Do Exophytic and Endophytic Patterns in Borderline Ovarian Tumors Have Different Prognostic Implications? A Large Multicentric Experience
Abstract
1. Introduction
2. Materials and Methods
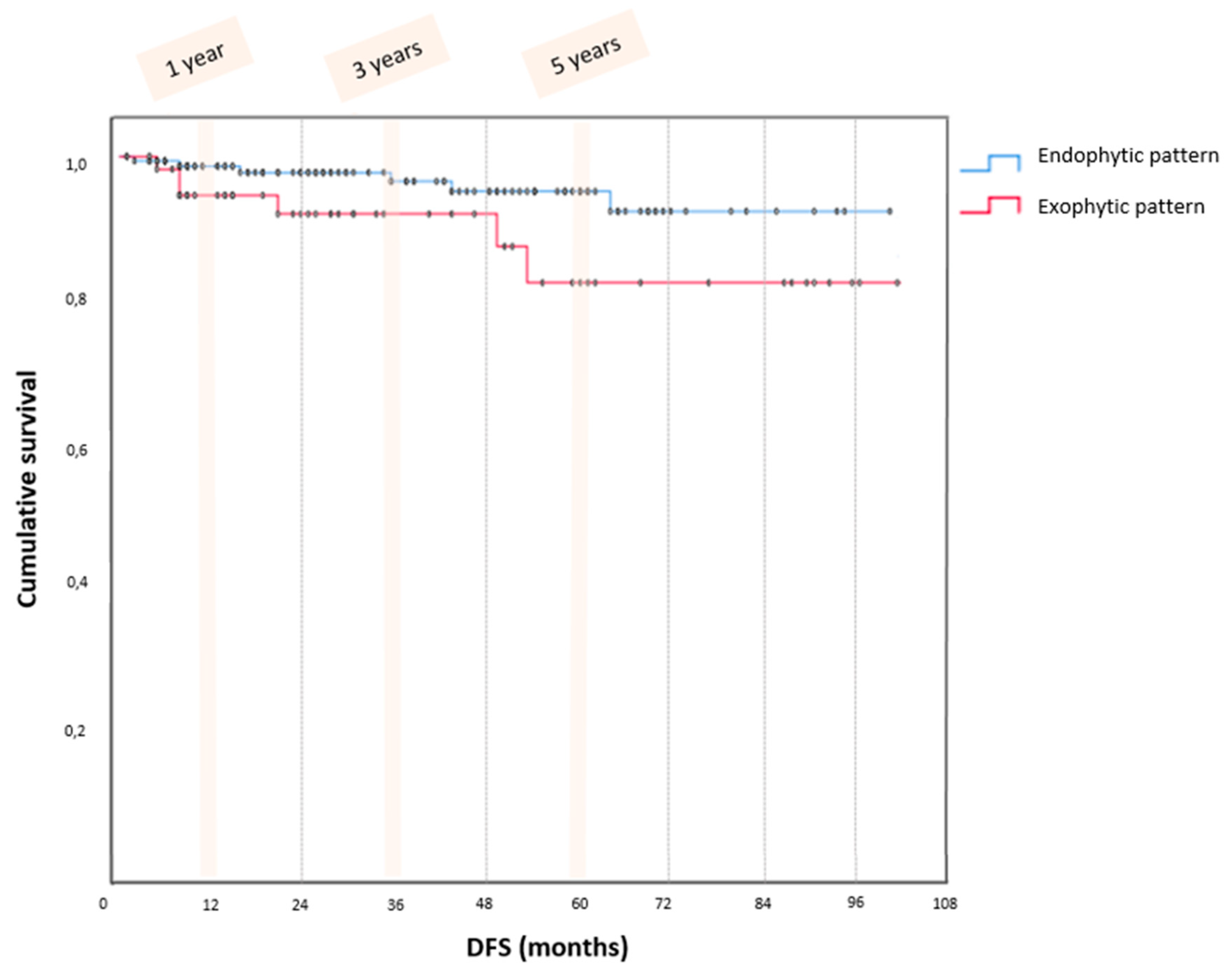
3. Results
4. Discussion
4.1. Summary of Main Results
4.2. Results in the Context of Published Literature
4.3. Strengths and Weaknesses
4.4. Implications for Practice and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skírnisdóttir, I.; Garmo, H.; Wilander, E.; Holmberg, L. Borderline ovarian tumors in Sweden 1960–2005: Trends in incidence and age at diagnosis compared to ovarian cancer. Int. J. Cancer 2008, 123, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Katsube, Y.; Berg, J.W.; Silverberg, S.G. Epidemiologic pathology of ovarian tumors: A histopathologic review of primary ovarian neoplasms diagnosed in the Denver Standard Metropolitan Statistical Area, 1 July–31 December 1969 and 1 July–31 December 1979. Int. J. Gynecol. Pathol. 1982, 1, 3–16. [Google Scholar] [CrossRef]
- Gizzo, S.; Berretta, R.; Di Gangi, S.; Guido, M.; Zanni, G.C.; Franceschetti, I.; Quaranta, M.; Plebani, M.; Nardelli, G.B.; Patrelli, T.S. Borderline ovarian tumors and diagnostic dilemma of intraoperative diagnosis: Could preoperative He4 assay and ROMA score assessment increase the frozen section accuracy? A multicenter case-control study. Biomed. Res. Int. 2014, 2014, 803598. [Google Scholar] [CrossRef] [PubMed]
- Seidman, J.D.; Russell, P.; Kurman, R.J. Surface epithelial tumors of the ovary. In Blaustein′s Pathology of the Female Genital Tract, 5th ed.; Kurman, R.J., Ed.; Springer: New York, NY, USA, 2002; p. 791. [Google Scholar]
- Moro, F.; Baima Poma, C.; Zannoni, G.F.; Vidal Urbinati, A.; Pasciuto, T.; Ludovisi, M.; Moruzzi, M.C.; Carinelli, S.; Franchi, D.; Scambia, G.; et al. Imaging in gynecological disease (12): Clinical and ultrasound features of invasive and non-invasive malignant serous ovarian tumors. Ultrasound Obs. Gynecol. 2017, 50, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Gershenson, D.; Lhomme, C.; Lecuru, F.; Ledermann, J.; Provencher, D.M.; Mezzanzanica, D.; Quinn, M.; Maenpaa, J.; Kim, J.