Biological Treatment for Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: Preliminary Real-World Results from a Tertiary Medical Center
Abstract
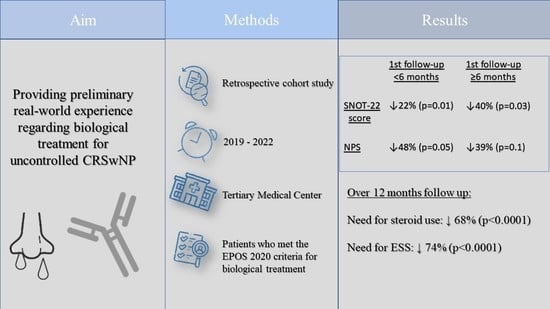
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, A.; Emmanuel, B.; Thomas, K.; Guiang, H. Systematic literature review of the epidemiology and clinical burden of chronic rhinosinusitis with nasal polyposis. Curr. Med. Res Opin. 2020, 36, 1897–1911. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International consensus statement on allergy and rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2021, 11, 213–739. [Google Scholar] [CrossRef] [PubMed]
- Adnane, C.; Adouly, T.; Khallouk, A.; Rouadi, S.; Abada, R.; Roubal, M.; Mahtar, M. Using preoperative unsupervised cluster analysis of chronic rhinosinusitis to inform patient decision and endoscopic sinus surgery outcome. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 879–885. [Google Scholar] [CrossRef]
- DeConde, A.S.; Mace, J.C.; Levy, J.M.; Rudmik, L.; Alt, J.A.; Smith, T.L. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope 2017, 127, 550–555. [Google Scholar] [CrossRef]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef]
- De Corso, E.; Bellocchi, G.; De Benedetto, M.; Lombardo, N.; Macchi, A.; Malvezzi, L.; Motta, G.; Pagella, F.; Vicini, C.; Passali, D. Biologics for severe uncontrolled chronic rhinosinusitis with nasal polyps: A change management approach. Consensus of the Joint Committee of Italian Society of Otorhinolaryngology on biologics in rhinology. ACTA Otorhinolaryngol. Ital. 2022, 42, 1–16. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef]
- Kilty, S.J.; Lasso, A. Canadian real-world study of access and clinical results using dupilumab for chronic rhinosinusitis with polyps. J. Otolaryngol.-Head Neck Surg. 2022, 51, 17. [Google Scholar] [CrossRef]
- De Corso, E.; Settimi, S.; Montuori, C.; Corbò, M.; Passali, G.C.; Porru, D.P.; Lo Verde, S.; Spanu, C.; Penazzi, D.; Di Bella, G.A.; et al. Effectiveness of Dupilumab in the Treatment of Patients with Severe Uncontrolled CRSwNP: A “Real-Life” Observational Study in the First Year of Treatment. J. Clin. Med. 2022, 11, 2684. [Google Scholar] [CrossRef]
- Bachert, C.; Zhang, N.; Cavaliere, C.; Weiping, W.; Gevaert, E.; Krysko, O. Biologics for chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2020, 145, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.Y.; Gevaert, P.; Heffler, E.; Hopkins, C.; Tversky, J.R.; Barker, P.; Cohen, D.; Emson, C.; et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2022, 149, 1309–1317.e12. [Google Scholar] [CrossRef] [PubMed]
- Han, J.K.; Bachert, C.; Fokkens, W.; Desrosiers, M.; Wagenmann, M.; Lee, S.E.; Smith, S.G.; Martin, N.; Mayer, B.; Yancey, S.W.; et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, H.H.; James, L.K. Chronic Rhinosinusitis with Nasal Polyps: Targeting IgE with Anti-IgE Omalizumab Therapy. Drug Des. Dev. Ther. 2020, 14, 5483. [Google Scholar] [CrossRef]
- Bachert, C.; Hellings, P.W.; Mullol, J.; Hamilos, D.L.; Gevaert, P.; Naclerio, R.M.; Joish, V.N.; Chao, J.; Mannent, L.P.; Amin, N.; et al. Dupilumab improves health-related quality of life in patients with chronic rhinosinusitis with nasal polyposis. Allergy 2020, 75, 148–157. [Google Scholar] [CrossRef]
- Fujieda, S.; Matsune, S.; Takeno, S.; Ohta, N.; Asako, M.; Bachert, C.; Inoue, T.; Takahashi, Y.; Fujita, H.; Deniz, Y.; et al. Dupilumab efficacy in chronic rhinosinusitis with nasal polyps from SINUS-52 is unaffected by eosinophilic status. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 186–196. [Google Scholar] [CrossRef]
- Soler, Z.M.; Sauer, D.A.; Mace, J.C.; Smith, T.L. Ethmoid Histopathology does not Predict Olfactory Outcomes after Endoscopic Sinus Surgery. Am. J. Rhinol. Allergy 2010, 24, 281–285. [Google Scholar] [CrossRef]
- Cantone, E.; De Corso, E.; Ricciardiello, F.; Di Nola, C.; Grimaldi, G.; Allocca, V.; Motta, G. Olfaction Recovery following Dupilumab Is Independent of Nasal Polyp Reduction in CRSwNP. J. Pers. Med. 2022, 12, 1215. [Google Scholar] [CrossRef]
- Mace, J.C.; Michael, Y.L.; Carlson, N.E.; Litvack, J.R.; Smith, T.L. Correlations between Endoscopy Score and Quality of Life Changes after Sinus Surgery. Arch. Otolaryngol. Neck Surg. 2010, 136, 340–346. [Google Scholar] [CrossRef]
- Schalek, P.; Hart, L.; Fuksa, J.; Guha, A. Quality of life in CRSwNP: Evaluation of ACCESS and Lund–Mackay computed tomography scores versus the QoL questionnaire. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 5721–5725. [Google Scholar] [CrossRef]
- Hopkins, C.; Gillett, S.; Slack, R.; Lund, V.J.; