Resectability, Resections, Survival Outcomes, and Quality of Life in Older Adult Patients with Metastatic Colorectal Cancer (the RAXO-Study)
Abstract
:1. Introduction
2. Materials and Methods
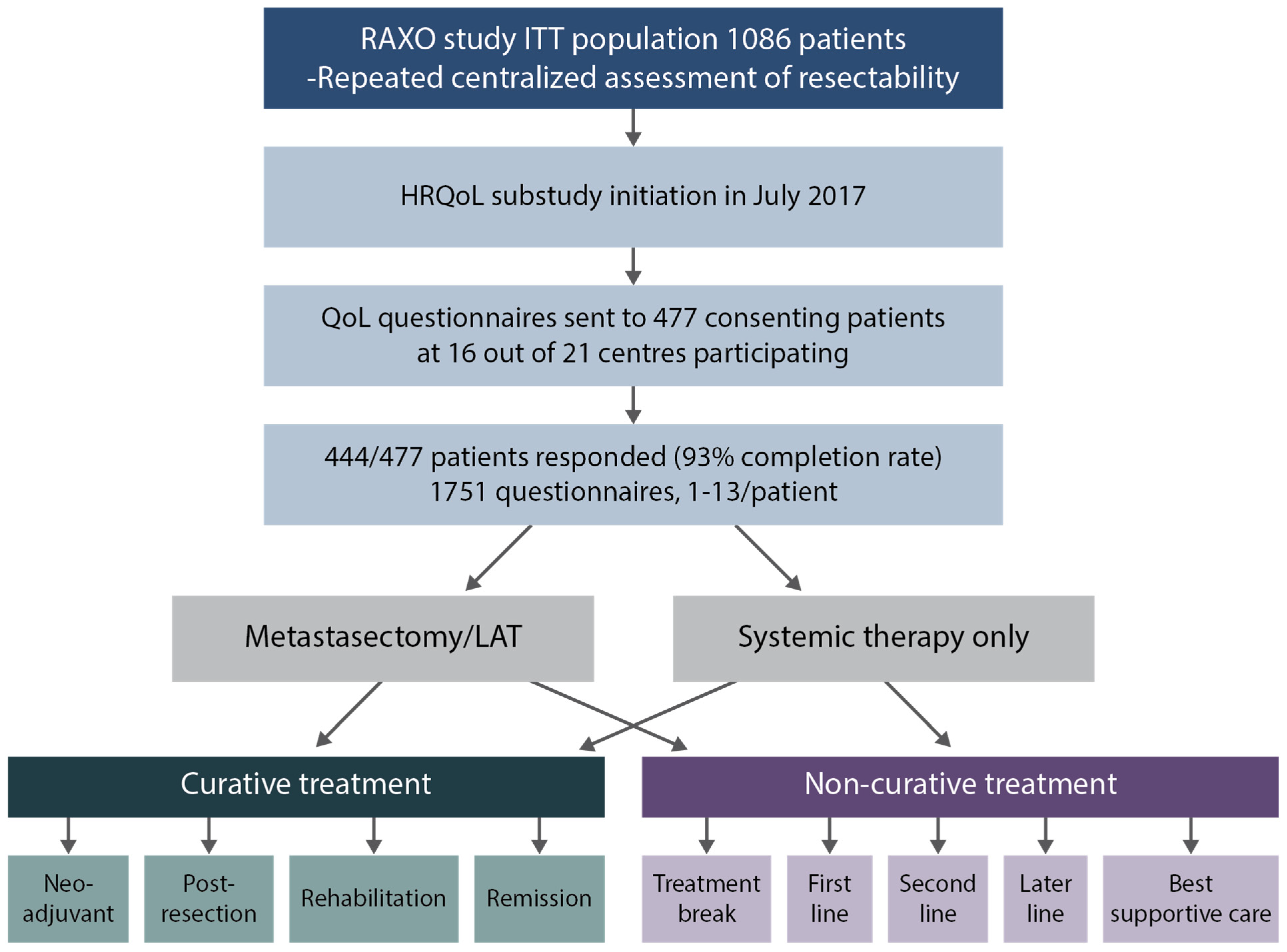
2.1. Quality of Life Questionnaires
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Assessment of Resectability
3.3. Resection Rates at Different Metastatic Sites
3.4. Systemic Therapy
3.5. Survival
3.6. Univariate and Multivariable Regression Analyses of OS
3.7. Surgical Complications and Adverse Events
3.8. HRQoL Index Scores
3.9. HRQoL Profile, Functioning, and Symptom Scales
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Aykut, B.; Lidsky, M.E. Colorectal Cancer Liver Metastases: Multimodal Therapy. Surg. Oncol. Clin. N. Am. 2023, 32, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bajorek, B. Defining “elderly” in clinical practice guidelines for pharmacotherapy. Pharm. Pract. 2014, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Frilling, A.; Elias, D.; Laurent, C.; Ramos, E.; Capussotti, L.; Poston, G.J.; Wicherts, D.A.; de Haas, R.J. Liver resection of colorectal metastases in elderly patients. Br. J. Surg. 2010, 97, 366–376. [Google Scholar] [CrossRef]
- Booth, C.M.; Nanji, S.; Wei, X.; Mackillop, W.J. Management and Outcome of Colorectal Cancer Liver Metastases in Elderly Patients. JAMA Oncol. 2015, 1, 1111. [Google Scholar] [CrossRef]
- Albertsmeier, M.; Engel, A.; Guba, M.O.; Stintzing, S.; Schiergens, T.S.; Schubert-Fritschle, G.; Hölzel, D.; Werner, J.; Angele, M.K.; Engel, J. Synchronous colorectal liver metastases: Focus on the elderly. Langenbeck’s Arch. Surg. 2017, 402, 1223–1232. [Google Scholar] [CrossRef]
- van Tuil, T.; Dhaif, A.A.; te Riele, W.W.; van Ramshorst, B.; van Santvoort, H.C. Systematic Review and Meta-Analysis of Liver Resection for Colorectal Metastases in Elderly Patients. Dig. Surg. 2019, 36, 111–123. [Google Scholar] [CrossRef]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Ardanaz, E.; et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE—5-a population-based study. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef]
- Montroni, I.; Ugolini, G.; Saur, N.M.; Spinelli, A.; Rostoft, S.; Millan, M.; Wolthuis, A.; Daniels, I.R.; Hompes, R.; Penna, M.; et al. Personalized management of elderly patients with rectal cancer: Expert recommendations of the European Society of Surgical Oncology, European Society of Coloproctology, International Society of Geriatric Oncology, and American College of Surgeons Commissi. Eur. J. Surg. Oncol. 2018, 44, 1685–1702. [Google Scholar] [CrossRef]
- Scotté, F.; Bossi, P.; Carola, E.; Cudennec, T.; Dielenseger, P.; Gomes, F.; Knox, S.; Strasser, F. Addressing the quality of life needs of older patients with cancer: A SIOG consensus paper and practical guide. Ann. Oncol. 2018, 29, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Rollet, Q.; Bouvier, V.; Moutel, G.; Launay, L.; Bignon, A.-L.; Bouhier-Leporrier, K.; Launoy, G.; Lièvre, A. Multidisciplinary team meetings: Are all patients presented and does it impact quality of care and survival—A registry-based study. BMC Health Serv. Res. 2021, 21, 1032. [Google Scholar] [CrossRef] [PubMed]
- Paillaud, E.; Canoui-Poitrine, F.; Varnier, G.; Anfasi-Ebadi, N.; Guery, E.; Saint-Jean, O.; Gisselbrecht, M.; Aparicio, T.; Bastuji-Garin, S.; Laurent, M.; et al. Preferences about information and decision-making among older patients with and without cancer. Age Ageing 2017, 46, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Lapinsky, E.; Man, L.C.; MacKenzie, A.R. Health-Related Quality of Life in Older Adults with Colorectal Cancer. Curr. Oncol. Rep. 2019, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Wedding, U.; Ködding, D.; Pientka, L.; Steinmetz, H.T.; Schmitz, S. Physicians’ judgement and comprehensive geriatric assessment (CGA) select different patients as fit for chemotherapy. Crit. Rev. Oncol. Hematol. 2007, 64, 1–9. [Google Scholar] [CrossRef]