-W.; et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian tumors of low malignant potential (borderline ovarian tumors). Int. J. Gynecol. Cancer 2014, 24 (Suppl. S3), S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Zanetta, G.; Rota, S.; Chiari, S.; Bonazzi, C.; Bratina, G.; Mangioni, C. Behavior of borderline tumors with particular interest to persistence, recurrence, and progression to invasive carcinoma: A prospective study. J. Clin. Oncol. 2001, 19, 2658–2664. [Google Scholar] [CrossRef]
- Morice, P.; Camatte, S.; El Hassan, J.; Pautier, P.; Duvillard, P.; Castaigne, D. Clinical outcomes and fertility after conservative treatment of ovarian borderline tumors. Fertil. Steril. 2001, 75, 92–96. [Google Scholar] [CrossRef]
- Lazarou, A.; Fotopoulou, C.; Coumbos, A.; Sehouli, J.; Vasiljeva, J.; Braicu, I.; Burger, H.; Kuehn, W. Long-term follow-up of borderline ovarian tumors clinical outcome and prognostic factors. Anticancer. Res. 2014, 34, 6725–6730. [Google Scholar]
- Seidman, J.D.; Kurman, R.J. Pathology of ovarian carcinoma. Hematol. Oncol. Clin. N. Am. 2003, 17, 909–925. [Google Scholar] [CrossRef]
- Uzan, C.; Berretta, R.; Rolla, M.; Gouy, S.; Fauvet, R.; Darai, E.; Duvillard, P.; Morice, P. Management and prognosis of endometrioid borderline tumors of the ovary. Surg. Oncol. 2012, 21, 178–184. [Google Scholar] [CrossRef]
- Fischerova, D.; Zikan, M.; Dundr, P.; Cibula, D. Diagnosis, treatment, and follow-up of borderline ovarian tumors. Oncologist 2012, 17, 1515–1533. [Google Scholar] [CrossRef] [PubMed]
- Trimble, C.L.; Trimble, E.L. Ovarian tumors of low malignant potential. Oncol. (Williston Park) 2003, 17, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Hart, W.R. Borderline epithelial tumors of the ovary. Mod. Pathol. 2005, 18 (Suppl. S2), S33–S50. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, D.W.; Yim, G.W.; Nam, E.J.; Kim, S.; Kim, Y.T. Staging laparoscopy for the management of early-stage ovarian cancer: A metaanalysis. Am. J. Obs. Gynecol. 2013, 209, 58.e1–58.e8. [Google Scholar] [CrossRef]
- Ludovisi, M.; Foo, X.; Mainenti, S.; Testa, A.C.; Arora, R.; Jurkovic, D. Ultrasound diagnosis of serous surface papillary borderline ovarian tumor: A case series with a review of the literature. J. Clin. Ultrasound. 2015, 43, 573–577. [Google Scholar] [CrossRef]
