New-Onset Atrial Fibrillation in the Setting of COVID-19 Infection Is a Predictor of Mortality in Hospitalized Patients: CovAF-Study
Abstract
:1. Introduction
2. Materials and Methods
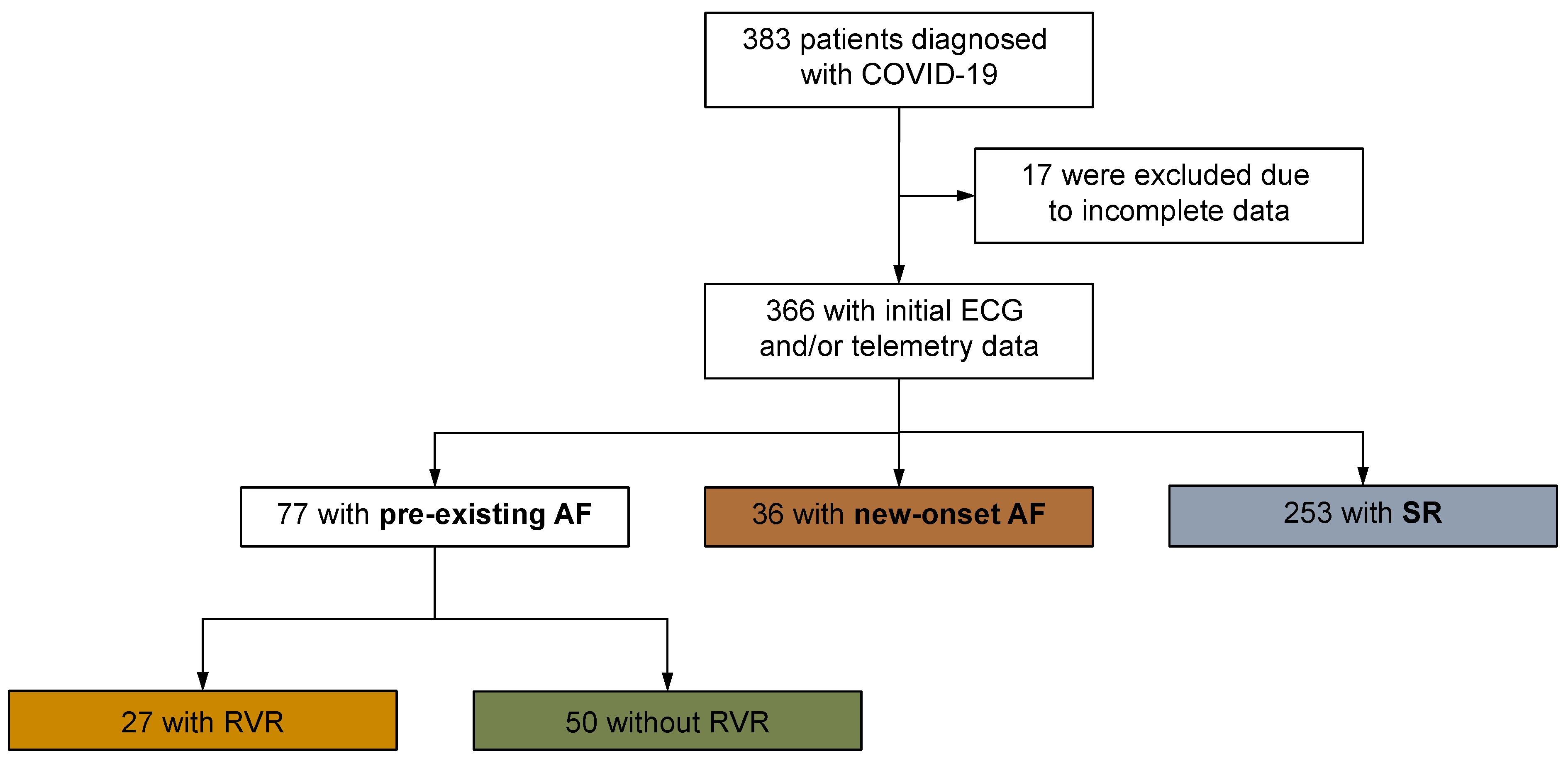
2.1. Study Design, Case Definition, and Control Selection
2.2. Ethical Statement
2.3. Laboratory Analyses
2.4. Assessment of Potential Covariates
2.5. Endpoints and Collection of Data
2.6. Statistical Analysis
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Batah, S.S.; Fabro, A.T. Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respir. Med. 2020, 176, 106239. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Carius, B.M.; Chavez, S.; Liang, S.Y.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Clinical update on COVID-19 for the emergency clinician: Presentation and evaluation. Am. J. Emerg. Med. 2022, 54, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions. 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html (accessed on 12 April 2023).