- Osterlund, P.; Salminen, T.; Soveri, L.-M.; Kallio, R.; Kellokumpu, I.; Lamminm, A.; Halonen, A.; Ristam, R.; Lantto, E.; Uutela, A.; et al. Repeated centralized multidisciplinary team assessment of resectability, clinical behavior, and outcomes in 1086 Finnish metastatic colorectal cancer patients (RAXO): A nationwide prospective intervention study. Lancet Reg. Health Eur. 2021, 3, 100049. [Google Scholar] [CrossRef]
- Sintonen, H. The 15D instrument of health-related quality of life: Properties and applications. Ann. Med. 2001, 33, 328–336. [Google Scholar] [CrossRef]
- Brooks, R. EuroQol: The current state of play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; Haes, J.C.J.M.d.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. JNCI J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Gujral, S.; Conroy, T.; Fleissner, C.; Sezer, O.; King, P.M.; Avery, K.N.L.; Sylvester, P.; Koller, M.; Sprangers, M.A.G.; Blazeby, J.M. Assessing quality of life in patients with colorectal cancer: An update of the EORTC quality of life questionnaire. Eur. J. Cancer 2007, 43, 1564–1573. [Google Scholar] [CrossRef]
- Lehtomäki, K.; Stedt, H.P.; Osterlund, E.; Muhonen, T.; Soveri, L.-M.; Halonen, P.; Salminen, T.K.; Kononen, J.; Kallio, R.; Ålgars, A.; et al. Health-Related Quality of Life in Metastatic Colorectal Cancer Patients Treated with Curative Resection and/or Local Ablative Therapy or Systemic Therapy in the Finnish RAXO-Study. Cancers 2022, 14, 1713. [Google Scholar] [CrossRef]
- Sedrak, M.S.; Freedman, R.A.; Cohen, H.J.; Muss, H.B.; Jatoi, A.; Klepin, H.D.; Wildes, T.M.; Le-Rademacher, J.G.; Kimmick, G.G.; Tew, W.P.; et al. Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J. Clin. 2021, 71, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.M.; Holmes, H.M.; Cheung, K.-L. Current Challenges Faced by Cancer Clinical Trials in Addressing the Problem of Under-Representation of Older Adults: A Narrative Review. Oncol. Ther. 2021, 9, 55–67. [Google Scholar] [CrossRef] [PubMed]
- De’Angelis, N.; Baldini, C.; Brustia, R.; Pessaux, P.; Sommacale, D.; Laurent, A.; Le Roy, B.; Tacher, V.; Kobeiter, H.; Luciani, A.; et al. Surgical and regional treatments for colorectal cancer metastases in older patients: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0230914. [Google Scholar] [CrossRef]
- Alabraba, E.; Gomez, D. Systematic Review of Treatments for Colorectal Metastases in Elderly Patients to Guide Surveillance Cessation Following Hepatic Resection for Colorectal Liver Metastases. Am. J. Clin. Oncol. 2021, 44, 210–223. [Google Scholar] [CrossRef]
- Angelsen, J.-H.; Horn, A.; Sorbye, H.; Eide, G.E.; Løes, I.M.; Viste, A. Population-based study on resection rates and survival in patients with colorectal liver metastasis in Norway. Br. J. Surg. 2017, 104, 580–589. [Google Scholar] [CrossRef]
- Kumar, R.; Jain, K.; Beeke, C.; Price, T.J.; Townsend, A.R.; Padbury, R.; Roder, D.; Young, G.P.; Richards, A.; Karapetis, C.S. A population-based study of metastatic colorectal cancer in individuals aged ≥ 80 years: Findings from the South Australian Clinical Registry for Metastatic Colorectal Cancer. Cancer 2013, 119, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Sjövall, A.; Järv, V.; Blomqvist, L.; Singnomklao, T.; Cedermark, B.; Glimelius, B.; Holm, T. The potential for improved outcome in patients with hepatic metastases from colon cancer: A population-based study. Eur. J. Surg. Oncol. 2004, 30, 834–841. [Google Scholar] [CrossRef]