- Hauptmann, S.; Friedrich, K.; Redline, R.; Avril, S. Ovarian borderline tumors in the 2014 WHO classification: Evolving concepts and diagnostic criteria. Virchows Arch. 2017, 470, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.R.; Scully, R.E. Mucinous tumors of the ovary: A clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘pseudomyxoma peritonei’. Am. J. Surg. Pathol. 2000, 24, 1447–1464. [Google Scholar] [CrossRef]
- Chiesa, A.G.; Deavers, M.T.; Veras, E.; Silva, E.G.; Gershenson, D.; Malpica, A. Ovarian intestinal type mucinous borderline tumors: Are we ready for a nomenclature change? Int. J. Gynecol. Pathol. 2010, 29, 108–112. [Google Scholar] [CrossRef]
- Ronnett, B.M.; Kajdacsy-Balla, A.; Gilks, C.B.; Merino, M.J.; Silva, E.; Werness, B.A.; Young, R.H. Mucinous borderline ovarian tumors: Points of general agreement and persistent controversies regarding nomenclature, diagnostic criteria, and behavior. Hum Pathol. 2004, 35, 949–960. [Google Scholar] [CrossRef]
- Shimamoto, A.; Isomoto, I.; Segawa, K.; Matsumoto, A.; Abe, K.; Uetani, M. The MRI findings in a case of ovarian mucinous borderline tumor mimicking a serous surface borderline tumor. Jpn. J. Radiol. 2014, 32, 552–555. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. WHO Classification of Tumours of Female Reproductive Organs; IARC: Lyon, France, 2014. [Google Scholar]
- Höhn, A.K.; Brambs, C.E.; Hiller, G.G.R.; May, D.; Schmoeckel, E.; Horn, L.C. 2020 WHO Classification of Female Genital Tumors. Geburtshilfe Frauenheilkd. 2021, 81, 1145–1153. [Google Scholar] [CrossRef]
- Timmerman, D.; Valentin, L.; Bourne, T.H.; Collins, W.P.; Verrelst, H.; Vergote, I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: A consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obs. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef]
- Mutch, D.G.; Prat, J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol. Oncol. 2014, 133, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Ardighieri, L.; Zeppernick, F.; Hannibal, C.G.; Vang, R.; Cope, L.; Junge, J.; Kjaer, S.K.; Kurman, R.J.; Shih, I.-M. Mutational analysis of BRAF and KRAS in ovarian serous borderline (atypical proliferative) tumours and associated peritoneal implants. J. Pathol. 2014, 232, 16–22. [Google Scholar] [CrossRef]