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, K.; Liu, X.; Zhuang, C.; Huang, X.; Huang, Y.; Yao, X.; Quan, J.; Lin, H.; Huang, S.; et al. The Protection of Naturally Acquired Antibodies Against Subsequent SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. Emerg. Microbes Infect. 2022, 11, 793–803. [Google Scholar] [CrossRef]
- Chung, M.K.; Zidar, D.A.; Bristow, M.R.; Cameron, S.J.; Chan, T.; Iii, C.V.H.; Kwon, D.H.; Singh, T.; Tilton, J.C.; Tsai, E.J.; et al. COVID-19 and Cardiovascular Disease. Circ. Res. 2021, 128, 1214–1236. [Google Scholar] [CrossRef]
- Bularga, A.; E Newby, D.; Chapman, A.R. Not to be sneezed at: Cardiovascular disease after COVID-19 infection. Heart 2022, 109, 84–85. [Google Scholar] [CrossRef]
- Coromilas, E.J.; Kochav, S.; Goldenthal, I.; Biviano, A.; Garan, H.; Goldbarg, S.; Kim, J.-H.; Yeo, I.; Tracy, C.; Ayanian, S.; et al. Worldwide Survey of COVID-19–Associated Arrhythmias. Circ. Arrhythmia Electrophysiol. 2021, 14, e009458. [Google Scholar] [CrossRef]
- Wang, L.; Hoang, L.; Aten, K.; Abualfoul, M.; Canela, V.; Prathivada, S.; Vu, M.; Zhao, Y.; Sidhu, M. Mortality and Major Adverse Cardiovascular Events in Hospitalized Patients with Atrial Fibrillation with COVID-19. Am. J. Cardiol. 2022, 189, 41–48. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Mathur, S.; Zhang, R.; Yan, V.K.C.; Lai, F.T.T.; Chui, C.S.L.; Li, X.; Wong, C.K.H.; Chan, E.W.Y.; Yiu, K.H.; et al. Association of COVID-19 with short- and long-term risk of cardiovascular disease and mortality: A prospective cohort in UK Biobank. Cardiovasc. Res. 2023, cvac195. [Google Scholar] [CrossRef]
- Piccini, J.P.; Russo, A.M.; Sharma, P.S.; Kron, J.; Tzou, W.; Sauer, W.; Park, D.S.; Birgersdotter-Green, U.; Frankel, D.S.; Healey, J.S.; et al. Advances in Cardiac Electrophysiology. Circ. Arrhythmia Electrophysiol. 2022, 15, e009911. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, D.; Heijman, J.; Hiram, R.; Na Li, N.; Nattel, S. Inflammatory signalling in atrial cardiomyocytes: A novel unifying principle in atrial fibrillation pathophysiology. Nat. Rev. Cardiol. 2022, 20, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Romiti, G.F.; Corica, B.; Lip, G.Y.H.; Proietti, M. Prevalence and Impact of Atrial Fibrillation in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2490. [Google Scholar] [CrossRef]
- Russo, V.; Silverio, A.; Scudiero, F.; Di Micco, P.; Di Maio, M. Pre-admission atrial fibrillation in COVID-19 patients: Prevalence and clinical impact. Eur. J. Intern. Med. 2021, 88, 133–135. [Google Scholar] [CrossRef]
