Current Insights into the Metabolome during Hypothermic Kidney Perfusion—A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction and Processing
2.4. Quality Assessment and Data Analysis
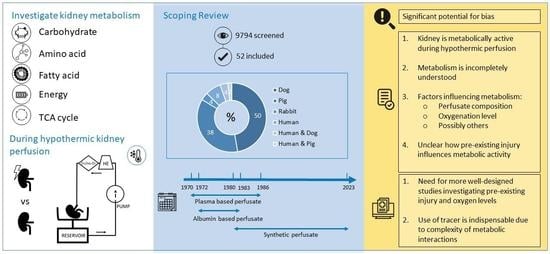
3. Results
3.1. Quality and Risk of Bias Assessment
3.2. Hypothermic Perfusion Set-up
3.3. Metabolism during Hypothermic Perfusion with Plasma-Based Perfusates
3.3.1. Carbohydrate Metabolism
3.3.2. Amino Acid Metabolism
3.3.3. Fatty Acid Metabolism
3.3.4. Energy Metabolism
3.4. Metabolism during Hypothermic Perfusion with Albumin-Based Perfusates
3.4.1. Carbohydrate Metabolism
3.4.2. Amino Acid Metabolism
3.4.3. Fatty Acid Metabolism
3.4.4. Metabolism of High-Energy Molecules
3.5. Metabolism during Hypothermic Perfusion with Synthetic Perfusates
3.5.1. Carbohydrate Metabolism
3.5.2. Amino Acid Metabolism
3.5.3. Fatty Acid Metabolism
3.5.4. Energy Metabolism
3.5.5. TCA Cycle Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Talbot, D.; Wilson, C.H. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 2019, 3, CD011671. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.H.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.; Squifflet, J.P.; van Heurn, E.; et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Moers, C.; Pirenne, J.; Paul, A.; Ploeg, R.J.; Na, N.A. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2012, 366, 770–771. [Google Scholar] [CrossRef]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.D.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.P.; Van Heurn, E.; Monbaliu, D.; et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: A multicenter, randomized, controlled trial. Ann. Surg. 2010, 252, 756–762. [Google Scholar] [CrossRef]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J.; COMPARE Trial Collaboration and Consortium for Organ Preservation in Europe (COPE). Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Nicholson, H.F.; Nicholson, M.L. Oxygenated kidney preservation techniques. Transplantation 2012, 93, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ preservation: Current concepts and new strategies for the next decade. Transfus. Med. Hemotherapy 2011, 38, 125–142. [Google Scholar] [CrossRef]
- Nath, J.; Guy, A.; Smith, T.B.; Cobbold, M.; Inston, N.G.; Hodson, J.; Tennant, D.A.; Ludwig, C.; Ready, A.R. Metabolomic perfusate analysis during kidney machine perfusion: The pig provides an appropriate model for human studies. PLoS ONE 2014, 9, e114818. [Google Scholar] [CrossRef]
- Jochmans, I. Improving Organ Preservation: The Trick Is to Keep (Cells) Breathing. Transplantation 2022, 106, 904–907. [Google Scholar] [CrossRef]
- Verstraeten, L.; Denabt, R.; Jochmans, I. Current insights into the metabolome during hypothermic isolated kidney perfusion—A scoping review. Open. Sci. Framew. 2022. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Verstraeten, L.; Denabt, R.; Jochmans, I. Replication Data for: Current Insights into the Metabolome during Hypothermic Kidney Perfusion—A Systematic Review. 2023. Available online: https://doi.org/10.48804/AMSYVO (accessed on 7 April 2023).
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Study Quality Assessment Tools, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 24 February 2023).
- Guy, A.J.; Nath, J.; Cobbold, M.; Ludwig, C.; Tennant, D.A.; Inston, N.G.; Ready, A.R. Metabolomic analysis of perfusate during hypothermic machine perfusion of human cadaveric kidneys. Transplantation 2015, 99, 754–759. [Google Scholar] [CrossRef]
- Faucher, Q.; Alarcan, H.; Sauvage, F.L.; Forestier, L.; Miquelestorena-Standley, E.; Nadal-Desbarats, L.; Arnion, H.; Venhard, J.C.; Brichart, N.; Bruyère, F.; et al. Perfusate Metabolomics Content and Expression of Tubular Transporters During Human Kidney Graft Preservation by Hypothermic Machine Perfusion. Transplantation 2022, 106, 1831–1843. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Dmochowski, J.R.; Murray, J.E.; Couch, N.P. Successful 24 hour renal perfusion-preservation with monitoring by surface electrometry during the storage interval. Surgery 1970, 67, 944–950. [Google Scholar] [PubMed]
- Bon, D.; Billault, C.; Thuillier, R.; Hebrard, W.; Boildieu, N.; Celhay, O.; Irani, J.; Seguin, F.; Hauet, T. Analysis of perfusates during hypothermic machine perfusion by NMR spectroscopy: A potential tool for predicting kidney graft outcome. Transplantation 2014, 97, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Gianello, P.; Vergauwen, M.; Mourad, N.I.; Buemi, A.; De Meyer, M.; Mourad, M. The effect on early renal function of various dynamic preservation strategies in a preclinical pig ischemia–reperfusion autotransplant model. Am. J. Transpl. 2018, 19, 752–762. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Mueller, M.; Aydin, S.; Dutkowski, P.; Gianello, P.; Mourad, M. Brief Bubble and Intermittent Surface Oxygenation Is a Simple and Effective Alternative for Membrane Oxygenation During Hypothermic Machine Perfusion in Kidneys. Transpl. Direct 2020, 6, e571. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Smith, T.; Gerin, I.; Joris, V.; Mueller, M.; Aydin, S.; Muller, X.; Schlegel, A.; Nath, J.; et al. Brief O2 uploading during continuous hypothermic machine perfusion is simple yet effective oxygenation method to improve initial kidney function in a porcine autotransplant model. Am. J. Transplant. 2020, 20, 2030–2043. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Smith, T.B.; Patel, K.; Craps, J.; Joris, V.; Aydin, S.; Ury, B.; Buemi, A.; De Meyer, M.; et al. Influence of Different Partial Pressures of Oxygen During Continuous Hypothermic Machine Perfusion in a Pig Kidney Ischemia-reperfusion Autotransplant Model. Transplantation 2020, 104, 731–743. [Google Scholar] [CrossRef]