- Ayhan, A.; Guven, S.; Guven, E.S.; Kucukali, T. Is there a correlation between tumor marker panel and tumor size and histopathology in well staged patients with borderline ovarian tumors? Acta Obs. Gynecol. Scand. 2007, 86, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Smith Sehdev, A.E.; Sehdev, P.S.; Kurman, R.J. Noninvasive and invasive micropapillary (low-grade) serous carcinoma of the ovary: A clinicopathologic analysis of 135 cases. Am. J. Surg. Pathol. 2003, 27, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, S.G.; Bell, D.A.; Kurman, R.J.; Seidman, J.D.; Prat, J.; Ronnett, B.M.; Copeland, L.; Silva, E.; Gorstein, F.; Young, R.H. Borderline ovarian tumors: Key points and workshop summary. Hum. Pathol. 2004, 35, 910–917. [Google Scholar] [CrossRef]
- Shih, K.K.; Zhou, Q.C.; Aghajanian, C.; Huh, J.; Soslow, R.A.; Morgan, J.C.; Iasonos, A.; Chi, D.S.; Barakat, R.R.; Abu-Rustum, N.R. Patterns of recurrence and role of adjuvant chemotherapy in stage II-IV serous ovarian borderline tumors. Gynecol. Oncol. 2010, 119, 270–273. [Google Scholar] [CrossRef]
- Gouy, S.; Maria, S.; Faron, M.; Maulard, A.; Pautier, P.; Leary, A.; Chargari, C.; Genestie, C.; Morice, P. Results After Conservative Surgery of Stage II/III Serous Borderline Ovarian Tumors. Ann. Surg. Oncol. 2021, 28, 3597–3604. [Google Scholar] [CrossRef]
- Longacre, T.A.; Wells, M. Serous tumors. In WHO Classification of Tumours of Female Reproductive Organs; Kurman, R.J., Carcangiu, M.L., Herrington, C.S., Young, R.H., Eds.; IARC Press: Lyon, France, 2014; pp. 15–24. [Google Scholar]
- Morice, P.; Uzan, C.; Fauvet, R.; Gouy, S.; Duvillard, P.; Darai, E. Borderline ovarian tumour: Pathological diagnostic dilemma and riCsk factors for invasive or lethal recurrence. Lancet Oncol. 2012, 13, e103–e115. [Google Scholar] [CrossRef]
- Shih, I. Ovarian serous low malignant potential (borderline) tumor—Does “micropapillary” matter? Gynecol. Oncol. 2010, 117, 1–3. [Google Scholar] [CrossRef]
- Genestie, C.; Auguste, A.; Al Battal, M.; Scoazec, J.Y.; Gouy, S.; Lacroix, L.; Morice, P.; Pautier, P.; Leary, A.; Devouassoux-Shisheboran, M. Histological classification of mucinous ovarian tumors: Inter-observer reproducibility, clinical relevance, and role of genetic biomarkers. Virchows Arch. 2021, 478, 885–891. [Google Scholar] [CrossRef]