- Mountantonakis, S.E.; Saleh, M.; Fishbein, J.; Gandomi, A.; Lesser, M.; Chelico, J.; Gabriels, J.; Qiu, M.; Epstein, L.M. Atrial fibrillation is an independent predictor for in-hospital mortality in patients admitted with SARS-CoV-2 infection. Heart Rhythm 2021, 18, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Gawałko, M.; Kapłon-Cieślicka, A.; Hohl, M.; Dobrev, D.; Linz, D. COVID-19 associated atrial fibrillation: Incidence, putative mechanisms and potential clinical implications. IJC Heart Vasc. 2020, 30, 100631. [Google Scholar] [CrossRef]
- Corica, B.; Tartaglia, F.; Oliva, A.; Raparelli, V.; Cangemi, R.; Basili, S.; Lip, G.Y.H.; Proietti, M.; Romiti, G.F. Prevalence of new-onset atrial fibrillation in hospitalized patients with community-acquired pneumonia: A systematic review and meta-analysis. Intern. Emerg. Med. 2022, 18, 127–135. [Google Scholar] [CrossRef]
- Wu, L.; Jiang, Z.; Meulendijks, E.R.; Baylan, U.; Waas, I.S.; Bugiani, M.; Tuinman, P.R.; Fronczek, J.; Heunks, L.M.; de Groot, J.R.; et al. Atrial inflammation and microvascular thrombogenicity are increased in deceased COVID-19 patients. Cardiovasc. Pathol. 2023, 64, 107524. [Google Scholar] [CrossRef]
- Parahuleva, M.S.; Kreutz, J.; Euler, G.; Terzieva, D.; Mardini, A.; Uchikova, E.; Parahuleva, N. Incidence of Atrial Fibrillation in Postmenopausal Women with Endometrial Cancer. J. Clin. Med. 2021, 10, 266. [Google Scholar] [CrossRef]
- Rosenblatt, A.G.; Ayers, C.R.; Rao, A.; Howell, S.J.; Hendren, N.S.; Zadikany, R.H.; Ebinger, J.E.; Daniels, J.D.; Link, M.S.; de Lemos, J.A.; et al. New-Onset Atrial Fibrillation in Patients Hospitalized With COVID-19: Results from the American Heart Association COVID-19 Cardiovascular Registry. Circ. Arrhythmia Electrophysiol. 2022, 15, e010666. [Google Scholar] [CrossRef]
- Sanz, A.P.; Tahoces, L.S.; Pérez, R.O.; Ferrer, E.G.; Recalde, Á.S.; Gómez, J.L.Z. New-onset atrial fibrillation during COVID-19 infection predicts poor prognosis. Cardiol. J. 2021, 28, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Battisti, V.; Costola, G.; Roncon, L.; Bilato, C. Increased risk of acute myocardial infarction after COVID-19 recovery: A systematic review and meta-analysis. Int. J. Cardiol. 2022, 372, 138–143. [Google Scholar] [CrossRef]
- Yang, H.; Liang, X.; Xu, J.; Hou, H.; Wang, Y. Meta-Analysis of Atrial Fibrillation in Patients With COVID-19. Am. J. Cardiol. 2021, 144, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Aibar, J.; Schulman, S. New-Onset Atrial Fibrillation in Sepsis: A Narrative Review. Semin. Thromb. Hemost. 2021, 47, 18–25. [Google Scholar] [CrossRef]
- Andrade, J.G.; Deyell, M.W.; Lee, A.Y.; Macle, L. Sex Differences in Atrial Fibrillation. Can. J. Cardiol. 2018, 34, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of Diagnosed Atrial Fibrillation in Adults: National implications for rhythm management and stroke prevention: The AnTico-agulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef]