- Finlayson, E.; Fan, Z.; Birkmeyer, J.D. Outcomes in octogenarians undergoing high-risk cancer operation: A national study. J. Am. Coll. Surg. 2007, 205, 729–734. [Google Scholar] [CrossRef]
- Phan, K.; An, V.V.G.; Ha, H.; Phan, S.; Lam, V.; Pleass, H. Hepatic resection for malignant liver tumours in the elderly: A systematic review and meta-analysis. ANZ J. Surg. 2015, 85, 815–822. [Google Scholar] [CrossRef]
- Niedersüß-Beke, D.; Orlinger, M.; Falch, D.; Heiler, C.; Piringer, G.; Thaler, J.; Hilbe, W.; Petzer, A.; Rumpold, H. Clinical Effectiveness of Oncological Treatment in Metastatic Colorectal Cancer Is Independent of Comorbidities and Age. Cancers 2021, 13, 2091. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Natoli, C.; Ciancola, F.; Gemma, D.; Pellegrino, A.; Pavese, I.; Garufi, C.; Di Lauro, L.; Corsi, D.; Signorelli, D.; et al. Treatment of Metastatic Colorectal Cancer Patients ≥75 Years Old in Clinical Practice: A Multicenter Analysis. PLoS ONE 2016, 11, e0157751. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, D.; Audisio, R.A.; Glimelius, B.; de Gramont, A.; Glynne-Jones, R.; Haller, D.; Köhne, C.-H.; Rostoft, S.; Lemmens, V.; Mitry, E.; et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.; Kleiss, M.; Lueck, R.; Detken, S.; Koenig, A.; Nietert, M.; Beissbarth, T.; Stanek, K.; Langer, C.; Ghadimi, M.; et al. The utilization of multidisciplinary tumor boards (MDT) in clinical routine: Results of a health care research study focusing on patients with metastasized colorectal cancer. Int. J. Colorectal Dis. 2017, 32, 1463–1469. [Google Scholar] [CrossRef]
- Fenton, H.M.; Taylor, J.C.; Lodge, J.P.A.; Toogood, G.J.; Finan, P.J.; Young, A.L.; Morris, E.J.A. Variation in the Use of Resection for Colorectal Cancer Liver Metastases. Ann. Surg. 2019, 270, 892–898. [Google Scholar] [CrossRef]
- Aubin, J.-M.; Bressan, A.K.; Grondin, S.C.; Dixon, E.; MacLean, A.R.; Gregg, S.; Tang, P.; Kaplan, G.G.; Martel, G.; Ball, C.G. Assessing resectability of colorectal liver metastases: How do different subspecialties interpret the same data? Can. J. Surg. 2018, 61, 251–256. [Google Scholar] [CrossRef]
- Mohile, S.G.; Mohamed, M.R.; Xu, H.; Culakova, E.; Loh, K.P.; Magnuson, A.; Flannery, M.A.; Obrecht, S.; Gilmore, N.; Ramsdale, E.; et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): A cluster-randomised study. Lancet 2021, 398, 1894–1904. [Google Scholar] [CrossRef]
- Soo, W.-K.; King, M.; Pope, A.; Parente, P.; Darzins, P.; Davis, I.D. Integrated geriatric assessment and treatment (INTEGERATE) in older people with cancer planned for systemic anticancer therapy. J. Clin. Oncol. 2020, 38, 12011. [Google Scholar] [CrossRef]
- Li, D.; Sun, C.-L.; Kim, H.; Soto-Perez-de-Celis, E.; Chung, V.; Koczywas, M.; Fakih, M.; Chao, J.; Cabrera Chien, L.; Charles, K.; et al. Geriatric Assessment-Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults With Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, e214158. [Google Scholar] [CrossRef]
- Verduzco-Aguirre, H.C.; Bolaño Guerra, L.M.; Culakova, E.; Chargoy, J.M.; Martínez-Said, H.; Quintero Beulo, G.; Mohile, S.G.; Soto-Perez-De-Celis, E. Barriers and Facilitators for the Implementation of Geriatric Oncology Principles in Mexico: A Mixed-Methods Study. JCO Glob. Oncol. 2022, 8, e2100390. [Google Scholar] [CrossRef]
- Rier, H.N.; Meinardi, M.C.; van Rosmalen, J.; Westerweel, P.E.; de Jongh, E.; Kitzen, J.J.E.M.; van den Bosch, J.; Trajkovic, M.; Levin, M.-D. Association Between Geriatric Assessment and Post-Chemotherapy Functional Status in Older Patients with Cancer. Oncologist 2022, 27, e878–e888. [Google Scholar] [CrossRef]