- Grundmann, R.; Berr, F.; Pitschi, H. Ninety six hour preservation of canine kidneys. Transplantation 1974, 17, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Halasz, N.A.; Collins, G.M. Fatty acid utilization during perfusion. Transplantation 1975, 20, 354–356. [Google Scholar] [PubMed]
- Pegg, D.E.; Foreman, J.; Rolles, K. Metabolism during preservation and viability of ischemically injured canine kidneys. Transplantation 1984, 38, 78–81. [Google Scholar] [PubMed]
- Slaattelid, O.; Flatmark, A.; Skrede, S. The importance of perfusate content of free fatty acids for dog kidney preservation. Scand. J. Clin. Lab. Investig. 1976, 36, 239–245. [Google Scholar] [CrossRef]
- Slaattelid, O.; Gronnerod, O.; Horn, A. Preservation of dog kidneys by hypothermic perfusion with glucose-free and glucose-rich perfusates. Eur. Surg. Res. 1976, 8, 515–527. [Google Scholar] [CrossRef]
- Collins, G.M.; Taft, P.; Green, R.D.; Ruprecht, R.; Halasz, N.A. Adenine nucleotide levels in preserved and ischemically injured canine kidneys. World J. Surg. 1977, 2, 237–243. [Google Scholar] [CrossRef]
- Grundmann, R.; Landes, T.; Pichlmaier, H. Hypothermic pulsatile perfusion of dog kidneys. Factors limiting successful preservation time. Transplantation 1972, 14, 742–747. [Google Scholar] [CrossRef]
- Kaminski, J.; Delpech, P.O.; Kaaki-Hosni, S.; Promeyrat, X.; Hauet, T.; Hannaert, P. Oxygen Consumption by Warm Ischemia-Injured Porcine Kidneys in Hypothermic Static and Machine Preservation. J. Surg. Res. 2019, 242, 78–86. [Google Scholar] [CrossRef]
- La Manna, G.; Conte, D.; Cappuccilli, M.L.; Nardo, B.; D’Addio, F.; Puviani, L.; Comai, G.; Bianchi, F.; Bertelli, R.; Lanci, N.; et al. An in vivo autotransplant model of renal preservation: Cold storage versus machine perfusion in the prevention of ischemia/reperfusion injury. Artif. Organs 2009, 33, 565–570. [Google Scholar] [CrossRef]
- McAnulty, J.F.; Southard, J.H.; Belzer, F.O. Comparison of the effects of adenine-ribose with adenosine for maintenance of ATP concentrations in 5-day hypothermically perfused dog kidneys. Cryobiology 1988, 25, 409–416. [Google Scholar] [CrossRef]
- Minor, T.; Sitzia, M.; Dombrowski, F. Kidney transplantation from non-heart-beating donors after oxygenated low-flow machine perfusion preservation with histidine-tryptophan-ketoglutarate solution. Transpl. Int. 2005, 17, 707–712. [Google Scholar] [CrossRef]
- Pegg, D.E.; Green, C.J. Renal preservation by hypothermic perfusion using a defined perfusion fluid. Cryobiology 1972, 9, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Simona, M.-S.; Alessandra, V.; Emanuela, C.; Elena, T.; Michela, M.; Fulvia, G.; Vincenzo, S.; Ilaria, B.; Federica, M.; Eloisa, A.; et al. Evaluation of Oxidative Stress and Metabolic Profile in a Preclinical Kidney Transplantation Model According to Different Preservation Modalities. Int. J. Mol. Sci. 2023, 24, 1029. [Google Scholar] [CrossRef] [PubMed]
- Venema, L.H.; Brat, A.; Moers, C.; Hart, N.A.; Ploeg, R.J.; Hannaert, P.; Minor, T.; Leuvenink, H. Effects of Oxygen During Long-term Hypothermic Machine Perfusion in a Porcine Model of Kidney Donation After Circulatory Death. Transplantation 2019, 103, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.H.; Armbruster, D.; Grebe, W.; Czerniak, A.; Isselhard, W. Effects of differences in substrate supply on the energy metabolism of hypothermically perfused canine kidneys. Cryobiology 1980, 17, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.H.; Isselhard, W.; Hauer, U.; Menge, M. Free fatty acid and glucose metabolism during hypothermic perfusion of canine kidneys. Eur. Surg. Res. 1979, 11, 107–121. [Google Scholar] [CrossRef]
- Belzer, F.O.; Glass, N.R.; Sollinger, H.W.; Hoffmann, R.M.; Southard, J.H. A new perfusate for kidney preservation. Transplantation 1982, 33, 322–323. [Google Scholar]
- Pedersen, F.B.; Hrynczuk, J.R.; Scheibel, J.H.; Sorensen, B.L. Urine production and metabolism of glucose and lactic acid in the kidney during 36 hours of cooling and perfusion with diluted plasma. Scand. J. Urol. Nephrol. 1973, 7, 68–73. [Google Scholar] [CrossRef]
- Lundstam, S.; Jagenburg, R.; Jonsson, O.; Lundholm, K.; Naucler, J.; Pettersson, S.; Schersten, T. Metabolism in the hypothermically perfused dog kidney. Incorporation rate of leucine and threonine into proteins. Eur. Surg. Res. 1977, 9, 206–216. [Google Scholar] [CrossRef]
- Lundstam, S.; Jagenburg, R.; Jonsson, O.; Lundholm, K.; Naucler, J.; Pettersson, S.; Schersten, T. Metabolism in the hypothermically perfused dog kidney. Utilization and production of amino acids. Eur. Surg. Res. 1977, 9, 191–205. [Google Scholar] [CrossRef]
