib’s effect on mania symptoms has only been examined in two double-blind randomized controlled trials. Study durations and study samples were 6 weeks (N = 46) and 6 ECT sessions (N = 35). Both studies used celecoxib in a dose of 400 mg/day, however, they differed in the main treatment—sodium valproate in one [59] and ECT in the other [60]. Celecoxib augmentation was found to be superior to placebo only in one of these two studies (1/2, 50%) [59].
A substantial heterogeneity (I2 = 67%, Chi2 = 3.06, df = 1, Tau2 = 0.23, p = 0.08) was found during analysis. Calculated SMD was −0.82 [95% CI: −1.62, −0.01], (p = 0.05) (Figure 5).
3.5.3. Clinical Studies—Safety of Celecoxib as an Added-on Treatment in Mood Disorders
Ten studies evaluated the incidence rates of adverse effects between celecoxib and placebo groups in major depression and bipolar disorder [48,50,51,52,53,54,56,57,58,59]. In all of the above studies no significant differences in the incidence rate of adverse effects between groups were observed, except skin and mucous membranes in one [54]. No serious adverse effects have been reported. Treatment with celecoxib did not affect cognition [48,54] or serum drug levels [51,52]. The acceptability of the treatment in both groups was similar in all studies.
3.5.4. Clinical Studies—Effect of Celecoxib Treatment on Inflammatory Markers in Patients with Mood Disorders
A total of 15 studies evaluated parameters related to inflammation (Table 2, Table 3 and Table 4) [48,50,54,55,58,60,61,62,63,64,65,66,67,68]. The most commonly studied parameters were: CRP, kynurenine pathway metabolites, IL-2, IL-1β, TNF-α, MFI (macrophage migration inhibitory factor) (Table 6). Overall, none of the parameters studied were found to be significantly different in the celecoxib-treated group compared to the control group in more than one study. Both IL-6 [50] and TNF-alpha [48] and CRP [61] levels were significantly different in only a single study; the other studies did not confirm such regularity [47,54,55,58]. Table 6 presents the pooled analysis of the studies on a given blood inflammatory parameter.

Table 6.
Effect of celecoxib on blood inflammatory parameters in patients with mood disorders.
3.5.5. Clinical Studies—Effectiveness of Celecoxib in Depressed Patients with Somatic Comorbidity
Three studies evaluating celecoxib’s effect on depressive symptoms in somatic disorders were double-blind RCTs involving a patient population with mild to moderate depression. Two of them concerned cancer patients [46,69], and one concerned depressed patients diagnosed with brucellosis [70]. Study duration and sample ranged from 6 weeks (N = 40), and 6 weeks (N = 52) to 8 weeks (N = 40). Celecoxib was administered at a dose of 400 mg; the control group used placebo or diclofenac.
In all three studies (3/3, 100%), celecoxib group showed significantly greater improvement in HAMD-17 score compared to controls. The pooled effect of celecoxib in two studies with cancer patients was significant [46,69]. The heterogeneity of these studies was small (I2 = 0%, Chi2 = 0.37, df = 1, Tau2 = 0.00). The standardized mean difference (SMD) was −1.06 [−1.50, −0.62]. Test for overall effect: Z = 4.72 (p < 0.00001).
The heterogeneity of studies evaluating the effect of celecoxib on depression symptoms in patients with somatic disorders was substantial (I2 = 59%, Chi2 = 4.83, df = 2, Tau2 = 0.17, p = 0.09). The standardized mean difference (SMD) was −1.35 [95% CI: −1.95, −0.75]. Test for overall effect: Z = 4.38 (p < 0.0001) (Figure 5).
3.5.6. Clinical Studies—Safety of Celecoxib in Depressed Patients with Somatic Comorbidity
No statistically significant differences in the frequency of adverse effects were observed between celecoxib and control groups [46,69,70].
3.5.7. Clinical Studies—Effect of Celecoxib Treatment on Inflammatory Markers in Depressed Patients with Somatic Comorbidity
Inflammatory parameters were not investigated in any of the studies on depressed patients with somatic comorbidities.
4. Discussion
According to recent literature, inflammation may play an important role in the pathogenesis and course of mood disorders [91]. This prompts the consideration of anti-inflammatory treatment as a potential therapeutic approach. Therefore, we decided to summarize in a systematic way the current literature data on one of the agents with anti-inflammatory activity—celecoxib as a treatment for mood disorders. The main finding of this study is the efficacy of celecoxib at a dose of 400 mg used for 6 weeks as an add-on treatment in major depression and mania, as well as in depression with comorbid somatic conditions used as the sole antidepressant treatment.
The rationale behind the antidepressant’s effectiveness was found in both preclinical and clinical studies. The meta-analysis indicated that celecoxib is an effective add-on treatment for major depression. This result is consistent with the previous reviews [34,39,40,92]. Only one out of the high-quality studies we identified was not consistent with this result [54]. We found that this study was the only study conducted in patients with treatment-resistant depression (TRD) and showed no benefits from celecoxib use in this population. As one-third of MD patients may be refractory to treatment, and the search for effective augmentation strategies is still ongoing [93,94], further studies are needed to determine whether celecoxib has a beneficial effect in treatment-resistant populations as well.
Studies of celecoxib in bipolar depression have yielded somewhat different results and these are inconclusive. In only one of the identified studies, an improvement in depressive symptoms was noted [56]. Interestingly, the opposite was found in the studies with the MD population; this particular study involved the TRD BD population [56]. Further studies to resolve these ambiguities are needed. In contrast to bipolar depression, in the case of mania, the results were more conclusive, indicating that celecoxib was effective in this indication as an add-on treatment. Our findings are consistent with a previously conducted meta-analysis [41].
It is worth mentioning that the efficacy of celecoxib should be evaluated along with treatment adherence. However, this was reported in 7 out of 16 RCTs [47,53,56,57,58,69,71]. It included patient–staff interviews [53,71] and pill counts [47,56,57,58,69,71]. Additionally, in two studies, the serum level of the main treatment (reboxetine and fluoxetine) was measured [51,52]. Although none of the above RCTs reported poor adherence, it should be noted that in the remaining studies, it was not reported. This issue was partly addressed during the risk of bias assessment, however, we cannot entirely exclude that this might have affected some results.