- Kenis, C.; Decoster, L.; Van Puyvelde, K.; De Grève, J.; Conings, G.; Milisen, K.; Flamaing, J.; Lobelle, J.-P.; Wildiers, H. Performance of two geriatric screening tools in older patients with cancer. J. Clin. Oncol. 2014, 32, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.V.; Agar, M.R.; Soo, W.-K.; To, T.; Phillips, J.L. Screening Tools for Identifying Older Adults With Cancer Who May Benefit From a Geriatric Assessment: A Systematic Review. JAMA Oncol. 2021, 7, 616–627. [Google Scholar] [CrossRef]
- Sponholz, S.; Schirren, M.; Oguzhan, S.; Schirren, J. Morbidity, mortality, and survival in elderly patients undergoing pulmonary metastasectomy for colorectal cancer. Int. J. Colorectal Dis. 2018, 33, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Kristjansson, S.R.; Nesbakken, A.; Jordhøy, M.S.; Skovlund, E.; Audisio, R.A.; Johannessen, H.-O.; Bakka, A.; Wyller, T.B. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: A prospective observational cohort study. Crit. Rev. Oncol. Hematol. 2010, 76, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.; Sevdalis, N. Understanding and improving multidisciplinary team working in geriatric medicine. Age Ageing 2019, 48, 498–505. [Google Scholar] [CrossRef]
- Dunne, D.F.J.; Jack, S.; Jones, R.P.; Jones, L.; Lythgoe, D.T.; Malik, H.Z.; Poston, G.J.; Palmer, D.H.; Fenwick, S.W. Randomized clinical trial of prehabilitation before planned liver resection. Br. J. Surg. 2016, 103, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Lund, C.M.; Vistisen, K.K.; Dehlendorff, C.; Rønholt, F.; Johansen, J.S.; Nielsen, D.L. Age-dependent differences in first-line chemotherapy in patients with metastatic colorectal cancer: The DISCO study. Acta Oncol. 2018, 57, 1445–1454. [Google Scholar] [CrossRef]
- Venderbosch, S.; Doornebal, J.; Teerenstra, S.; Lemmens, W.; Punt, C.J.A.; Koopman, M. Outcome of first line systemic treatment in elderly compared to younger patients with metastatic colorectal cancer: A retrospective analysis of the CAIRO and CAIRO2 studies of the Dutch Colorectal Cancer Group (DCCG). Acta Oncol. 2012, 51, 831–839. [Google Scholar] [CrossRef]
- Winther, S.B.; Liposits, G.; Skuladottir, H.; Hofsli, E.; Shah, C.-H.; Poulsen, L.Ø.; Ryg, J.; Osterlund, P.; Berglund, Å.; Qvortrup, C.; et al. Reduced-dose combination chemotherapy (S-1 plus oxaliplatin) versus full-dose monotherapy (S-1) in older vulnerable patients with metastatic colorectal cancer (NORDIC9): A randomised, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2019, 4, 376–388. [Google Scholar] [CrossRef]
- Liposits, G.; Eshøj, H.R.; Möller, S.; Winther, S.B.; Skuladottir, H.; Ryg, J.; Hofsli, E.; Shah, C.-H.; Poulsen, L.Ø.; Berglund, Å.; et al. Quality of Life in Vulnerable Older Patients with Metastatic Colorectal Cancer Receiving Palliative Chemotherapy—The Randomized NORDIC9-Study. Cancers 2021, 13, 2604. [Google Scholar] [CrossRef] [PubMed]
- Lund, C.M.; Nielsen, D.; Dehlendorff, C.; Christiansen, A.B.; Rønholt, F.; Johansen, J.S.; Vistisen, K.K. Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colorectal cancer: The ACCORE study. ESMO Open 2016, 1, e000087. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.; Merino, S.; Caro, A.; Feliu, F.; Escuder, J.; Francesch, T. Treatment of colorectal cancer in the elderly. World J. Gastrointest. Oncol. 2015, 7, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Wiering, B.; Oyen, W.J.G.; Adang, E.M.M.; van der Sijp, J.R.M.; Roumen, R.M.; de Jong, K.P.; Ruers, T.J.M.; Krabbe, P.F.M. Long-term global quality of life in patients treated for colorectal liver metastases. Br. J. Surg. 2011, 98, 565–571. [Google Scholar] [CrossRef]