- Pegg, D.E.; Wusteman, M.C.; Foreman, J. Metabolism of normal and ischemically injured rabbit kidneys during perfusion for 48 hours at 10 C. Transplantation 1981, 32, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Verkh, L.; Freier, D.T.; Celik, C. Changes in concentration of amino acids and other metabolites during hypothermic perfusion of the canine kidney. Cryobiology 1986, 23, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; Baldassare, M.; Vasuri, F.; Pasquinelli, G.; Laggetta, M.; Valente, S.; De Pace, V.; Neri, F.; Siniscalchi, A.; Zanfi, C.; et al. Strategies to Restore Adenosine Triphosphate (ATP) Level After More than 20 Hours of Cold Ischemia Time in Human Marginal Kidney Grafts. Ann. Transplant. 2018, 23, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Lundstam, S.; Claes, G.; Jonsson, O.; Naucler, J.; Pettersson, S.; Schersten, T. metabolic studies on kidney in hypothermic perfusion. J. D. Urol. Et. De. Nephrol. 1975, 81, 716–720. [Google Scholar]
- Lundstam, S.; Claes, G.; Jonsson, O.; Pettersson, S.; Schersten, T. Metabolism in the hypothermically perfused kidney. Production and utilization of lactate and utilization of acetate in the dog kidney. Eur. Surg. Res. 1976, 8, 300–310. [Google Scholar] [CrossRef]
- Pettersson, S.; Claes, G.; Schersten, T. Fatty acid and glucose utilization during continuous hypothermic perfusion of dog kidney. Eur. Surg. Res. 1974, 6, 79–94. [Google Scholar] [CrossRef]
- Skrede, S.; Slaattelid, O. Fatty acid metabolism during hypothermic perfusion of the isolated dog kidney. Scand. J. Clin. Lab. Invest. 1979, 39, 765–771. [Google Scholar] [CrossRef]
- Lazeyras, F.; Buhler, L.; Vallee, J.P.; Hergt, M.; Nastasi, A.; Ruttimann, R.; Morel, P.; Buchs, J.B. Detection of ATP by “in line” 31P magnetic resonance spectroscopy during oxygenated hypothermic pulsatile perfusion of pigs’ kidneys. Magn. Reson. Mater. Phys. Biol. Med. 2012, 25, 391–399. [Google Scholar] [CrossRef]
- Boudjema, K.; Lindell, S.L.; Southard, J.H.; Belzer, F.O. Changes in glutathione concentration in hypothermically perfused dog kidneys. J. Lab. Clin. Med. 1991, 117, 131–137. [Google Scholar]
- Southhard, J.H.; Kuniyoshi, M.; Lutz, M.F.; Ametani, M.; Belzer, F.O. Comparison of the effect of 3- and 5-day hypothermic perfusion of dog kidneys on metabolism of tissue slices. Cryobiology 1984, 21, 285–295. [Google Scholar] [CrossRef]
- Kleist, H.; Jonsson, O.; Lundstam, S.; Naucler, J.; Pettersson, S.; Schersten, T. Metabolism in the hypothermically perfused kidney: Utilization of mevalonate in the human and in the dog kidney. Eur. Surg. Res. 1982, 14, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Southard, J.H.; Ametani, M.S.; Lutz, M.F.; Belzer, F.O. Effects of hypothermic perfusion of kidneys on tissue and mitochondrial phospholipids. Cryobiology 1984, 21, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Southard, J.H.; Lutz, M.F.; Ametani, M.S.; Belzer, F.O. Stimulation of ATP synthesis in hypothermically perfused dog kidneys by adenosine and PO4. Cryobiology 1984, 21, 13–19. [Google Scholar] [CrossRef]
- Collste, H.; Bergström, J.; Hultman, E.; Melin, B. ATP in the cortex of canine kidneys undergoing hypothermic storage. Life Sci. 1971, 10, 1201–1206. [Google Scholar] [CrossRef]
- Kahng, M.W.; Trifillis, A.L.; Hall-Craggs, M.; Regec, A.; Trump, B.F. Biochemical and morphological studies on human kidneys preserved for transplantation. Am. J. Clin. Pathol. 1983, 80, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Baicu, S.C.; Simmons, P.M.; Campbell, L.H.; Taylor, M.J.; Brockbank, K.G. Interstitial fluid analysis for assessment of organ function. Clin. Transplant. 2004, 18 (Suppl. S12), 16–21. [Google Scholar] [CrossRef]
- Baicu, S.C.; Taylor, M.J.; Brockbank, K.G. Modulating biochemical perturbations during 72-hour machine perfusion of kidneys: Role of preservation solution. Cryobiology 2007, 54, 114–120. [Google Scholar] [CrossRef]
- Nath, J.; Smith, T.; Hollis, A.; Ebbs, S.R.; Canbilen, S.W.; Tennant, D.A.; Ready, A.R.; Ludwig, C. 13 C glucose labelling studies using 2D NMR are a useful tool for determining ex vivo whole organ metabolism during hypothermic machine perfusion of kidneys. Transplant. Res. 2016, 5, 7. [Google Scholar] [CrossRef]
- Nath, J.; Smith, T.B.; Patel, K.; Ebbs, S.R.; Hollis, A.; Tennant, D.A.; Ludwig, C.; Ready, A.R. Metabolic differences between cold stored and machine perfused porcine kidneys: A (1)H NMR based study. Cryobiology 2017, 74, 115–120. [Google Scholar] [CrossRef]
- Hamaoui, K.; Gowers, S.; Damji, S.; Rogers, M.; Leong, C.L.; Hanna, G.; Darzi, A.; Boutelle, M.; Papalois, V. Rapid sampling microdialysis as a novel tool for parenchyma assessment during static cold storage and hypothermic machine perfusion in a translational ex vivo porcine kidney model. J. Surg. Res. 2016, 200, 332–345. [Google Scholar] [CrossRef]