The included trials ranged in duration from 6 to 12 weeks aiming to assess the efficacy of celecoxib in the short to medium term. However, anti-inflammatory agents may have long-term positive effects. In a recent review, low-dose aspirin was found to reduce the risk of reoccurrence of all affective episodes of bipolar disorder [95]. In this context, studies with long-term follow-up, targeting the assessment of recurrent affective episodes would be required to evaluate the potential efficacy of celecoxib in the treatment of relapses. Obviously, this is where the safety of such treatment comes into consideration, especially when considering long-term therapy. The safety of celecoxib in mood disorders has also been raised in this review. Several years ago, the FDA imposed a black box warning about the drug’s cardiovascular risk. This is particularly relevant to patients suffering from bipolar disorder who are at heightened risk of cardiovascular events, which remain a leading cause of death in this population [96,97,98]. However, based on the PRECISION study, the FDA has backed down on this warning in recent years. Celecoxib, at a dose of 2 × 100 mg, had the same effect on cardiovascular risk as other NSAIDs [99]. As a result of this review, we concluded that celecoxib could be used safely in mood disorders at a dose of 400 mg per day for 6–12 weeks. Furthermore, 400 mg/day was safe for patients with somatic conditions taking it for 6–8 weeks. Cardiovascular complications were not reported. However, the risk of cardiovascular complications may rise with an increasing dose and length of treatment, thus it has been recommended to use it for the shortest possible time and at the lowest effective daily dose (the maximum recommended daily dose is 400 mg for all indications). Furthermore, it is critical to avoid administering this medication to patients with contraindications, including those with hypersensitivity to the active ingredient, sulfonamides or other nonsteroidal anti-inflammatory drugs, active gastric or duodenal ulcer disease, or pregnant and breastfeeding patients. Long-term studies can be considered to determine whether celecoxib is effective in recurrent mood disorders. However, this would require an evaluation of a safe and effective dose for long-term use in this indication.
Celecoxib’s doses in mood disorders should be investigated. According to clinical studies, this drug provides greater pain relief and inflammation reduction at higher doses, but at the same time increases adverse effects. The majority of studies we found used a celecoxib dose of 400 mg, while two used 200–400 mg [58,71] and one used 200 mg [53]. Therefore, the dose–effect relationship cannot be concluded on this basis. To estimate this connection, further studies including various doses of celecoxib should be performed. It should be noted that, currently, the maximum dose recommended for all indications according to the Summary of Product Characteristics is 400 mg.
As a result of its anti-inflammatory and analgesic properties, celecoxib is often used to treat somatic diseases such as rheumatoid arthritis, osteoarthritis, and neuralgia [100,101,102]. According to our meta-analysis, celecoxib at a dose of 400 mg/d used for 6–8 weeks as the sole treatment in patients with the somatic disease and comorbid depression was significantly more effective in antidepressant efficacy than place and the comparator (diclofenac). Nevertheless, we identified only three studies involving patient populations with brucellosis, colorectal cancer, and breast cancer [47,69,70]. Although brucellosis is an infectious disease, neurobrucellosis can also clinically manifest as depression [103]. In light of the high incidence and mortality of different types of cancer in recent years, celecoxib’s efficacy in this patient population seems to be important information [104,105]. Patients with cancer are more likely to experience depression and chronic pain compared to the general population [106,107]. It has been shown that celecoxib is a good therapeutic option for reducing pain, as well as improving mood. An analysis of pooled data from five post approval trials also showed that this drug significantly reduced depressive symptoms in patients with osteoarthritis at a dose of 200 mg daily [108]. Considering that depression can accompany many diseases, it is important to study celecoxib’s use in patients with other somatic conditions, in particular when treatments are based primarily on pain management. Comparison with other commonly used NSAIDs, such as diclofenac, may also provide important information. Although celecoxib appears to improve depression symptoms in somatic patients, none of the papers we reviewed examined the drug’s association with inflammatory markers. As it seems that this could provide important data regarding a possible common underlying origin of both conditions, there is a need for further research on these issues in this group of patients.
The potential therapeutic effect of celecoxib is likely to be due to its ability to act via COX-2 and its effect on the arachidonic pathway [109]. Moreover, inhibition of this enzyme might directly affect the serotonergic system in the central nervous system [110]. Celecoxib inhibits COX-2 selectively, therefore PEG2 levels are decreased and the balance of pro-inflammatory and anti-inflammatory cytokines is altered. There are various pathways through which inflammation can be modulated, affecting the concentrations of inflammatory and neurotrophic markers. IL-1, TNFα, IFNγ, NGF, or microglia activation in depression, as well as IL-4, TNFα, and IL-10 in mania, were normalized by celecoxib in preclinical studies. Nevertheless, the results of preclinical studies have not been confirmed in clinical trials. Celecoxib treatment only improved peripheral IL-6 levels in depression [50] and TNF-alpha levels in mania [48], but these are only single clinical studies on these markers. There were negative results for other substances (such as kynurenine pathway metabolites) or unclear results (such as CRP). Our findings from preclinical studies can be used to identify future research directions in clinical trials. For instance, preclinical studies have demonstrated positive results regarding central IFNγ and NGF for depression [81,87], as well as peripheral IL-10 for mania [89]. To confirm these findings, translational studies would be needed. Attwells et al. proposed a new approach to predicting the effects of celecoxib treatment in their open-label study using PET method [71]. Celecoxib was more effective in treating patients with severe gliosis determined by using translocator protein total distribution volume in the anterior cingulate cortex and prefrontal cortex. There is a need for further randomized controlled studies to confirm this method’s effectiveness in predicting anti-inflammatory responses. However, mood disorders, particularly MDD, are very complicated and varied conditions. Although genetic, neurobiological, or environmental factors are known to have a significant influence, the pathophysiology of this disorder is not entirely understood, as we previously mentioned. MDD patients’ immunological states are inconsistent with notable interindividual variations. This necessitates careful interpretation of the outcomes of numerous therapies on this patient group [111]. Finding markers that identify MDD patients who respond better to a specific adjunctive therapy prove crucial for personalizing therapy. However, the results of the studies in this field are still unclear. The development of predictors of treatment response should be conducted to identify patients with inflammatory phenotypes who will benefit from celecoxib augmentation.
Finally, it is important to consider celecoxib’s pharmacokinetic interaction with antidepressants as another possible mechanism explaining its positive effect on depression symptoms. Most antidepressants are metabolized by two major metabolic enzyme systems: cytochrome P450 (CYP) or UDP- glucuronosyltransferases (UGT) [112]. Celecoxib is mainly metabolized by CYP2C9 in the liver. Among its pharmacokinetic interactions, inhibition of CYP2D6 and inhibition of CYP2C19 contribute to the suppression of the metabolism of substances catalyzed by these enzymes. Among these compounds are antidepressants, including those identified in our review: CYP2D6 metabolizes fluoxetine and vortioxetine, while CYP2C19 metabolizes sertraline. In the only study we found which assessed fluoxetine levels in both celecoxib and control groups, no difference was found between them [51]. It is further surprising that there is no clear drug–drug association since fluoxetine is also metabolized by CYP2C9, which is the main metabolic pathway for celecoxib [112]. Therefore, the pharmacokinetic responses that might be expected were not observed. Furthermore, there was no difference in reboxetine concentrations between celecoxib and control groups [52]. This finding is, however, backed up by the fact that the drug is not metabolized by CYP2D6. As a result of these findings, celecoxib did not elevate antidepressant levels. There is also evidence that anti-inflammatory drugs that affect COX enzymes, such as aspirin, can also be used as an effective augmentation method, although they affect CYP in very different ways—they do not affect CYP2D6 and instead induce CYP2C19 activity [113]. Evaluation of the serum concentration of celecoxib and the main antidepressant treatment will be critical in future studies to confirm and clarify these findings.