- Langenhoff, B.S.; Krabbe, P.F.M.; Peerenboom, L.; Wobbes, T.; Ruers, T.J.M. Quality of life after surgical treatment of colorectal liver metastases. Br. J. Surg. 2006, 93, 1007–1014. [Google Scholar] [CrossRef]
- Rees, J.R.; Blazeby, J.M.; Brookes, S.T.; John, T.; Welsh, F.K.; Rees, M. Patient-reported outcomes in long-term survivors of metastatic colorectal cancer needing liver resection. Br. J. Surg. 2014, 101, 1468–1474. [Google Scholar] [CrossRef]
- Studer, P.; Horn, T.; Haynes, A.; Candinas, D.; Banz, V.M. Quality of life after hepatic resection. Br. J. Surg. 2018, 105, 237–243. [Google Scholar] [CrossRef]
- Cashin, P.; Mahteme, H.; Syk, I.; Frödin, J.; Glimelius, B.; Graf, W. Quality of life and cost effectiveness in a randomized trial of patients with colorectal cancer and peritoneal metastases. Eur. J. Surg. Oncol. 2018, 44, 983–990. [Google Scholar] [CrossRef]
- Welter, S.; Schwan, A.; Cheufou, D.; Darwiche, K.; Christoph, D.; Eberhardt, W.; Weinreich, G.; Stamatis, G. Midterm changes in quality of life: A prospective evaluation after open pulmonary metastasectomy. Ann. Thorac. Surg. 2013, 95, 1006–1011. [Google Scholar] [CrossRef]
- Souwer, E.T.D.; Oerlemans, S.; van de Poll-Franse, L.V.; van Erning, F.N.; van den Bos, F.; Schuijtemaker, J.S.; van den Berkmortel, F.W.P.J.; Ten Bokkel Huinink, D.; Hamaker, M.E.; Dekker, J.W.T.; et al. The impact of colorectal surgery on health-related quality of life in older functionally dependent patients with cancer—A longitudinal follow-up study. J. Geriatr. Oncol. 2019, 10, 724–732. [Google Scholar] [CrossRef]
- Cummings, A.; Grimmett, C.; Calman, L.; Patel, M.; Permyakova, N.V.; Winter, J.; Corner, J.; Din, A.; Fenlon, D.; Richardson, A.; et al. Comorbidities are associated with poorer quality of life and functioning and worse symptoms in the 5 years following colorectal cancer surgery: Results from the ColoREctal Well-being (CREW) cohort study. Psychooncology. 2018, 27, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Rønning, B.; Wyller, T.B.; Nesbakken, A.; Skovlund, E.; Jordhøy, M.S.; Bakka, A.; Rostoft, S. Quality of life in older and frail patients after surgery for colorectal cancer-A follow-up study. J. Geriatr. Oncol. 2016, 7, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.C.; Coburn, N.G.; Devitt, K.S.; Serrano, P.E.; Moulton, C.-A.; Cleary, S.P.; Law, C.; Moore, M.J.; Gallinger, S. Survival Following Resection of Intra- and Extra-Hepatic Metastases from Colorectal Cancer: A Phase II Trial. Ann. Surg. Oncol. 2016, 23, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Lapid, M.I.; Rummans, T.A.; Boeve, B.F.; McCormick, J.K.; Pankratz, V.S.; Cha, R.H.; Smith, G.E.; Ivnik, R.J.; Tangalos, E.G.; Petersen, R.C. What is the quality of life in the oldest old? Int. Psychogeriatr. 2011, 23, 1003–1010. [Google Scholar] [CrossRef]
- Folprecht, G.; Seymour, M.T.; Saltz, L.; Douillard, J.-Y.; Hecker, H.; Stephens, R.J.; Maughan, T.S.; Van Cutsem, E.; Rougier, P.; Mitry, E.; et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: Combined analysis of 2,691 patients in randomized controlled trials. J. Clin. Oncol. 2008, 26, 1443–1451. [Google Scholar] [CrossRef]
- Kabbinavar, F.F.; Hurwitz, H.I.; Yi, J.; Sarkar, S.; Rosen, O. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: Pooled analysis of cohorts of older patients from two randomized clinical trials. J. Clin. Oncol. 2009, 27, 199–205. [Google Scholar] [CrossRef]
- Sorbye, H.; Pfeiffer, P.; Cavalli-Björkman, N.; Qvortrup, C.; Holsen, M.H.; Wentzel-Larsen, T.; Glimelius, B. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer 2009, 115, 4679–4687. [Google Scholar] [CrossRef]