- Huang, J.S.; Downes, G.L.; Belzer, F.O. Utilization of fatty acids in perfused hypothermic dog kidney. J. Lipid Res. 1971, 12, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Smith, T.B.; Neil, D.A.H.; Thakker, A.; Tsuchiya, Y.; Higgs, E.B.; Hodges, N.J.; Ready, A.R.; Nath, J.; Ludwig, C. The Effects of Oxygenation on Ex Vivo Kidneys Undergoing Hypothermic Machine Perfusion. Transplantation 2019, 103, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Buchs, J.B.; Lazeyras, F.; Ruttimann, R.; Nastasi, A.; Morel, P. Oxygenated hypothermic pulsatile perfusion versus cold static storage for kidneys from non heart-beating donors tested by in-line ATP resynthesis to establish a strategy of preservation. Perfusion 2011, 26, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, A.; Klauser, A.; Songeon, J.; Agius, T.; Nastasi, A.; Ruttiman, R.; Moll, S.; Meier, R.P.H.; Buhler, L.; Corpataux, J.M.; et al. Ex Vivo Analysis of Kidney Graft Viability Using 31P Magnetic Resonance Imaging Spectroscopy. Transplantation 2020, 104, 1825–1831. [Google Scholar] [CrossRef]
- Martinez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- van de Poll, M.C.G.; Soeters, P.B.; Deutz, N.E.P.; Fearon, K.C.H.; Dejong, C.H.C. Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am. J. Clin. Nutr. 2004, 79, 185–197. [Google Scholar] [CrossRef]
- Pitts, R.F.; Stone, W.J. Renal metabolism of alanine. J. Clin. Invest. 1967, 46, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Husen, P.; Boffa, C.; Jochmans, I.; Krikke, C.; Davies, L.; Mazilescu, L.; Brat, A.; Knight, S.; Wettstein, D.; Cseprekal, O.; et al. Oxygenated End-Hypothermic Machine Perfusion in Expanded Criteria Donor Kidney Transplant: A Randomized Clinical Trial. JAMA Surg. 2021, 156, 517–525. [Google Scholar] [CrossRef]
- Giraud, S.; Favreau, F.; Chatauret, N.; Thuillier, R.; Maiga, S.; Hauet, T. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: The preclinical model. J. Biomed. Biotechnol. 2011, 2011, 532127. [Google Scholar] [CrossRef]
- Lieberthal, W.; Nigam, S.K. Acute renal failure. II. Experimental models of acute renal failure: Imperfect but indispensable. Am. J. Physiol. Renal Physiol. 2000, 278, F1–F12. [Google Scholar] [CrossRef]
- Karangwa, S.A.; Dutkowski, P.; Fontes, P.; Friend, P.J.; Guarrera, J.V.; Markmann, J.F.; Mergental, H.; Minor, T.; Quintini, C.; Selzner, M.; et al. Machine Perfusion of Donor Livers for Transplantation: A Proposal for Standardized Nomenclature and Reporting Guidelines. Am. J. Transplant 2016, 16, 2932–2942. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.F. Removal of Fatty Acids from Serum Albumin by Charcoal Treatment. J. Biol. Chem. 1967, 242, 173–181. [Google Scholar] [CrossRef] [PubMed]

| Reference | Severity Injury | O2 | Metabolites Provided in Perfusion Solution at Start | Findings |
|---|---|---|---|---|
| Carbohydrate metabolism | ||||
| Alexander 1970 [17] | Minimal | Yes | acid citrate dextrose, no extra Gluc | P: = in lactate; + in pyruvate; + in lactate/pyruvate ratio (first 30 min) then return to normal |
| Grundmann 1972 [29] | Minimal | Yes | dextrose | P: + in dextrose (10%); lactate/pyruvate ratio: − (first hours), plateau, + (after 24 h)) |
| Pedersen 1973 [40] | Minimal | Yes/No | Gluc | P: = in Gluc (similar with/without O2); − in lactate (greater decrease with O2) |
| Grundmann 1974 [23] | Minimal | Yes | dextrose | P: + in lactate/pyruvate ratio |
| Kahng 1983 [57] | Injured | Yes | Gluc | T: lactate highest measured metabolite; lactate/pyruvate ratio +/− 70 |
| Pegg 1984 [25] | Minimal/ Injured | Yes | Gluc, mann, caprylate, protein | P: − in Gluc; + in lactate; + in pyruvate |
| Verkh 1986 [44] | Minimal | Yes | dextrose | P: − in Gluc; + in lactate; + in pyruvate; lactate/pyruvate ratio increased by 11.3% |
| Amino Acid metabolism | ||||
| Verkh 1986 [44] | Minimal | Yes | dextrose | P: + in Ala, Glu, Ser, Gly, Val, Thr, (iso)Leu, Met, Asp, Phe, Lys, (-OH)Pro, Tyr, Arg, His |
| Fatty Acid metabolism | ||||
| Huang 1971 [63] | Minimal | Yes | acid citrate dextrose, mann, sodium oleate | P: no oleate: −25% lipids (TG, less phospholipids); with oleate: − in TG but less vs. no oleate T: no oleate: −35% lipids (neutral lipids and TG − most, total phospholipids − by only 27%); with oleate: phospholipids − by only 8% |
| Grundmann 1972 [29] | Minimal | Yes | dextrose | P: − in unesterified FA |
| Energy metabolism | ||||
| Collste 1971 [56] | Minimal/ Injured | Yes | acid citrate dextrose, dextrose | T: minimal injury: constant + in ATP level; injury: ATP − during warm ischemia and partial restoration of ATP with perfusion |
| Kahng 1983 [57] | Injured | Yes | Gluc | T: variable nucleotide contents with wide ranges in each nucleotide |
| Pegg 1984 [25] | Injured | Yes | Gluc, mann, caprylate, protein | T: no restoration of adenine nucleotides |
| Reference | Severity Injury | O2 | Metabolites Provided in Perfusion Solution at Start | Findings |
|---|---|---|---|---|
| Carbohydrate metabolism | ||||
| Pegg 1972 [34] | Minimal | Yes | Gluc | P: = in Gluc; no measurable + pyruvate; lactate levels remained below 0.5 mM |
| Grundmann 1974 [23] | Minimal | Yes | FA-rich (human albumin), (labeled) Gluc, acetate, lactate | P: + in lactate/pyruvate ratio; perfusate exchange |
| Pettersson 1974 [48] | Minimal | Yes | Gluc-rich (Gluc), octanoate | P: − in Gluc; + in lactate and lactate/pyruvate ratio Label (P): Gluc label mainly seen in lactate and to a low extent in glycogen and CO2 |
| Lundstam 1975 [46] | NA | Yes | (labeled) Gluc, linoleate, palmitate, myristic acid, caprylate | P: − in Gluc; + in lactate (lower in FA-free with higher Gluc oxidation) Label (P): modest incorporation of Gluc label in CO2, similar findings human and dog |