Several studies have demonstrated that inflammation pathways play a critical role in improving symptoms of mood disorders. According to a recent systematic review, aspirin, which inhibits both COX-1 and COX-2, is an effective and safe adjunctive treatment option for MDD and BD in adults [95]. Other anti-inflammatory drugs, such as minocycline or N-acetylcysteine, are also beneficial to patients with mood disorders [114,115]. Additionally, in some cases, combined anti-inflammatory treatments may be effective. We identified one study in which minocycline + celecoxib was no better than placebo [58], however, in another NSAID study with aspirin, it was made more effective when combined with N-acetylcysteine [116]. The benefits of possible combinations have been assessed in further studies.
5. Conclusions
This study suggests the antidepressant efficacy of celecoxib at a dose of 400 mg used for 6 weeks as an add-on treatment for major depression and mania. Furthermore, celecoxib in the above dosage used as sole treatment was also effective in reducing depressive symptoms in depressed patients with somatic comorbidity. No conclusive evidence on the antidepressant efficacy of celecoxib in bipolar depression was found. Celecoxib at a dose of 400 mg/d used for up to 12 weeks appears to be a safe treatment for patients with mood disorders. Although an association between celecoxib response and inflammatory parameters has been found in preclinical studies, this has not been confirmed in clinical trials. Therefore, based on the available studies to date, it is not possible to identify a marker of inflammation in the given types of depression and affective episodes in stratifying patients on this basis.
Further high-quality RCTs are needed to evaluate celecoxib efficacy in bipolar depression. Other identified research gaps include evaluating the efficacy of celecoxib in treatment-resistant depression (TRD), the efficacy in preventing relapse in recurrent mood disorders, and finally, the association of celecoxib treatment with inflammatory cytokines, particularly in patients with comorbid somatic disorders.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12103497/s1. Supplementary Material File S1: Full search strategy for each database and registry. Supplementary Material File S2: In vivo studies in animal models of depression and mania (n = 19).
Author Contributions
Conceptualization, A.G. and M.D.; Data Curation, A.G., Z.S. and M.D.; Writing—Original Draft Preparation, A.G., Z.S. and M.D.; Writing—Review and Editing, M.D., A.G. and Z.S.; Formal analysis, M.D. and A.G.; Visualization, A.G. and M.D.; Supervision, P.M. and A.Z.A.; Project Administration, M.D. and P.M.; Funding Acquisition, M.D. and A.Z.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical concerns.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global Prevalence and Burden of Depressive and Anxiety Disorders in 204 Countries and Territories in 2020 Due to the COVID-19 Pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Mental Disorders Collaborators. Global, Regional, and National Burden of 12 Mental Disorders in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Psychiatr. 2022, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Tondo, L.; Visioli, C.; Preti, A.; Baldessarini, R.J. Bipolar Disorders Following Initial Depression: Modeling Predictive Clinical Factors. J. Affect. Disord. 2014, 167, 44–49. [Google Scholar] [CrossRef]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 Guidelines for the Management of Patients with Bipolar Disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- Hidalgo-Mazzei, D.; Berk, M.; Cipriani, A.; Cleare, A.J.; Florio, A.D.; Dietch, D.; Geddes, J.R.; Goodwin, G.M.; Grunze, H.; Hayes, J.F.; et al. Treatment-Resistant and Multi-Therapy-Resistant Criteria for Bipolar Depression: Consensus Definition. Br. J. Psychiatr. 2019, 214, 27–35. [Google Scholar] [CrossRef]
- Borbély, É.; Simon, M.; Fuchs, E.; Wiborg, O.; Czéh, B.; Helyes, Z. Novel Drug Developmental Strategies for Treatment-Resistant Depression. Br. J. Pharmacol. 2022, 179, 1146–1186. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, M.P.; Fenoy, A.J.; Carvalho, A.F.; Soares, J.C.; Quevedo, J. Deep Brain Stimulation for Treatment-Resistant Depression: An Integrative Review of Preclinical and Clinical Findings and Translational Implications. Mol. Psychiatr. 2018, 23, 1094–1112. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Karrouri, R.; Hammani, Z.; Benjelloun, R.; Otheman, Y. Major Depressive Disorder: Validated Treatments and Future Challenges. World J. Clin. Cases 2021, 9, 9350–9367. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Lane, H.-Y.; Lin, C.-H. New Treatment Strategies of Depression: Based on Mechanisms Related to Neuroplasticity. Neural Plast. 2017, 2017, 4605971. [Google Scholar] [CrossRef]
- Inserra, A.; Rogers, G.B.; Licinio, J.; Wong, M.-L. The Microbiota-Inflammasome Hypothesis of Major Depression. Bioessays 2018, 40, e1800027. [Google Scholar] [CrossRef]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a Glutamate Hypothesis of Depression: An Emerging Frontier of Neuropsychopharmacology for Mood Disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef] [PubMed]
- SayuriYamagata, A.; Brietzke, E.; Rosenblat, J.D.; Kakar, R.; McIntyre, R.S. Medical Comorbidity in Bipolar Disorder: The Link with Metabolic-Inflammatory Systems. J. Affect. Disord. 2017, 211, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Lin, X.; Jiang, D.; Tian, H.; Xu, Y.; Wang, L.; Ji, F.; Zhou, C.; Song, X.; Zhuo, C. Depression and Cardiovascular Disease: Shared Molecular Mechanisms and Clinical Implications. Psychiatr. Res. 2020, 285, 112802. [Google Scholar] [CrossRef] [PubMed]
- Andrejew, R.; Oliveira-Giacomelli, Á.; Ribeiro, D.E.; Glaser, T.; Arnaud-Sampaio, V.F.; Lameu, C.; Ulrich, H. The P2X7 Receptor: Central Hub of Brain Diseases. Front. Mol. Neurosci. 2020, 13, 124. [Google Scholar] [CrossRef]