- Nunes, L.; Aasebø, K.; Mathot, L.; Ljungström, V.; Edqvist, P.-H.; Sundström, M.; Dragomir, A.; Pfeiffer, P.; Ameur, A.; Ponten, F.; et al. Molecular characterization of a large unselected cohort of metastatic colorectal cancers in relation to primary tumor location, rare metastatic sites and prognosis. Acta Oncol. 2020, 59, 417–426. [Google Scholar] [CrossRef]
- Saridaki, Z.; Tzardi, M.; Sfakianaki, M.; Papadaki, C.; Voutsina, A.; Kalykaki, A.; Messaritakis, I.; Mpananis, K.; Mavroudis, D.; Stathopoulos, E.; et al. BRAFV600E Mutation Analysis in Patients with Metastatic Colorectal Cancer (mCRC) in Daily Clinical Practice: Correlations with Clinical Characteristics, and Its Impact on Patients’ Outcome. PLoS ONE 2013, 8, e84604. [Google Scholar] [CrossRef]
- Fischer, L.E.; Stintzing, S.; von Weikersthal, L.F.; Modest, D.P.; Decker, T.; Kiani, A.; Kaiser, F.; Al-Batran, S.-E.; Heintges, T.; Lerchenmüller, C.; et al. Efficacy of FOLFIRI plus cetuximab vs FOLFIRI plus bevacizumab in 1st-line treatment of older patients with RAS wild-type metastatic colorectal cancer: An analysis of the randomised trial FIRE-3. Br. J. Cancer 2022, 127, 836–843. [Google Scholar] [CrossRef]
- Gajra, A.; Jeune-Smith, Y.; Fortier, S.; Feinberg, B.; Phillips, E.; Balanean, A.; Klepin, H.D. The Use and Knowledge of Validated Geriatric Assessment Instruments Among US Community Oncologists. JCO Oncol. Pract. 2022, 18, e1081–e1090. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Chemotherapy for colorectal cancer in the elderly. World J. Gastroenterol. 2015, 21, 5158–5166. [Google Scholar] [CrossRef] [PubMed]





| Adults | Older Adults | p-Value | ||||
|---|---|---|---|---|---|---|
| n = 905 (83%) | n = 181 (17%) | |||||
| Age | Median (range) | 64 | (24–75) | 78 | (75–90) | <0.001 |
| Sex | Male | 549 | 61% | 107 | 59% | 0.698 |
| Female | 356 | 39% | 74 | 41% | ||
| ECOG | PS 0 | 266 | 29% | 29 | 16% | <0.001 |
| PS 1 | 500 | 55% | 100 | 55% | ||
| PS 2–3 | 139 | 15% | 52 | 29% | ||
| Charlson comorbidity index * | 0 | 719 | 79% | 115 | 64% | <0.001 |
| 1 to 2 | 179 | 20% | 65 | 36% | ||
| 3 to 5 | 7 | 1% | 1 | 1% | ||
| Second cancer | Non-colorectal cancer | 106 | 12% | 37 | 20% | 0.002 |
| Presentation of metastases | Synchronous | 632 | 70% | 104 | 57% | 0.001 |
| Metachronous | 273 | 30% | 77 | 43% | ||
| Primary location | Right colon | 247 | 27% | 63 | 35% | 0.127 |
| Left colon | 338 | 37% | 58 | 32% | ||
| Rectum | 316 | 35% | 58 | 32% | ||
| Multiple | 4 | 0% | 2 | 1% | ||
| Surgery of primary | Operated upfront | 592 | 65% | 133 | 74% | 0.035 |
| Not operated or later | 313 | 35% | 48 | 27% | ||
| Number of metastatic sites | 1 site | 497 | 55% | 89 | 49% | 0.153 |
| 2 sites | 255 | 28% | 64 | 35% | ||
| 3 to 6 sites | 153 | 17% | 28 | 16% | ||
| Liver metastases | Liver-limited | 365 | 40% | 65 | 36% | 0.345 |
| Liver and extrahepatic | 310 | 34% | 72 | 40% | ||
| Lung metastases | Lung-limited | 56 | 6% | 10 | 6% | 0.048 |
| Lung and extrapulmonary | 207 | 23% | 57 | 32% | ||
| Other metastases | Peritoneal metastases | 144 | 16% | 31 | 17% | 0.685 |
| Distant lymph nodes | 235 | 26% | 40 | 22% | 0.275 | |
| Other metastases | 153 | 17% | 30 | 17% | 0.913 | |
| Estimated glomerular | ≥90 mL/min/1.73 m2 | 463 | 52% | 17 | 10% | <0.001 |
| filtration rate | 60–89 mL/min/1.73 m2 | 343 | 38% | 79 | 44% | |
| 30–59 mL/min/1.73 m2 | 84 | 9% | 82 | 46% | ||
| <30 mL/min/1.73 m2 | 4 | 0% | 1 | 1% | ||
| Mutational status | RAS +/− BRAF wt | 365 | 40% | 63 | 35% | 0.169 |
| RAS mt | 441 | 49% | 98 | 54% | ||
| BRAF mt | 82 | 9% | 11 | 6% | ||