- Segal, G.H.; Hart, W.R. Ovarian serous tumors of low malignant potential (serous borderline tumors). The relationship of exophytic surface tumor to peritoneal “implants”. Am. J. Surg. Pathol. 1992, 16, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Bell, D.A.; Welch, W.R.; Berkowitz, R.S.; Mok, S.C. Evidence for the multifocal origin of bilateral and advanced human serous borderline ovarian tumors. Cancer Res. 1998, 58, 2328–2330. [Google Scholar]
- Heublein, S.; Grasse, K.; Hessel, H.; Burges, A.; Lenhard, M.; Engel, J.; Kirchner, T.; Jeschke, U.; Mayr, D. KRAS, BRAF genotyping reveals genetic heterogeneity of ovarian borderline tumors and associated implants. BMC Cancer 2013, 13, 483. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Roth, L.M.; Younger, C.; Michael, H.; Abdul-Karim, F.W.; Zhang, S.; Ulbright, T.M.; Eble, J.N.; Cheng, L. Molecular evidence for the independent origin of extra-ovarian papillary serous tumors of low malignant potential. J. Natl. Cancer Inst. 2001, 93, 1147–1152. [Google Scholar] [CrossRef][Green Version]
- Morice, P.; Camatte, S.; Rey, A.; Atallah, D.; Lhommé, C.; Pautier, P.; Pomel, C.; Coté, J.-F.; Haie-Meder, C.; Duvillard, P.; et al. Prognostic factors for patients with advanced stage serous borderline tumours of the ovary. Ann. Oncol. 2003, 14, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Seidman, J.D.; Kurman, R.J. Ovarian serous borderline tumors: A critical review of the literature with emphasis on prognostic indicators. Hum. Pathol. 2000, 31, 539–557. [Google Scholar] [CrossRef]
- Chui, M.H.; Xing, D.; Zeppernick, F.; Wang, Z.Q.; Hannibal, C.G.; Frederiksen, K.; Kjaer, S.K.; Cope, L.; Kurman, R.J.; Shih, I.M.; et al. Clinicopathologic and Molecular Features of Paired Cases of Metachronous Ovarian Serous Borderline Tumor and Subsequent Serous Carcinoma. Am. J. Surg. Pathol. 2019, 43, 1462–1472. [Google Scholar] [CrossRef]
- Sadlecki, P.; Antosik, P.; Grzanka, D.; Grabiec, M.; Walentowicz-Sadlecka, M. KRAS mutation testing in borderline ovarian tumors and low-grade ovarian carcinomas with a rapid, fully integrated molecular diagnostic system. Tumour Biol. 2017, 39, 1010428317733. [Google Scholar] [CrossRef] [PubMed]