- Li, H.; Sagar, A.P.; Kéri, S. Microglial Markers in the Frontal Cortex Are Related to Cognitive Dysfunctions in Major Depressive Disorder. J. Affect. Disord 2018, 241, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Gritti, D.; Delvecchio, G.; Ferro, A.; Bressi, C.; Brambilla, P. Neuroinflammation in Major Depressive Disorder: A Review of PET Imaging Studies Examining the 18-KDa Translocator Protein. J. Affect. Disord. 2021, 292, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Snijders, G.J.L.J.; Sneeboer, M.A.M.; Fernández-Andreu, A.; Udine, E.; Psychiatric donor program of the Netherlands Brain Bank (NBB-Psy); Boks, M.P.; Ormel, P.R.; van Berlekom, A.B.; van Mierlo, H.C.; Böttcher, C.; et al. Distinct Non-Inflammatory Signature of Microglia in Post-Mortem Brain Tissue of Patients with Major Depressive Disorder. Mol. Psychiatr. 2021, 26, 3336–3349. [Google Scholar] [CrossRef]
- Agarwal, K.; Manza, P.; Chapman, M.; Nawal, N.; Biesecker, E.; McPherson, K.; Dennis, E.; Johnson, A.; Volkow, N.D.; Joseph, P.V. Inflammatory Markers in Substance Use and Mood Disorders: A Neuroimaging Perspective. Front. Psychiatr. 2022, 13, 647. [Google Scholar] [CrossRef]
- Aronica, R.; Enrico, P.; Squarcina, L.; Brambilla, P.; Delvecchio, G. Association between Diffusion Tensor Imaging, Inflammation and Immunological Alterations in Unipolar and Bipolar Depression: A Review. Neurosci. Biobehav. Rev. 2022, 143, 104922. [Google Scholar] [CrossRef]
- Saccaro, L.F.; Crokaert, J.; Perroud, N.; Piguet, C. Structural and Functional MRI Correlates of Inflammation in Bipolar Disorder: A Systematic Review. J. Affect. Disord. 2023, 325, 83–92. [Google Scholar] [CrossRef]
- Enache, D.; Pariante, C.M.; Mondelli, V. Markers of Central Inflammation in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Studies Examining Cerebrospinal Fluid, Positron Emission Tomography and Post-Mortem Brain Tissue. Brain. Behav. Immun. 2019, 81, 24–40. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Sayana, P.; Pinjari, O.F.; Ahmad, N.; da Rosa, M.I.; Quevedo, J.; Barichello, T. Postmortem Evidence of Brain Inflammatory Markers in Bipolar Disorder: A Systematic Review. Mol. Psychiatr. 2020, 25, 94–113. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.M.; Pocivavsek, A.; Nicholson, J.D.; Notarangelo, F.M.; Langenberg, P.; McMahon, R.P.; Kleinman, J.E.; Hyde, T.M.; Stiller, J.; Postolache, T.T.; et al. Reduced Kynurenine Pathway Metabolism and Cytokine Expression in the Prefrontal Cortex of Depressed Individuals. J. Psychiatr. Neurosci. 2016, 41, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A Meta-Analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons between Schizophrenia, Bipolar Disorder and Depression. Mol. Psychiatr. 2016, 21, 1696–1709. [Google Scholar] [CrossRef]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.A.; Dalton, B. Cytokine Research in Depression: Principles, Challenges, and Open Questions. Front. Psychiatr. 2019, 10, 30. [Google Scholar] [CrossRef]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory Markers in Depression: A Meta-Analysis of Mean Differences and Variability in 5,166 Patients and 5,083 Controls. Brain. Behav. Immun 2020, 87, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Tatay-Manteiga, A.; Balanzá-Martínez, V.; Bristot, G.; Tabarés-Seisdedos, R.; Kapczinski, F.; Cauli, O. Clinical Staging and Serum Cytokines in Bipolar Patients during Euthymia. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2017, 77, 194–201. [Google Scholar] [CrossRef]
- Jones, G.H.; Vecera, C.M.; Pinjari, O.F.; Machado-Vieira, R. Inflammatory Signaling Mechanisms in Bipolar Disorder. J. Biomed. Sci. 2021, 28, 45. [Google Scholar] [CrossRef]
- Hang, X.; Zhang, Y.; Li, J.; Li, Z.; Zhang, Y.; Ye, X.; Tang, Q.; Sun, W. Comparative Efficacy and Acceptability of Anti-Inflammatory Agents on Major Depressive Disorder: A Network Meta-Analysis. Front. Pharmacol. 2021, 12, 691200. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Ramamoorthy, K.; Loke, W.; Lee, M.W.L.; Yeo, W.S.; Lim, D.Y.; Sivalingam, V. Clinical Role of Aspirin in Mood Disorders: A Systematic Review. Brain Sci. 2019, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Sethi, R.; Gómez-Coronado, N.; Walker, A.J.; Robertson, O.D.; Agustini, B.; Berk, M.; Dodd, S. Neurobiology and Therapeutic Potential of Cyclooxygenase-2 (COX-2) Inhibitors for Inflammation in Neuropsychiatric Disorders. Front. Psychiatr. 2019, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.S.; Arteaga-Henríquez, G.; Fouad Algendy, A.; Siepmann, T.; Illigens, B.M.W. Anti-Inflammatory Treatment Efficacy in Major Depressive Disorder: A Systematic Review of Meta-Analyses. Neuropsychiatr. Dis. Treat. 2023, 19, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kulesza, A.; Paczek, L.; Burdzinska, A. The Role of COX-2 and PGE2 in the Regulation of Immunomodulation and Other Functions of Mesenchymal Stromal Cells. Biomedicines 2023, 11, 445. [Google Scholar] [CrossRef]
- Pepine, C.J.; Gurbel, P.A. Cardiovascular Safety of NSAIDs: Additional Insights after PRECISION and Point of View. Clin. Cardiol. 2017, 40, 1352–1356. [Google Scholar] [CrossRef]
- Obeid, S.; Libby, P.; Husni, E.; Wang, Q.; Wisniewski, L.M.; Davey, D.A.; Wolski, K.E.; Xia, F.; Bao, W.; Walker, C.; et al. Cardiorenal Risk of Celecoxib Compared with Naproxen or Ibuprofen in Arthritis Patients: Insights from the PRECISION Trial. Eur. Hear. J. Cardiovasc. Pharmacother. 2022, 8, 611–621. [Google Scholar] [CrossRef]
- Kittur, M.E.; Jones, B.D.M.; Dai, N.; Mahboob, M.; Husain, M.I. Repurposing Anti-Inflammatory Agents for Mood Disorders: An Updated Review of Current Evidence. Curr. Treat. Options Psychiatr. 2022, 9, 346–362. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Q.; Wang, Q. Effect of Celecoxib on Improving Depression: A Systematic Review and Meta-Analysis. World J. Clin. Cases 2022, 10, 7872–7882. [Google Scholar] [CrossRef]
- Bavaresco, D.V.; Colonetti, T.; Grande, A.J.; Colom, F.; Valvassori, S.S.; Quevedo, J.; da Rosa, M.I. Efficacy of Celecoxib Adjunct Treatment on Bipolar Disorder: Systematic Review and Meta-Analysis. CNS. Neurol. Disord. Drug Targets 2019, 18, 19–28. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions: Online Version 5.1.0. Cochrane. Colab. 2011. Available online: www.training.cochrane.org/handbook (accessed on 24 September 2014).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.; Higgins, J.; Sterne, J.A.C.; Higgins, J.P.T.; Reeves, B.C. Development Group for ACROBAT-NRSI. A Cochrane Risk of Bias Assessment Tool: For Non-Randomized Studies of Interventions (ACROBAT-NRSI), Version 1.0.0. Available online: http://www.bristo (accessed on 24 September 2014).