| Slaattelid 1976(2) * [27] | Minimal | Yes | Gluc-free (mann) | P: + in Gluc if animal received Gluc infusion before procurement; T: − in Gluc, − in renal glycogen, higher glucose when Gluc infusion before procurement |
| Slaattelid 1976(2) * [27] | Minimal | Yes | (labeled) Gluc, linoleate, palmitate, caprylate | P: − in Gluc; + in lactate; Label (P): − in labeled Gluc with modest (3%) metabolization to lactate and CO2; T: high concentration of Gluc |
| Lundstam 1976 * [47] | Minimal | Yes | FA-free (bovine albumin), (labeled) Gluc, lactate | P: − in Gluc (mainly initially) + in lactate (lowest in FA free perfusate) Label (P): incorporation of Gluc carbon into CO2 (greater in FA-free perfusate the first 3 days); − in labeled lactate with recovery in CO2 and Gluc |
| Lundstam 1976 * [47] | Minimal | Yes | FA-rich (human albumin), (labeled) Gluc, acetate and lactate | P: constant − in Gluc; + in lactate (most pronounced with acetate, FA-rich perfusate) Label (P): incorporation of Gluc carbon into CO2 (less in FA-rich perfusate) − in labeled lactate with recovery in CO2 and Gluc |
| Lundstam 1977(2) * [42] | Minimal | Yes | AA (labeled Gluc) | P: slight − in Gluc; + in lactate (first 4 days), plateau or decrease hereafter; Label (P): − in specific activity Gluc |
| Lundstam 1977(2) * [42] | Minimal | Yes | no AA, (labeled) Gluc, caprylate, cycloleucine | P: greater − in Gluc; continuous + in lactate |
| Fischer 1979 [38] | Minimal | Yes | dextrose | T: − in Gluc (non-significant); no + in lactate |
| Fischer 1980 [37] | Minimal | Yes | Gluc, caprylate | T: significant − in Gluc; stable lactate levels up to 72 h with + thereafter |
| Kahng 1983 [57] | Injured | Yes | Gluc | T: lactate highest measured metabolite; lactate/pyruvate ratio +/− 70 |
| Amino Acid metabolism | ||||
| Lundstam 1977(1) [41] | Minimal | Yes | Gluc, 17 L-AA *, (labeled) leucine, threonine | P: − in Leu, slight + in Thr; Label (P): − in specific activity Leu and Thr; Label (T): carbon incorporation Leu > Thr in protein, Carbon Leu >> Thr incorporation in CO2 |
| Lundstam 1977(2) * [42] | Minimal | Yes | 17 L-AA *,(labeled) Gluc, caprylate, cycloleucine | P: rapid − in Gln, Pro, Gly, Asp, Arg, slower − in (iso)Leu, Met, Val; − in Ser; = His, Cys, Lys, Phe; + for Thr, Tyr, Orn, Ala, taurine, ammonia and urea, + in Glu first days, − after 4th day |
| Lundstam 1977(2) * [42] | Minimal | Yes | no AA, (labeled) Gluc, caprylate, cycloleucine | P: + in almost all AA and ammonia (most pronounced for Ala and taurine) no + for Gln, Pro, Asp, Cys; + in nitrogen |
| Fatty acid metabolism | ||||
| Pettersson 1974 [48] | Minimal | Yes | (labeled) Gluc, linoleate, palmitate, myristic acid, caprylate | P: − in FFA (fast − in short-chain FA (caprylate, after 2 days − in middle-chain FA (laureate and myristic acid), slow − in long-chain FA (palmitate, oleate, linoleate, stearate); Label (P): high incorporation of caprylate label in Gluc and lactate; T: = in TG and phospholipids; − cholesterol after 6 days; Label (T): caprylate mostly oxidized to CO2 until day 4, then + oxidation of myristic acid to CO2; low incorporation of labeled palmitate into CO2; labeled linoleate, palmitate and myristic acid incorporated mainly in phospholipids and TG |
| Lundstam 1975 [46] | NA | Yes | (labeled) Gluc, linoleate, palmitate, caprylate | P: fast − in FA (fast − caprylate, slow followed by fast − (after 2–4 days) for laureate and myristic acid and slow − in palmitate, oleate and linoleate); Label (P): fast incorporation of caprylate in CO2 (first 3 days), from day 4 faster incorporation of myristic acid label in CO2, low incorporation of label from long-chain FA in CO2 |
| Halasz 1975 [24] | Minimal | Yes | mann, defatted albumin | P: + in FFA |
| Lundstam 1976 * [47] | Minimal | Yes | FA-free (bovine albumin), (labeled) Gluc, lactate | T: − in phospholipids |
| Lundstam 1976 * [47] | Minimal | Yes | FA-rich (human albumin), (labeled) Gluc, acetate and lactate | Label (P): − in labeled acetate with incorporation in CO2 (first 2–4 days), Gluc and lactate; T: = in cholesterol |
| Slaattelid 1976(1) * [26] | Minimal | Yes | FFA-rich, (labeled) palmitate, Gluc | P: fast linear − in FFA; Label (P): slow − in labeled palmitate |
| Slaattelid 1976(1) * [26] | Minimal | Yes | FFA-poor, (labeled) Gluc | P: low FFA was remained |
| Slaattelid 1976(2) [27] | Minimal | Yes | Gluc-free (mann)/Gluc-rich (labeled Gluc) | P: − in FFA |
| Lundstam 1977(2) [42] | Minimal | Yes | 17 L-AA **/no AA,(labeled) Gluc, caprylate, cycloleucine | P: higher − in FA with AA; rapid − in caprylate (depleted day 3–4); Label (P): high incorporation of labeled caprylate in CO2 |
| Fischer 1979 [38] | Minimal | Yes | Gluc, caprylate | P: first 24 h: − in caprylate; no − in long-chain FFA (+ in palmitate and oleate, no − in stearate and linoleate); after 24 h: depletion of caprylate and − in long-chain FFA |
| Skrede 1979 [49] | Minimal | Yes | Gluc, labeled palmitate and linoleate, caprylate | P: − in caprylate, + in all long-chain FA and arachidonic acid; Label (P): only traces of palmitate and linoleate label in CO2; T: 10% − in total phospholipids, − in all FA except arachidonic acid, = in cholesterol and TG; Label (T): palmitate and linoleate label in tissue lipids (higher in phospholipids than TG) |