- Sadlecki, P.; Walentowicz, P.; Bodnar, M.; Marszalek, A.; Grabiec, M.; Walentowicz-Sadlecka, M. Determination of BRAF V600E (VE1) protein expression and BRAF gene mutation status in codon 600 in borderline and low-grade ovarian cancers. Tumour Biol. 2017, 39, 1010428317706230. [Google Scholar] [CrossRef] [PubMed]
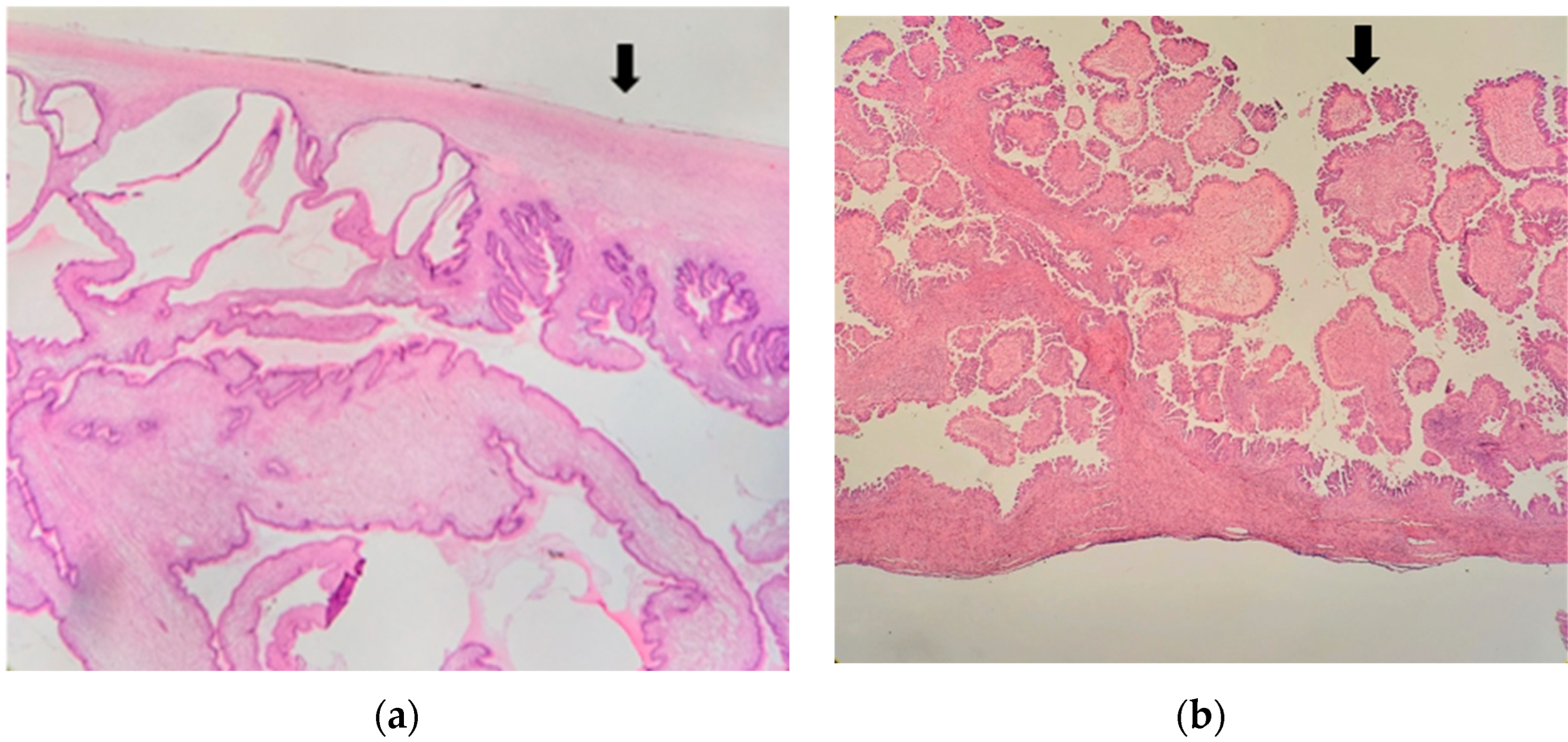
| Total n; % | Endophytic Endophytic Pattern n; % | Exophytic Pattern n; % | p | |
|---|---|---|---|---|
| 229; 100.0 | 169; 73.8 | 60; 26.2 | ||
| Median Age | 49 (18–90) | 50 (18–90) | 41 (18–82) | 0.001 |
| Median BMI | 25 (17–50) | 25 (17–40) | 24 (19–50) | 0.680 |
| BMI > 30 | 33; 14.4 | 20; 17.8 | 3; 5.0 | 0.016 |
| Menopausal state | 104; 45.4 | 88; 52.1 | 16; 26.7 | 0.001 |
| No Parity | 100; 43.7 | 65; 38.5 | 35; 58.3 | 0.008 |
| Abnormal Ca125 | 84; 36.7 | 53; 31.4 | 31; 51.7 | 0.003 |
| Fertility sparing surgery | 80; 34.9 | 51; 30.2 | 29; 48.3 | 0.011 |
| Total n; % | Endophytic Pattern n; % | Exophytic Pattern n; % | p | |
|---|---|---|---|---|
| Histologic subtype | ||||
| Serous | 134; 58.5 | 87; 51.5 | 47; 79.7 | <0.001 |
| Mucinous | 87; 38.0 | 75; 44.4 | 12; 20.3 | 0.001 |
| Others | 7; 3.1 | 7; 4.1 | 0; - | 0.109 |
| FIGO stage | <0.001 | |||
| IA | 160; 69.9 | 156; 92.3 | 4; 6.7 | |
| IB | 14; 6.1 | 13; 7.7 | 1; 1.7 | |
| IC | 35; 15.3 | 0; - | 35; 58.3 | |
| IIA | 4; 1.7 | 0; - | 4; 6.7 | |
| IIB | 1; 0.4 | 0; - | 1; 1.7 | |
| IIIB | 4; 1.7 | 0; - | 4; 6.7 | |
| IIIC | 11; 4.8 | 0; - | 11; 18.3 | |
| Peritoneal implants | 11; 4.5 | 0;- | 11;18.3 | <0.001 |
| Invasive implants | 3; 1.3 | 0; - | 3; 5.0 | 0.003 |
| Pcytologycitology in peritoneal washing | 13; 5.7 | 1; 0.6 | 12; 20.0 | <0.001 |
| Total n; % | Endophytic Pattern n; % | Exophytic Pattern n; % | p | |
|---|---|---|---|---|
| Median diameter | 80 (15–370) | 82 (18–370) | 67.5 (15–270) | 0.564 |
| Diameter > 100 mm | 86; 37.6 | 70; 41.4 | 16; 26.7 | 0.043 |
| Diameter solid component mm | 22 (5–180) | 20 (5–85) | 33 (5–180) | 0.070 |
| Solid component > 10 mm | 89; 40.1 | 59; 34.9 | 30; 50.0 | 0.017 |
| Color Score | 0.404 | |||