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, P.; Arya, P.; Esfandbod, M.; Kaviani, A.; Najafi, M.; Kashani, L.; Zeinoddini, A.; Emami, S.A.; Akhondzadeh, S. Celecoxib Versus Diclofenac in Mild to Moderate Depression Management Among Breast Cancer Patients: A Double-Blind, Placebo-Controlled, Randomized Trial. Ann. Pharmacother. 2015, 49, 953–961. [Google Scholar] [CrossRef]
- Kargar, M.; Yousefi, A.; Mojtahedzadeh, M.; Akhondzadeh, S.; Artounian, V.; Abdollahi, A.; Ahmadvand, A.; Ghaeli, P. Effects of Celecoxib on Inflammatory Markers in Bipolar Patients Undergoing Electroconvulsive Therapy: A Placebo-Controlled, Double-Blind, Randomised Study. Swiss Med. Wkly. 2014, 144, w13880. [Google Scholar] [CrossRef]
- Banaha, N.; Ghaeli, P.; Yousefi, A.; Artounian, V.; Afzali, M.H.; Ghasemi, M. Effects of Celecoxib on Electroconvulsive Therapy-Induced Cognitive Impairment in Patients with Major Depressive Disorder: A Pilot, Double-Blind, Placebo-Controlled Trial. Acta. Med. Iran. 2019, 57, 627–634. [Google Scholar] [CrossRef]
- Abbasi, S.-H.; Hosseini, F.; Modabbernia, A.; Ashrafi, M.; Akhondzadeh, S. Effect of Celecoxib Add-on Treatment on Symptoms and Serum IL-6 Concentrations in Patients with Major Depressive Disorder: Randomized Double-Blind Placebo-Controlled Study. J. Affect. Disord. 2012, 141, 308–314. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Jafari, S.; Raisi, F.; Nasehi, A.A.; Ghoreishi, A.; Salehi, B.; Mohebbi-Rasa, S.; Raznahan, M.; Kamalipour, A. Clinical Trial of Adjunctive Celecoxib Treatment in Patients with Major Depression: A Double Blind and Placebo Controlled Trial. Depress. Anxiety 2009, 26, 607–611. [Google Scholar] [CrossRef]
- Muller, N.; Schwarz, M.J.; Dehning, S.; Douhe, A.; Cerovecki, A.; Goldstein-Muller, B.; Spellmann, I.; Hetzel, G.; Maino, K.; Kleindienst, N.; et al. The Cyclooxygenase-2 Inhibitor Celecoxib Has Therapeutic Effects in Major Depression: Results of a Double-Blind, Randomized, Placebo Controlled, Add-on Pilot Study to Reboxetine. Mol. Psychiatr. 2006, 11, 680–684. [Google Scholar] [CrossRef]
- Majd, M.; Hashemian, F.; Hosseinib, S.M.; Shariatpanahi, M.V.; Sharifid, A. A Randomized, Double-Blind, Placebo-Controlled Trial of Celecoxib Augmentation of Sertraline in Treatment of Drug-Naive Depressed Women: A Pilot Study. Iran. J. Pharm. Res. 2015, 14, 891–899. [Google Scholar]
- Baune, B.T.; Sampson, E.; Louise, J.; Hori, H.; Schubert, K.O.; Clark, S.R.; Mills, N.T.; Fourrier, C. No Evidence for Clinical Efficacy of Adjunctive Celecoxib with Vortioxetine in the Treatment of Depression: A 6-Week Double-Blind Placebo Controlled Randomized Trial. Eur. Neuropsychopharmacol. 2021, 53, 34–46. [Google Scholar] [CrossRef]
- Simon, M.S.; Burger, B.; Weidinger, E.; Arteaga-Henríquez, G.; Zill, P.; Musil, R.; Drexhage, H.A.; Müller, N. Efficacy of Sertraline Plus Placebo or Add-On Celecoxib in Major Depressive Disorder: Macrophage Migration Inhibitory Factor as a Promising Biomarker for Remission After Sertraline-Results from a Randomized Controlled Clinical Trial. Front. Psychiatr. 2021, 12, 615261. [Google Scholar] [CrossRef]
- Halaris, A.; Cantos, A.; Johnson, K.; Hakimi, M.; Sinacore, J. Modulation of the Inflammatory Response Benefits Treatment-Resistant Bipolar Depression: A Randomized Clinical Trial. J. Affect. Disord. 2020, 261, 145–152. [Google Scholar] [CrossRef]
- Nery, F.G.; Monkul, E.S.; Hatch, J.P.; Fonseca, M.; Zunta-Soares, G.B.; Frey, B.N.; Bowden, C.L.; Soares, J.C. Celecoxib as an Adjunct in the Treatment of Depressive or Mixed Episodes of Bipolar Disorder: A Double-Blind, Randomized, Placebo-Controlled Study. Hum. Psychopharmacol. 2008, 23, 87–94. [Google Scholar] [CrossRef]
- Husain, M.I.; Chaudhry, I.B.; Khoso, A.B.; Husain, M.O.; Hodsoll, J.; Ansari, M.A.; Naqvi, H.A.; Minhas, F.A.; Carvalho, A.F.; Meyer, J.H.; et al. Minocycline and Celecoxib as Adjunctive Treatments for Bipolar Depression: A Multicentre, Factorial Design Randomised Controlled Trial. Lancet Psychiatr. 2020, 7, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Arabzadeh, S.; Ameli, N.; Zeinoddini, A.; Rezaei, F.; Farokhnia, M.; Mohammadinejad, P.; Ghaleiha, A.; Akhondzadeh, S. Celecoxib Adjunctive Therapy for Acute Bipolarmania: A Randomized, Double-Blind, Placebo-Controlled Trial. Bipolar Disord. 2015, 17, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Kargar, M.; Yoosefi, A.; Akhondzadeh, S.; Artonian, V.; Ashouri, A.; Ghaeli, P. Effect of Adjunctive Celecoxib on BDNF in Manic Patients Undergoing Electroconvulsive Therapy: A Randomized Double Blind Controlled Trial. PharmacoPsychiatr. 2015, 48, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Edberg, D.; Hoppensteadt, D.; Walborn, A.; Fareed, J.; Sinacore, J.; Halaris, A. Plasma C-Reactive Protein Levels in Bipolar Depression during Cyclooxygenase-2 Inhibitor Combination Treatment. J. Psychiatr. Res. 2018, 102, 1–7. [Google Scholar] [CrossRef]