| Kleist 1982 [53] | Minimal | Yes | Gluc, 17 L-AA ** | Label (P): − in mevalonate label, only small amounts in CO2; T: no − in cholesterol when mevalonate added; Label (T): mevalonate label in total lipid fraction of kidney cortex, (80% recovered in cholesterol and cholesterol precursors and 20% in FA containing lipids) |
| Energy metabolism | ||||
| Collins 1977 * [28] | Minimal | Yes | mann | T: = in TAN levels during 72 h perfusion |
| Collins 1977 * [28] | Injured | Yes | mann | T: warm ischemia: − in ATP, ADP and TAN (first 15′ + in AMP, thereafter −); perfusion: regeneration of ATP and rise in energy charge (ATP+ 1/2 ADP/TAN) to near normal within first hour (no significant regeneration of TAN) |
| Fischer 1980 [37] | Minimal | Yes | Gluc-rich (Gluc), octanoate | T: − in TAN and ATP; energy charge was optimal in both groups, significantly lower without Gluc after 72 h) |
| Kahng 1983 [57] | Injured | Yes | Gluc | T: variable nucleotide contents with wide ranges in each nucleotide |
| Reference | Severity Injury | O2 | Metabolites Provided in Perfusion Solution at Start | Findings |
|---|---|---|---|---|
| Carbohydrate metabolism | ||||
| Fischer 1980 [37] | Minimal | Yes | Gluc-free (mann) | T: low Gluc and slight − in Gluc; stable lactate levels (+/− zero) without + |
| Pegg 1981 [43] | Minimal/ Injured | Yes | Haemaccel-based; Gluc (+/− caprylate, acetate, pyruvate) | P: − Gluc utilization with acetate/pyruvate, − Gluc with caprylate, less lactate accumulation with higher pO2 and/or hypoxanthine, + in lactate and pyruvate with caprylate |
| Pegg 1984 [25] | Injured | Yes | Haemaccel-based; Gluc, mann, caprylate, protein | P: − in Gluc; + in lactate; + in pyruvate |
| Baicu 2004 [58] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine), +/− fructose-1,6-biphosphate (FDP) | P: = in Gluc; = in pyruvate (constant low value) Interstitial fluid (micro dialysis): + in pyruvate (higher concentrations FDP- kidneys) |
| Baicu 2006 * [59] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: = in Gluc, perfusate replacement every 24 h |
| Baicu 2006 * [59] | Injured | No | Unisol-UHK, sucrose, mann, Gluc, GSH, adenosine | P: − in Gluc (perfusate replacement every 24 h) |
| Bon 2014 [18] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in lactate |
| Nath 2014 [8] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: = in Gluc; + in lactate; = in mann and rib; pig and human comparable |
| Guy 2015 [15] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in Gluc; + in lactate; = in mann and rib |
| Nath 2016(1) [60] | Injured | No | MPS ((labeled)Gluc, mann, rib, GSH, adenine) | P: + in lactate; Label (P): + in labeled lactate from labeled Gluc; Label (T): labeled lactate present in kidney cortex |
| Nath 2016(2) [61] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in lactate; T: lactate in HMP was lower than at start; Total metabolite amount (T + P): + in lactate |
| Hamaoui 2016 [62] | Injured | NA | Belzer-UWMP, raffinose, GSH, adenosine | P: + in Gluc levels (first 3 h), − thereafter; + in lactate Micro dialysis: + in cortical lactate (after 1.5 h) |
| Ravaioli 2018 [45] | Injured | No/Yes | Celsior, mann, glutamate, histidine, GSH | P: + in lactate |
| Darius 2018 [19] | Injured | No/Yes | MPS (Gluc, mann, rib, GSH, adenine) | P: + in Gluc (no difference O2 vs. non- O2) |
| Patel 2019 [64] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) + (labeled)Gluc | Label (P): + in labeled lactate from labeled Gluc (less + in O2 vs. steady + in air); Label (T): lower cortical concentrations of labeled lactate in O2 vs. air. |
| Darius 2020(1) [21] | Injured | Yes/No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in lactate and + in mann (in groups with pre-or end-O2) (all O2 strategies resulted in lower concentrations of lactate) no differences in perfusate Gluc between O2 conditions; T: = in lactate |
| Darius 2020(2) [22] | Injured | Yes/No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in Gluc (independent of O2 content); lower lactate in HMPO2 high vs. HMP; T: + in lactate |
| Darius 2020(3) [20] | Injured | Yes/No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in Gluc (independent of O2 content); T: = in lactate |
| Faucher 2022 [16] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: = in Gluc and mann; + in lactate; − in rib |
| Mrakic-Sposta 2023 [35] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in lactate; T: + in lactate |
| Amino Acid metabolism | ||||
| Boudjema 1991 [51] | Minimal | Yes | UWMP(Gluc, GSH, raffinose, rib, adenine, adenosine) | P: reduced GSH disappeared from perfusate in 24 h; T: loss of GSH from the cortex tissue, if reduced GSH administered then less GSH loss in tissue; adding glycine, glutamate, cysteine stimulated GSH synthesis while GSSG did not prevent GSH loss. |
| Baicu 2004 [58] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine), +/− fructose-1,6-biphosphate (FDP) | P: + in Glu |
| Baicu 2006 * [59] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in Glu and ammonia (the first hours) |
| Baicu 2006 * [59] | Injured | No | Unisol-UHK, sucrose, mann, Gluc, GSH, adenosine | P: Baseline Gln detected; + in Glu (higher in UHK than Belzer MPS) + in NH4+ (higher in UHK than Belzer MPS) |