- Musikantow, D.R.; Turagam, M.K.; Sartori, S.; Chu, E.; Kawamura, I.; Shivamurthy, P.; Bokhari, M.; Oates, C.; Zhang, C.; Pumill, C.; et al. Atrial Fibrillation in Patients Hospitalized With COVID-19: Incidence, Predictors, Outcomes, and Comparison to Influenza. JACC Clin. Electrophysiol. 2021, 7, 1120–1130. [Google Scholar] [CrossRef]
- Kallistratos, M.; Poulimenos, L.; Manolis, A. Atrial fibrillation and arterial hypertension. Pharmacol. Res. 2018, 128, 322–326. [Google Scholar] [CrossRef]
- Horio, T.; Iwashima, Y.; Kamide, K.; Tokudome, T.; Yoshihara, F.; Nakamura, S.; Kawano, Y. Chronic kidney disease as an independent risk factor for new-onset atrial fibrillation in hypertensive patients. J. Hypertens. 2010, 28, 1738–1744. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Ho, S.-W.; Wang, Y.-H.; Leong, P.-Y.; Wei, J.C.-C. Risk of atrial fibrillation in patients with pneumonia. Heart Lung 2022, 52, 110–116. [Google Scholar] [CrossRef]
- Wollborn, J.; Karamnov, S.; Fields, K.G.; Yeh, T.; Muehlschlegel, J.D. COVID-19 increases the risk for the onset of atrial fibrillation in hospitalized patients. Sci. Rep. 2022, 12, 12014. [Google Scholar] [CrossRef] [PubMed]
- Kichloo, A.; Dettloff, K.; Aljadah, M.; Albosta, M.; Jamal, S.; Singh, J.; Wani, F.; Kumar, A.; Vallabhaneni, S.; Khan, M.Z. COVID-19 and Hypercoagulability: A Review. Clin. Appl. Thromb. 2020, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Babapoor-Farrokhran, S.; Gill, D.; Walker, J.; Rasekhi, R.T.; Bozorgnia, B.; Amanullah, A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020, 253, 117723. [Google Scholar] [CrossRef]
- Boos, C.J. Infection and atrial fibrillation: Inflammation begets AF. Eur. Heart J. 2020, 41, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Kurtoğlu, E.; Afsin, A.; Aktaş, I.; Aktürk, E.; Kutlusoy, E.; Çağaşar, Ö. Altered cardiac autonomic function after recovery from COVID-19. Ann. Noninvasive Electrocardiol. 2021, 27, e12916. [Google Scholar] [CrossRef]
- Zhan, Y.; Yue, H.; Liang, W.; Wu, Z. Effects of COVID-19 on Arrhythmia. J. Cardiovasc. Dev. Dis. 2022, 9, 292. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Zanon, F.; Zuliani, G.; Roncon, L. Pre-existing atrial fibrillation is associated with increased mortality in COVID-19 Patients. J. Interv. Card. Electrophysiol. 2021, 62, 231–238. [Google Scholar] [CrossRef]
- Aydemir, S.; Aksakal, E.; Aydınyılmaz, F.; Gülcü, O.; Saraç, I.; Aydın, S.Ş.; Doğan, R.; Lazoğlu, M.; Kalkan, K. Does new onset and pre-existing atrial fibrillation predict mortality in COVID-19 patients? Egypt. Heart J. 2022, 74, 53. [Google Scholar] [CrossRef]



| Control Group | Pre-AF no RVR | p-Value | New-AF | p-Value | Pre-AF with RVR | p-Value | |