- Musil, R.; Schwarz, M.J.; Riedel, M.; Dehning, S.; Cerovecki, A.; Spellmann, I.; Arolt, V.; Müller, N. Elevated Macrophage Migration Inhibitory Factor and Decreased Transforming Growth Factor-Beta Levels in Major Depression—No Influence of Celecoxib Treatment. J. Affect. Disord. 2011, 134, 217–225. [Google Scholar] [CrossRef]
- Krause, D.; Myint, A.-M.; Schuett, C.; Musil, R.; Dehning, S.; Cerovecki, A.; Riedel, M.; Arolt, V.; Schwarz, M.J.; Müller, N. High Kynurenine (a Tryptophan Metabolite) Predicts Remission in Patients with Major Depression to Add-on Treatment with Celecoxib. Front. Psychiatr. 2017, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Castillo, M.F.R.; Murphy, M.; Schwarz, M.; Moll, N.; Martin, B.; Weidinger, E.; Leitner, B.; Mueller, N.; Halaris, A. Effects of Inflammation Modulation on Tryptophan and Kynurenine Pathway Regulation in Treatment Resistant Bipolar Depression. Neurol. Psychiatr. Brain Res. 2019, 33, 65–72. [Google Scholar] [CrossRef]
- Murata, S.; Murphy, M.; Hoppensteadt, D.; Fareed, J.; Welborn, A.; Halaris, A. Effects of Adjunctive Inflammatory Modulation on IL-1β in Treatment Resistant Bipolar Depression. Brain Behav. Immun. 2020, 87, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Edberg, D.; Hoppensteadt, D.; Walborn, A.; Fareed, J.; Sinacore, J.; Halaris, A. Plasma MCP-1 Levels in Bipolar Depression during Cyclooxygenase-2 Inhibitor Combination Treatment. J. Psychiatr. Res. 2020, 129, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Murphy, M.; Khanna, R.; Hoppensteadt, D.; Fareed, J.; Halaris, A. Elevated Salivary Cortisol Predicts Response to Adjunctive Immune Modulation in Treatment-Resistant Bipolar Depression. J. Affect. Disord. Rep. 2021, 4, 100117. [Google Scholar] [CrossRef]
- Castillo, M.F.R.; Cohen, A.; Edberg, D.; Hoppensteadt, D.; Fareed, J.; Martin, B.; Halaris, A. Vascular Endothelial Growth Factor in Bipolar Depression: A Potential Biomarker for Diagnosis and Treatment Outcome Prediction. Psychiatr. Res. 2020, 284, 112781. [Google Scholar] [CrossRef]
- Alamdarsaravi, M.; Ghajar, A.; Noorbala, A.A.; Arbabi, M.; Emami, A.; Shahei, F.; Mirzania, M.; Jafarinia, M.; Afarideh, M.; Akhondzadeh, S. Efficacy and Safety of Celecoxib Monotherapy for Mild to Moderate Depression in Patients with Colorectal Cancer: A Randomized Double-Blind, Placebo Controlled Trial. Psychiatr. Res. 2017, 255, 59–65. [Google Scholar] [CrossRef]
- Jafari, S.; Ashrafizadeh, S.-G.; Zeinoddini, A.; Rasoulinejad, M.; Entezari, P.; Seddighi, S.; Akhondzadeh, S. Celecoxib for the Treatment of Mild-to-Moderate Depression Due to Acute Brucellosis: A Double-Blind, Placebo-Controlled, Randomized Trial. J. Clin. Pharm. Ther. 2015, 40, 441–446. [Google Scholar] [CrossRef]
- Attwells, S.; Setiawan, E.; Rusjan, P.M.; Xu, C.; Hutton, C.; Rafiei, D.; Varughese, B.; Kahn, A.; Kish, S.J.; Vasdev, N.; et al. Translocator Protein Distribution Volume Predicts Reduction of Symptoms During Open-Label Trial of Celecoxib in Major Depressive Disorder. Biol. Psychiatr. 2020, 88, 649–656. [Google Scholar] [CrossRef]
- Myint, A.M.; Steinbusch, H.W.M.; Goeghegan, L.; Luchtman, D.; Kim, Y.K.; Leonard, B.E. Effect of the COX-2 Inhibitor Celecoxib on Behavioural and Immune Changes in an Olfactory Bulbectomised Rat Model of Depression. Neuroimmunomodulation 2007, 14, 65–71. [Google Scholar] [CrossRef]
- Guo, J.Y.; Li, C.Y.; Ruan, Y.P.; Sun, M.; Qi, X.L.; Zhao, B.S.; Luo, F. Chronic Treatment with Celecoxib Reverses Chronic Unpredictable Stress-Induced Depressive-like Behavior via Reducing Cyclooxygenase-2 Expression in Rat Brain. Eur. J. Pharmacol. 2009, 612, 54–60. [Google Scholar] [CrossRef]
- Prakash, R.; Ramanathan, M. Effect of COX-Inhibitors Attentuated LPS Induced Behavioural Alterations in Male Wistar Rats. J. Pharm. Sci. Res. 2013, 5, 226–230. [Google Scholar]
- Maciel, I.S.; Silva, R.B.M.; Morrone, F.B.; Calixto, J.B.; Campos, M.M. Synergistic Effects of Celecoxib and Bupropion in a Model of Chronic Inflammation-Related Depression in Mice. PLoS ONE 2013, 8, e77227. [Google Scholar] [CrossRef]
- Kurhe, Y.; Mahesh, R.; Gupta, D. Effect of a Selective Cyclooxygenase Type 2 Inhibitor Celecoxib on Depression Associated with Obesity in Mice: An Approach Using Behavioral Tests. Neurochem. Res. 2014, 39, 1395–1402. [Google Scholar] [CrossRef]
- Santiago, R.M.; Barbiero, J.K.; Martynhak, B.J.; Boschen, S.L.; Da Silva, L.M.; Werner, M.F.P.; Da Cunha, C.; Andreatini, R.; Lima, M.M.S.; Vital, M.A.B.F.; et al. Antidepressant-like Effect of Celecoxib Piroxicam in Rat Models of Depression. Eur. Neuropsychopharmacol. 2014, 121, S218. [Google Scholar] [CrossRef] [PubMed]
- Costa-Nunes, J.P.; Cline, B.H.; Araújo-Correia, M.; Valencą, A.; Markova, N.; Dolgov, O.; Kubatiev, A.; Yeritsyan, N.; Steinbusch, H.W.M.; Strekalova, T. Animal Models of Depression and Drug Delivery with Food as an Effective Dosing Method: Evidences from Studies with Celecoxib and Dicholine Succinate. Biomed. Res. Int. 2015, 2015, 596126. [Google Scholar] [CrossRef]