| Bon 2014 [18] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in Val, Ala, Gly, Glu |
| Nath 2014 [8] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in Gly, Glu, Ala, (iso)Leu, Val; = in Tyr; − in GSH |
| Guy 2015 [15] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in Ala, Gly, Glu, (iso)Leu, Tyr, Val; − in reduced GSH; GSSG not detected |
| Nath 2016(1) [60] | Injured | No | MPS ((labeled)Gluc, mann, rib, GSH, adenine) | P: + in Ala; Label (P): + in labeled Ala from labeled Gluc; Label (T): labeled Ala from Gluc present in kidney cortex, labeled Glu in small amounts (<0.5% of total Glu) |
| Nath 2016(2) [61] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in Ala, Glu; − in reduced GSH; T: Absence of reduced GSH Total metabolite amount (T + P): + in Glu, Ala, Asp, Leu, Tyr |
| Patel 2019 [64] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) + (labeled)Gluc | P: Ala (+ air/= O2), Asp (= air/+ O2), Glu (= air/− O2), Glyl (= air/O2); − in GSH (in both oxygenation conditions); Label (P): + in labeled Ala (reduction in + in O2 vs. steady + in air); T: higher concentrations of Asp, Tyr, Val, Gly; Ala in cortex in O2 vs. air, lower Gllu in cortex in O2 vs. air; higher cortex GSH in O2 vs. air; Label (T): higher concentrations of [4,5-13C] Glu in cortex in O2 vs. air |
| Darius 2020(1) [21] | Injured | Yes/No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in Ala, Asp, Gly, (iso)Leu, Glu; − in GSH (pre/end O2); T: − in Glu (lower in O2) |
| Darius 2020(2) [22] | Injured | Yes/No | MPS (Gluc, mann, rib, GSH, adenine) | P: GSH (no difference between O2 conditions) T: = in Glu (lower in O2) |
| Darius 2020(3) [20] | Injured | Yes/No | MPS (Gluc, mann, rib, GSH, adenine) | T: − in Glu (lower when O2 was added for 2 h by membrane oxygenator than with bubble and intermittent surface oxygenation or when no O2 was given) |
| Faucher 2022 [16] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in Trp, Asp, Ser, Gly, Thr, Glu, Ala, (Orn), Pro, Lys, His, Arg, Val, Met, Tyr, (iso)Leu, Phe, taurine; + in AA correlated with perfusion duration; = in GSH (reduced form from MPS); − in GSSG |
| Mrakic-Sposta 2023 [35] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in Val, Ala; − in total GSH levels; T: + in Val, Ala |
| Fatty acid metabolism | ||||
| Southard 1984(1) [54] | Minimal | Yes | Gluconate based; Gluc, GSH | T: − in phospholipids (first 24 h) thereafter +; isolated mitochondria: initial − in phospholipids with + after 3 days (FFA show gradual +) |
| Nath 2014 [8] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: = in acetate (human + pig); + (human), = (pig) in 3-hydroxybutyrate |
| Guy 2015 [15] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: = in acetate; + in the ketone body 3-hydroxybutyrate |
| Nath 2016(1) [60] | Injured | No | MPS ((labeled)Gluc, mann, rib, GSH, adenine) | P: = in acetate; Label (P): = in labeled acetate from labeled Gluc (concentration = 1.25% at all time points) |
| Nath 2016(2) [61] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | Total metabolite amount (T + P): + in acetate |
| Patel 2019 [64] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) + (labeled)Gluc | P: − air = O2 in acetate; T: higher cortical concentration of acetate in O2 |
| Darius 2020(1) [21] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) | P: − in acetate (pre-or end-O2) |
| Darius 2020(2) [22] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) | P: acetate (lower in O2 groups) |
| Mrakic-Sposta 2023 [35] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in acetate |
| Energy metabolism | ||||
| Fischer 1980 [37] | Minimal | Yes | Gluc-free (mann) | T: − in TAN and ATP; energy charge (EC= ATP +1/2 ADP/TAN) optimal in both groups, (EC lower in the group without Gluc after 72 h) |
| Pegg 1981 [43] | Minimal/ Injured | Yes | Haemaccel-based; Gluc (+/− caprylate, acetate, pyruvate) | T: − in TAN, ATP, ATP/ADP ratio with WI (high-energy phosphate stores depleted when Gluc was sole energy source and pO2 150 mmHg) When O2 tension was 600 mmHg and with Gluc, caprylate, hypoxanthine added, 5′nucleotide adenine levels maintained close to normal values; with 60′ WIT: total AN level was restored to normal (only the ATP/ADP ratio was depressed) after 48 h of perfusion |
| Pegg 1984 [25] | Injured | Yes | Haemaccel-based; Gluc, mann, caprylate, protein | T: significant AN restoration |
| Southard 1984(2) [55] | Minimal | Yes | Gluconate based; Gluc, GSH, no/adenosine | P: − in adenosine; T: − in ATP (loss can be prevented by including both adenosine (10 mM) and PO4 (25 mM) |
| Southard 1984(3) [52] | Minimal | Yes | Gluconate based, Gluc, GSH, adenosine | T: higher ATP content in cortex tissue after 3 days perfusion than control (perfusion with adenosine and PO4), concentration of ATP in cortex tissue from 5-day perfused kidneys was less than control |
| McAnulty 1988 [32] | Minimal | Yes | Gluconate based, Gluc, GSH, rib + adenine/adenosine | P: almost complete degradation of adenosine (5 d); + in hypoxanthine and inosine; only 10% loss of adenine (no large + in purine end products); T: higher ATP and TAN in cortical tissue in adenine/rib kidneys than in adenosine kidneys after 5 days |