| Not tested | 17 | 2% | 9 | 5% | - | |
| Anemia | Hemoglobin <11 g/dL | 163 | 18% | 25 | 14% | 0.173 |
| Leukocytosis | Leukocytes >109/L | 163 | 18% | 22 | 12% | 0.056 |
| Thrombocytosis | Thrombocytes >4009/L | 257 | 28% | 35 | 19% | 0.012 |
| Hypoalbuminemia | Albumin <30 g/L | 90 | 16% | 14 | 16% | 0.915 |
| Alkaline phosphatase | >105 U/L | 318 | 35% | 55 | 31% | 0.211 |
| Carcinoembryonic antigen | >5 µ/L | 622 | 70% | 136 | 76% | 0.110 |
| Cancer antigen 19-9 | >26 kU/L | 301 | 55% | 44 | 51% | 0.481 |
| Adults | Older Adults | p-Value | |||
|---|---|---|---|---|---|
| 905 | % | 181 | % | ||
| Liver resections/LAT | 279 | 31% | 37 | 20% | 0.005 |
| Lung resection/LAT | 74 | 8% | 7 | 4% | 0.044 |
| Cytoreductive surgery | 44 | 5% | 4 | 2% | 0.113 |
| Local, lymphadenectomy, gynecologic, urologic, or other | 66 | 7% | 9 | 5% | 0.261 |
| Metastasectomy and/or LAT | Systemic Therapy Only or BSC Only | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adults | Older Adults | p-Value | Adults | Older Adults | p-Value | |||||
| 354 | 39% | 45 | 25% | 551 | 61% | 136 | 75% | |||
| Metastasectomy only | 36 | 10% | 6 | 13% | 0.515 | |||||
| Adjuvant | 236 | 67% | 27 | 60% | 0.374 | |||||
| Neoadjuvant | 136 | 38% | 21 | 47% | 0.286 | 23 | 4% | 11 | 8% | 0.059 |
| Conversion | 126 | 36% | 14 | 31% | 0.553 | 86 | 16% | 20 | 15% | 0.794 |
| Disease control | 427 | 77% | 97 | 71% | 0.130 | |||||
| Best supportive care | 15 | 3% | 8 | 6% | 0.067 | |||||
| A | Adults (n = 886) | Older Adults (n = 171) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |||||
| Age (years) | 1.00 | 1.00 | - | 1.01 | 0.313 | 1.12 | 1.06 | - | 1.17 | <0.001 | |
| ECOG | PS 0 | 1.00 | <0.001 | 1.00 | 0.001 | ||||||
| PS 1 | 1.72 | 1.41 | - | 2.10 | <0.001 | 1.79 | 1.09 | - | 2.93 | 0.021 | |
| PS 2–3 | 3.48 | 2.70 | - | 4.49 | <0.001 | 2.69 | 1.57 | - | 4.63 | <0.001 | |
| Treatment groups | R0–1 resection | 1.00 | <0.001 | 1.00 | <0.001 | ||||||
| R2 resection/LAT | 2.53 | 1.72 | - | 3.73 | <0.001 | 2.63 | 1.12 | - | 6.18 | 0.026 | |
| Systemic therapy only | 6.75 | 5.35 | - | 8.52 | <0.001 | 5.62 | 3.24 | - | 9.75 | <0.001 | |
| Charlson comorbidity | 0 | 1.00 | 0.241 | 1.00 | 0.583 | ||||||
| index | 1–2 | 1.16 | 0.95 | - | 1.42 | 0.135 | 1.19 | 0.84 | - | 1.68 | 0.325 |
| 3–5 | 1.47 | 0.61 | - | 3.55 | 0.393 | 0.76 | 0.11 | - | 5.50 | 0.789 | |
| Primary tumor | Left-sided | 1.00 | <0.001 | 1.00 | 0.008 | ||||||
| location | Right-sided | 1.67 | 1.40 | - | 1.99 | <0.001 | 1.60 | 1.13 | - | 2.26 | 0.008 |
| Primary tumor | Yes | 1.00 | <0.001 | 1.00 | 0.041 | ||||||
| operated | No | 2.04 | 1.73 | - | 2.41 | <0.001 | 1.49 | 1.02 | - | 2.18 | 0.041 |
| Mutational status | RAS +/− BRAF wt | 1.00 | <0.001 | 1.00 | <0.001 | ||||||
| RAS mt | 1.24 | 1.04 | - | 1.48 | 0.014 | 1.05 | 0.73 | - | 1.52 | 0.793 | |
| BRAF mt | 2.66 | 2.00 | - | 3.52 | <0.001 | 2.15 | 1.05 | - | 4.42 | 0.037 | |
| Not tested | 0.28 | 0.10 | - | 0.75 | 0.012 | 6.73 | 2.92 | - | 15.48 | <0.001 | |
| B | |||||||||||
| Multivariable | |||||||||||
| Age (years) | 0.99 | 0.99 | - | 1.00 | 0.238 | 1.07 | 1.01 | - | 1.13 | 0.016 | |
| ECOG | PS 0 | 1 | <0.001 | 1 | 0.016 | ||||||
| PS 1 | 1.43 | 1.17 | - | 1.75 | 0.001 | 1.58 | 0.96 | - | 2.63 | 0.075 | |
| PS 2–3 | 2.46 | 1.88 | - | 3.21 | <0.001 | 2.28 | 1.29 | - | 4.02 | 0.004 | |
| Treatment groups | R0–1 resection | 1 | <0.001 | 1 | <0.001 | ||||||
| R2 resection/LAT | 2.23 | 1.51 | - | 3.32 | <0.001 | 2.76 | 1.17 | - | 6.51 | 0.020 | |
| Systemic therapy only | 5.58 | 4.38 | - | 7.10 | <0.001 | 4.66 | 2.65 | - | 8.18 | <0.001 | |
| Charlson comorbidity | 0 | 1 | 0.856 | 1 | 0.587 | ||||||