- Fischer, C.W.; Eskelund, A.; Budac, D.P.; Tillmann, S.; Liebenberg, N.; Elfving, B.; Wegener, G. Interferon-Alpha Treatment Induces Depression-like Behaviour Accompanied by Elevated Hippocampal Quinolinic Acid Levels in Rats. Behav. Brain Res. 2015, 293, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Morgese, M.G.; Schiavone, S.; Bove, M.; Mhillaj, E.; Tucci, P.; Trabace, L. Sub-Chronic Celecoxib Prevents Soluble Beta Amyloid-Induced Depressive-like Behaviour in Rats. J. Affect. Disord. 2018, 238, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Feng, Y.-B.; Wang, L.; Shen, J.; Li, Y.; Fan, C.; Wang, P.; Yu, S.Y. COX-2 Inhibition Rescues Depression-like Behaviors via Suppressing Glial Activation, Oxidative Stress and Neuronal Apoptosis in Rats. Neuropharmacology 2019, 160, 107779. [Google Scholar] [CrossRef] [PubMed]
- Mesripour, A.; Shahnooshi, S.; Hajhashemi, V. Celecoxib, Ibuprofen, and Indomethacin Alleviate Depression-like Behavior Induced by Interferon-Alfa in Mice. J. Complement. Integr. Med. 2020, 17, 20190016. [Google Scholar] [CrossRef] [PubMed]
- de Munter, J.; Babaevskaya, D.; Wolters, E.C.; Pavlov, D.; Lysikova, E.; Kalueff, V.A.; Gorlova, A.; Oplatchikova, M.; Pomytkin, I.A.; Proshin, A.; et al. Molecular and Behavioural Abnormalities in the FUS-Tg Mice Mimic Frontotemporal Lobar Degeneration: Effects of Old and New Anti-Inflammatory Therapies. J. Cell. Mol. Med. 2020, 24, 10251–10257. [Google Scholar] [CrossRef]
- Feng, X.; Fan, Y.; Chung, C.Y. Mefenamic Acid Can Attenuate Depressive Symptoms by Suppressing Microglia Activation Induced upon Chronic Stress. Brain Res. 2020, 1740, 146846. [Google Scholar] [CrossRef] [PubMed]
- Mesripour, A.; Gasemi, F. The NSAIDs Ibuprofen and Celecoxib and the TNF-α Blocker Etanercept Prevented Cyclosporine A-Induced Depression-Like Behavior in Mice. Hacettepe. Univ. J. Fac. Pharm. 2021, 41, 133–142. [Google Scholar] [CrossRef]
- Strekalova, T.; Pavlov, D.; Trofimov, A.; Anthony, D.C.; Svistunov, A.; Proshin, A.; Umriukhin, A.; Lyundup, A.; Lesch, K.-P.; Cespuglio, R. Hippocampal Over-Expression of Cyclooxygenase-2 (COX-2) Is Associated with Susceptibility to Stress-Induced Anhedonia in Mice. Int. J. Mol. Sci. 2022, 23, 2061. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Xiang, Y.Z.; Manku, M. Increased Phospholipase A2 Activity and Inflammatory Response but Decreased Nerve Growth Factor Expression in the Olfactory Bulbectomized Rat Model of Depression: Effects of Chronic Ethyl-Eicosapentaenoate Treatment. J. Neurosci. 2009, 29, 14–22. [Google Scholar] [CrossRef]
- Alboni, S.; Benatti, C.; Capone, G.; Tascedda, F.; Brunello, N. Neither All Anti-Inflammatory Drugs nor All Doses Are Effective in Accelerating the Antidepressant-like Effect of Fluoxetine in an Animal Model of Depression. J. Affect. Disord. 2018, 235, 124–128. [Google Scholar] [CrossRef]
- Valvassori, S.S.; Dal-Pont, G.C.; Tonin, P.T.; Varela, R.B.; Ferreira, C.L.; Gava, F.F.; Andersen, M.L.; Soares, J.C.; Quevedo, J. Coadministration of Lithium and Celecoxib Attenuates the Behavioral Alterations and Inflammatory Processes Induced by Amphetamine in an Animal Model of Mania. Pharmacol. Biochem. Behav. 2019, 183, 56–63. [Google Scholar] [CrossRef]
- Valvassori, S.S.; Tonin, P.T.; Dal-Pont, G.C.; Varela, R.B.; Cararo, J.H.; Garcia, A.F.; Gava, F.F.; Menegas, S.; Soares, J.C.; Quevedo, J. Coadministration of Lithium and Celecoxib Reverses Manic-like Behavior and Decreases Oxidative Stress in a Dopaminergic Model of Mania Induced in Rats. Transl. Psychiatr. 2019, 9, 297. [Google Scholar] [CrossRef]
- Bauer, M.E.; Teixeira, A.L. Inflammation in Psychiatric Disorders: What Comes First? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Faridhosseini, F.; Sadeghi, R.; Farid, L.; Pourgholami, M. Celecoxib: A New Augmentation Strategy for Depressive Mood Episodes. A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Hum. Psychopharmacol. 2014, 29, 216–223. [Google Scholar] [CrossRef]
- Nuñez, N.A.; Joseph, B.; Pahwa, M.; Kumar, R.; Resendez, M.G.; Prokop, L.J.; Veldic, M.; Seshadri, A.; Biernacka, J.M.; Frye, M.A.; et al. Augmentation Strategies for Treatment Resistant Major Depression: A Systematic Review and Network Meta-Analysis. J. Affect. Disord. 2022, 302, 385–400. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatr. 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Dominiak, M.; Gędek, A.; Sikorska, M.; Mierzejewski, P.; Wojnar, M.; Antosik-Wójcińska, A.Z. Acetylsalicylic Acid and Mood Disorders: A Systematic Review. Pharmaceuticals 2022, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.L.; Schenck, L.A.; Kruse, J.L.; Klaas, J.P.; Chamberlain, A.M.; Bobo, W.V.; Bellivier, F.; Leboyer, M.; Roger, V.L.; Brown, R.D.J.; et al. Long-Term Risk of Myocardial Infarction and Stroke in Bipolar I Disorder: A Population-Based Cohort Study. J. Affect. Disord. 2016, 194, 120–127. [Google Scholar] [CrossRef]