| Minor 2005 * [33] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) | T: improved energy status with O2 perfusion with Belzer (2.43 +- 0.23 µmol ATP/g) vs. HTK (1.18 +- 0.12 µmol ATP/g) |
| Minor 2005 * [33] | Injured | Yes | HTK (mann, histidine, tryptophan | T: improved energy status with O2 perfusion with Belzer (2.43 +- 0.23 µmol ATP/g) vs. HTK (1.18 +- 0.12 µmol ATP/g) |
| La Manna 2009 [31] | Injured | NA | Belzer solution, not further specified | T: − in ATP levels |
| Buchs 2011 [65] | Minimal/ Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) | T: + in ATP (only with O2 perfusion); − in ATP with WI |
| Lazeyras 2012 [50] | Minimal | Yes | MPS (Gluc, mann, rib, GSH, adenine) | T: ATP depletion during cold storage with almost complete ATP recovery during cold perfusion; ATP detection only with higher O2 concentrations |
| Nath 2014 [8] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in hypoxanthine and inosine; = in adenine |
| Guy 2015 [15] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in inosine and hypoxanthine; = in adenine |
| Nath 2016(2) [61] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | Total metabolite amount (T + P): + in hypoxanthine; − in inosine |
| Ravaioli 2018 [45] | Injured | No/Yes | Celsior, mann, glutamate, histidine, GSH, ketoglutarate) | T: − in ATP (non-O2 perfusion); + in ATP (O2 and hyperbaric perfusion); ATP higher in O2 and hyperbaric vs. non-O2 perfusion |
| Patel 2019 [64] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) + (labeled)Gluc | T: higher ATP, ADP in cortex in O2 vs. air; AMP comparable between O2 vs. air; no difference in adenosine between O2 and air |
| Kaminski 2019 [30] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | T: decrease in ATP with 60′ WI; + in ATP with hypothermic perfusion after 60′ WI |
| Venema 2019 [36] | Injured | No/Yes | MPS (Gluc, mann, rib, GSH, adenine) | T: ATP depletion after 30′ WI; no + in ATP during 24 h non-O2 perfusion; + in ATP during 24 h O2 (21% or 100%) perfusion |
| Darius 2020(1) [21] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) | P: + in hypoxanthine; = in adenine (pre- or end O2); T: + in ATP and ADP in pre-O2 group; − in AMP in all groups; no differences in AMP in O2 vs. non-O2 group |
| Darius 2020(2) [22] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) | P: no difference in adenine, hypoxanthine with different O2 conc; T: + in ATP and ADP; = in AMP; no differences in ATP, ADP and AMP with different O2 conc |
| Darius 2020(3) [20] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) | T: + in ATP and ADP in O2 groups; − in AMP; ATP higher in O2 vs. no O2; no difference in ADP, AMP in O2 vs. non- O2 |
| Longchamp 2020 [66] | Min/Inj | Yes | MPS (Gluc, mann, rib, GSH, adenine) | T: ATP + (in the presence of O2); 0′ WI: ATP remained stable up to 22 h of perfusion; − in PME; 60′ WI: − in total ATP but = in PME (containing AMP) |
| Faucher 2022 [16] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in inosine, xanthosine, (hypo)xanthine and adenosine; − in adenine and rib |
| TCA cycle metabolites | ||||
| Nath 2014 [8] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in fumarate; = in citrate |
| Guy 2015 [15] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in citrate |
| Nath 2016(2) [61] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | Total metabolite amount (T + P): + in fumarate, succinate |
| Patel 2019 [64] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) + (labeled)Gluc | P: no difference in fumarate between O2 conditions; T: no difference in fumarate between O2 conditions; Label (T): Higher labeled succinate in O2 vs. air, labeling of citrate and malate in cortex and medulla |
| Darius 2020(1) [21] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) | P: = in succinate (pre- or end O2); T: − in succinate (in O2 group) |
| Darius 2020(2) [22] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) | P: succinate (lower in O2); T: − in succinate (lower in O2 groups) |
| Darius 2020(3) [20] | Injured | Yes | MPS (Gluc, mann, rib, GSH, adenine) | T: − in succinate (lower in 2 h O2 group) |
| Faucher 2022 [16] | Injured | No | MPS (Gluc, mann, rib, GSH, adenine) | P: + in alpha-keto-glutarate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verstraeten, L.; Den abt, R.; Ghesquière, B.; Jochmans, I. Current Insights into the Metabolome during Hypothermic Kidney Perfusion—A Scoping Review. J. Clin. Med. 2023, 12, 3613. https://doi.org/10.3390/jcm12113613
Verstraeten L, Den abt R, Ghesquière B, Jochmans I. Current Insights into the Metabolome during Hypothermic Kidney Perfusion—A Scoping Review. Journal of Clinical Medicine. 2023; 12(11):3613. https://doi.org/10.3390/jcm12113613
Chicago/Turabian StyleVerstraeten, Laurence, Rutger Den abt, Bart Ghesquière, and Ina Jochmans. 2023. "Current Insights into the Metabolome during Hypothermic Kidney Perfusion—A Scoping Review" Journal of Clinical Medicine 12, no. 11: 3613. https://doi.org/10.3390/jcm12113613
APA StyleVerstraeten, L., Den abt, R., Ghesquière, B., & Jochmans, I. (2023). Current Insights into the Metabolome during Hypothermic Kidney Perfusion—A Scoping Review. Journal of Clinical Medicine, 12(11), 3613. https://doi.org/10.3390/jcm12113613