|---|---|---|---|---|---|---|---|
| Number of COVID-19 patients 1 | n = 253 (69.1%) | n = 50 (13.7%) | n = 36 (9.8%) | n = 27 (7.4%) | |||
| Age 2 | 66.4 (17.0) | 80.2 (8.7) | <0.001 + | 75.5 (12.0) | <0.001 + | 79.7 (10.5) | <0.001 + |
| Male sex 1 | 156 (61.7%) | 30 (60.0%) | 0.826 * | 29 (80.6%) | 0.027 * | 11 (40.7%) | 0.036 * |
| Duration of stay 3 | 9.0 (4.0–16.0) | 9.0 (4.0–20.0) | 0.561 # | 11.50 (4.0–21.0) | 0.188 # | 11.0 (3.0–19.0) | 0.682 # |
| Body mass index 3 | 27.8 (24.5–31.8) | 28.4 (24.0–32.5) | 0.852 # | 27.5 (24.4–32.2) | 0.733 # | 27.8 (25.2–31.5) | 0.861 # |
| Diabetes mellitus type I 1 | 3 (1.2%) | 0 (0%) | 1 § | 1 (2.8%) | 0.414 § | 0 (0%) | 1 § |
| Diabetes mellitus type II 1 | 66 (26.1%) | 24 (48,0%) | 0.002 * | 14 (38.9%) | 0.109 * | 12 (44.4%) | 0.043 * |
| Bronchial asthma 1 | 18 (7.1%) | 3 (6.0%) | 1 § | 0 (0.0%) | 0.142 § | 1 (3.7%) | 1 § |
| COPD 1 | 18 (7.1%) | 12 (24.0%) | 0.001 § | 1 (2.8%) | 0.485 § | 4 (14.8%) | 0.247 § |
| DVT/PE 1 | 21 (8.3%) | 3 (6.0%) | 0.777 § | 3 (8.3%) | 1 § | 3 (11.1%) | 0.714 § |
| Kidney failure 1 | 40 (15.8%) | 18 (36.0%) | 0.001 * | 14 (38.9%) | 0.001 * | 7 (25.9%) | 0.182 § |
| Arterial hypertension 1 | 153 (60.5%) | 40 (80.0%) | 0.009 * | 28 (77.8%) | 0.045 * | 23 (85.2%) | 0.012 * |
| Coronary heart disease 1 | 41 (16.2%) | 21 (42.0%) | <0.001 * | 5 (13.9%) | 0.723 * | 8 (29.6%) | 0.106 § |
| Hypercholesterolemia 1 | 32 (12.6%) | 8 (16.0%) | 0.523 * | 5 (13.9%) | 0.792 § | 5 (18.5%) | 0.374 § |
| Cardiomyopathy 1 | 28 (11.1%) | 22 (44.0%) | <0.001 * | 6 (16.7%) | 0.403 § | 6 (22.2%) | 0.115 § |
| Nicotine abuse 1 | 36 (14.2%) | 10 (20.4%) | 0.271 * | 1 (2.8%) | 0.061 § | 3 (11.1%) | 1 § |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parahuleva, M.S.; Harbaum, L.; Patsalis, N.; Parahuleva, N.; Arndt, C.; Lüsebrink, U.; Schieffer, B.; Kreutz, J. New-Onset Atrial Fibrillation in the Setting of COVID-19 Infection Is a Predictor of Mortality in Hospitalized Patients: CovAF-Study. J. Clin. Med. 2023, 12, 3500. https://doi.org/10.3390/jcm12103500
Parahuleva MS, Harbaum L, Patsalis N, Parahuleva N, Arndt C, Lüsebrink U, Schieffer B, Kreutz J. New-Onset Atrial Fibrillation in the Setting of COVID-19 Infection Is a Predictor of Mortality in Hospitalized Patients: CovAF-Study. Journal of Clinical Medicine. 2023; 12(10):3500. https://doi.org/10.3390/jcm12103500
Chicago/Turabian StyleParahuleva, Mariana S., Lukas Harbaum, Nikolaos Patsalis, Nikoleta Parahuleva, Christian Arndt, Ulrich Lüsebrink, Bernhard Schieffer, and Julian Kreutz. 2023. "New-Onset Atrial Fibrillation in the Setting of COVID-19 Infection Is a Predictor of Mortality in Hospitalized Patients: CovAF-Study" Journal of Clinical Medicine 12, no. 10: 3500. https://doi.org/10.3390/jcm12103500