- Foroughi, M.; Medina Inojosa, J.R.; Lopez-Jimenez, F.; Saeidifard, F.; Suarez, L.; Stokin, G.B.; Prieto, M.L.; Rocca, W.A.; Frye, M.A.; Morgan, R.J. Association of Bipolar Disorder with Major Adverse Cardiovascular Events: A Population-Based Historical Cohort Study. Psychosom. Med. 2022, 84, 97–103. [Google Scholar] [CrossRef]
- Lambert, A.M.; Parretti, H.M.; Pearce, E.; Price, M.J.; Riley, M.; Ryan, R.; Tyldesley-Marshall, N.; Avşar, T.S.; Matthewman, G.; Lee, A.; et al. Temporal Trends in Associations between Severe Mental Illness and Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. PLoS Med. 2022, 19, e1003960. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Husni, M.E.; Libby, P.A.; Yeomans, N.D.; Lincoff, A.M.; Lϋscher, T.F.; Menon, V.; Brennan, D.M.; Wisniewski, L.M.; Nissen, S.E.; et al. The Risk of Major NSAID Toxicity with Celecoxib, Ibuprofen, or Naproxen: A Secondary Analysis of the PRECISION Trial. Am. J. Med. 2017, 130, 1415–1422.e4. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Lin, Y.; Fang, Y.; Li, X.; Li, X.-N.; Yang, Y.; Liu, N.; Jiang, X.; Yu, Y.; Zhou, Y.; et al. Clinical Efficacy Evaluation and Prevention of Adverse Reactions in a Randomized Trial of a Combination of Three Drugs in the Treatment of Cancerous Pudendal Neuralgia. Ann. Palliat. Med. 2021, 10, 5754–5762. [Google Scholar] [CrossRef]
- Huang, H.; Luo, M.; Liang, H.; Pan, J.; Yang, W.; Zeng, L.; Liang, G.; Hou, S.; Zhao, J.; Liu, J. Meta-Analysis Comparing Celecoxib with Diclofenac Sodium in Patients with Knee Osteoarthritis. Pain Med. 2021, 22, 352–362. [Google Scholar] [CrossRef]
- Fidahic, M.; Jelicic Kadic, A.; Radic, M.; Puljak, L. Celecoxib for Rheumatoid Arthritis. Cochrane Database Syst. Rev. 2017, 6, CD012095. [Google Scholar] [CrossRef]
- Esmael, A.; Elsherif, M.; Elegezy, M.; Egilla, H. Cognitive Impairment and Neuropsychiatric Manifestations of Neurobrucellosis. Neurol. Res. 2021, 43, 1–8. [Google Scholar] [CrossRef]
- Santucci, C.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Pastorino, U.; Boffetta, P.; Negri, E.; Bosetti, C.; La Vecchia, C. Progress in Cancer Mortality, Incidence, and Survival: A Global Overview. Eur. J. cancer. Prev. Off. J. Eur. Cancer. Prev. Organ. 2020, 29, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Linden, W.; Vodermaier, A.; Mackenzie, R.; Greig, D. Anxiety and Depression after Cancer Diagnosis: Prevalence Rates by Cancer Type, Gender, and Age. J. Affect. Disord. 2012, 141, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Casavilca-Zambrano, S.; Custodio, N.; Liendo-Picoaga, R.; Cancino-Maldonado, K.; Esenarro, L.; Montesinos, R.; Bertani, S.; Fejerman, L.; Guerchet, M.; Vidaurre, T. Depression in Women with a Diagnosis of Breast Cancer. Prevalence of Symptoms of Depression in Peruvian Women with Early Breast Cancer and Related Sociodemographic Factors. Semin. Oncol. 2020, 47, 293–301. [Google Scholar] [CrossRef]
- Iyengar, R.L.; Gandhi, S.; Aneja, A.; Thorpe, K.; Razzouk, L.; Greenberg, J.; Mosovich, S.; Farkouh, M.E. NSAIDs Are Associated with Lower Depression Scores in Patients with Osteoarthritis. Am. J. Med. 2013, 126, 1017.E11–1017.E18. [Google Scholar] [CrossRef]
- Regulska, M.; Szuster-Głuszczak, M.; Trojan, E.; Leśkiewicz, M.; Basta-Kaim, A. The Emerging Role of the Double-Edged Impact of Arachidonic Acid- Derived Eicosanoids in the Neuroinflammatory Background of Depression. Curr. Neuropharmacol. 2021, 19, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, M.; Vitale, G.; Pini, L.A. Effect of Rofecoxib on Nociception and the Serotonin System in the Rat Brain. Inflamm. Res. 2002, 51, 154–159. [Google Scholar] [CrossRef]
- Ng, Q.X.; Lim, Y.L.; Yaow, C.Y.L.; Ng, W.K.; Thumboo, J.; Liew, T.M. Effect of Probiotic Supplementation on Gut Microbiota in Patients with Major Depressive Disorders: A Systematic Review. Nutrients 2023, 15, 1351. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [CrossRef]
- Chen, X.-P.; Tan, Z.-R.; Huang, S.-L.; Huang, Z.; Ou-Yang, D.-S.; Zhou, H.-H. Isozyme-Specific Induction of Low-Dose Aspirin on Cytochrome P450 in Healthy Subjects. Clin. Pharmacol. Ther. 2003, 73, 264–271. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Efficacy and Tolerability of Minocycline for Depression: A Systematic Review and Meta-Analysis of Clinical Trials. J. Affect. Disord. 2018, 227, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Miyake, N.; Okuya, M.; Sakuma, K.; Iwata, N. N-Acetylcysteine as an Adjunctive Treatment for Bipolar Depression and Major Depressive Disorder: A Systematic Review and Meta-Analysis of Double-Blind, Randomized Placebo-Controlled Trials. Psychopharmacology 2020, 237, 3481–3487. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.E.; Green, C.; Colpo, G.D.; Teixeira, A.L.; Selvaraj, S.; Durkin, K.; Zunta-Soares, G.B.; Soares, J.C. A Double-Blind, Randomized, Placebo-Controlled Study of Aspirin and N-Acetylcysteine as Adjunctive Treatments for Bipolar Depression. J. Clin. Psychiatr. 2018, 80, 459. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).