| Color score 1 | 105; 45.9 | 81; 47.9 | 24; 40.0 | 0.606 |
| Color score 2 | 90; 39.3 | 65; 38.5 | 25; 41.7 | 0.383 |
| Color score 3 | 21; 9.2 | 16; 9.5 | 5; 8.3 | 0.847 |
| Color score 4 | 3; 1.3 | 1; 0.6 | 2; 3.3 | 0.100 |
| N of locules | ||||
| <10 Loculi | 66; 28.8 | 52; 30.8 | 14; 23.3 | 0.301 |
| >10 Loculi | 29; 12.7 | 21; 12.4 | 8; 13.3 | 0.823 |
| N of papillae | ||||
| No papillae | 116; 50.7 | 86; 50.9 | 30; 50.0 | 0.991 |
| <4 papillae | 96; 41.9 | 72; 42.6 | 24; 40.0 | 0.792 |
| >4 papillae | 9; 3.9 | 6; 3.6 | 3; 5.0 | 0.603 |
| Ascitis | 19; 8.3 | 14; 8.3 | 5; 8.3 | 0.983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capozzi, V.A.; Scarpelli, E.; Monfardini, L.; Mandato, V.D.; Merisio, C.; Uccella, S.; Sozzi, G.; Ceccaroni, M.; Chiantera, V.; Giordano, G.; et al. Do Exophytic and Endophytic Patterns in Borderline Ovarian Tumors Have Different Prognostic Implications? A Large Multicentric Experience. J. Clin. Med. 2023, 12, 3544. https://doi.org/10.3390/jcm12103544
Capozzi VA, Scarpelli E, Monfardini L, Mandato VD, Merisio C, Uccella S, Sozzi G, Ceccaroni M, Chiantera V, Giordano G, et al. Do Exophytic and Endophytic Patterns in Borderline Ovarian Tumors Have Different Prognostic Implications? A Large Multicentric Experience. Journal of Clinical Medicine. 2023; 12(10):3544. https://doi.org/10.3390/jcm12103544
Chicago/Turabian StyleCapozzi, Vito Andrea, Elisa Scarpelli, Luciano Monfardini, Vincenzo Dario Mandato, Carla Merisio, Stefano Uccella, Giulio Sozzi, Marcello Ceccaroni, Vito Chiantera, Giovanna Giordano, and et al. 2023. "Do Exophytic and Endophytic Patterns in Borderline Ovarian Tumors Have Different Prognostic Implications? A Large Multicentric Experience" Journal of Clinical Medicine 12, no. 10: 3544. https://doi.org/10.3390/jcm12103544
APA StyleCapozzi, V. A., Scarpelli, E., Monfardini, L., Mandato, V. D., Merisio, C., Uccella, S., Sozzi, G., Ceccaroni, M., Chiantera, V., Giordano, G., Della Corte, L., Conte, C., Cianci, S., Ghi, T., & Berretta, R. (2023). Do Exophytic and Endophytic Patterns in Borderline Ovarian Tumors Have Different Prognostic Implications? A Large Multicentric Experience. Journal of Clinical Medicine, 12(10), 3544. https://doi.org/10.3390/jcm12103544