| index | 1–2 | 1.04 | 0.85 | - | 1.27 | 0.709 | 0.82 | 0.56 | - | 1.21 | 0.327 |
| 3–5 | 1.22 | 0.50 | - | 2.99 | 0.663 | 0.67 | 0.09 | - | 4.95 | 0.691 | |
| Primary tumor | Left-sided | 1 | <0.001 | 1 | 0.352 | ||||||
| location | Right-sided | 1.70 | 1.39 | - | 2.07 | <0.001 | 1.21 | 0.81 | - | 1.83 | 0.352 |
| Primary tumor | Yes | 1 | <0.001 | 1 | 0.019 | ||||||
| operated | No | 1.57 | 1.32 | - | 1.87 | <0.001 | 1.65 | 1.09 | - | 2.51 | 0.019 |
| Mutational status | RAS +/− BRAF wt | 1 | 0.026 | 1 | 0.001 | ||||||
| RAS mt | 1.14 | 0.96 | - | 1.37 | 0.139 | 0.96 | 0.65 | - | 1.44 | 0.855 | |
| BRAF mt | 1.45 | 1.06 | - | 1.97 | 0.020 | 2.70 | 1.24 | - | 5.88 | 0.012 | |
| Not tested | 0.45 | 0.16 | - | 1.21 | 0.113 | 3.71 | 1.53 | - | 9.01 | 0.004 | |
| Adults | Older Adults | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 397 | Mean | SD | 95% CI | n = 47 | Mean | SD | 95% CI | ∆ | p-Value | |
| 15D | ||||||||||
| Neoadjuvant | 52 | 0.884 | 0.097 | 0.857–0.911 | 4 | 0.959 | 0.066 | 0.853–1.064 | 0.075 | 0.056 |
| Post resection | 54 | 0.872 | 0.091 | 0.846–0.896 | 4 | 0.932 | 0.055 | 0.845–1.019 | 0.060 | 0.209 |
| Rehabilitation | 55 | 0.907 | 0.071 | 0.887–0.925 | 5 | 0.931 | 0.055 | 0.863–0.999 | 0.023 | 0.514 |
| Remission | 125 | 0.896 | 0.091 | 0.879–0.912 | 7 | 0.882 | 0.111 | 0.779–0.985 | −0.014 | 0.831 |
| First-line | 148 | 0.860 | 0.090 | 0.850–0.880 | 21 | 0.870 | 0.080 | 0.830–0.900 | 0.010 | 0.937 |
| Second-line | 104 | 0.859 | 0.090 | 0.842–0.877 | 10 | 0.836 | 0.071 | 0.785–0.877 | −0.023 | 0.356 |
| Later-line | 93 | 0.849 | 0.097 | 0.829–0.869 | 12 | 0.877 | 0.072 | 0.831–0.923 | 0.027 | 0.435 |
| Best supportive care | 22 | 0.782 | 0.116 | 0.730–0.833 | 13 | 0.730 | 0.132 | 0.650–0.810 | −0.052 | 0.302 |
| GHS | ||||||||||
| Neoadjuvant | 52 | 67.5 | 20.7 | 61.3–73.7 | 4 | 78.1 | 11.5 | 59.9–96.4 | 10.66 | 0.366 |
| Post resection | 54 | 74.5 | 17.7 | 69.1–79.0 | 4 | 94.4 | 9.6 | 70.5–118.3 | 19.90 | 0.037 |
| Rehabilitation | 55 | 79.2 | 15.0 | 74.9–83.1 | 5 | 72.9 | 17.2 | 45.6–100.3 | −6.30 | 0.524 |
| Remission | 125 | 76.1 | 18.6 | 72.8–79.5 | 7 | 62.2 | 20.3 | 40.9–83.5 | −13.91 | 0.055 |
| First-line | 148 | 66.6 | 18.2 | 63.1–70.1 | 21 | 68.1 | 11.9 | 62.6–73.7 | 1.54 | 0.712 |
| Second-line | 104 | 68.3 | 18.2 | 64.5–72.0 | 10 | 61.7 | 16.8 | 49.7–73.7 | −6.60 | 0.234 |
| Later-line | 93 | 66.1 | 17.8 | 62.4–69.8 | 12 | 73.8 | 16.8 | 63.7–84.0 | 7.73 | 0.129 |
| Best supportive care | 22 | 52.6 | 19.6 | 43.7–61.5 | 13 | 56.8 | 23.1 | 42.9–70.8 | 4.26 | 0.576 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehtomäki, K.; Soveri, L.-M.; Osterlund, E.; Lamminmäki, A.; Uutela, A.; Heervä, E.; Halonen, P.; Stedt, H.; Aho, S.; Muhonen, T.; et al. Resectability, Resections, Survival Outcomes, and Quality of Life in Older Adult Patients with Metastatic Colorectal Cancer (the RAXO-Study). J. Clin. Med. 2023, 12, 3541. https://doi.org/10.3390/jcm12103541
Lehtomäki K, Soveri L-M, Osterlund E, Lamminmäki A, Uutela A, Heervä E, Halonen P, Stedt H, Aho S, Muhonen T, et al. Resectability, Resections, Survival Outcomes, and Quality of Life in Older Adult Patients with Metastatic Colorectal Cancer (the RAXO-Study). Journal of Clinical Medicine. 2023; 12(10):3541. https://doi.org/10.3390/jcm12103541
Chicago/Turabian StyleLehtomäki, Kaisa, Leena-Maija Soveri, Emerik Osterlund, Annamarja Lamminmäki, Aki Uutela, Eetu Heervä, Päivi Halonen, Hanna Stedt, Sonja Aho, Timo Muhonen, and et al. 2023. "Resectability, Resections, Survival Outcomes, and Quality of Life in Older Adult Patients with Metastatic Colorectal Cancer (the RAXO-Study)" Journal of Clinical Medicine 12, no. 10: 3541. https://doi.org/10.3390/jcm12103541







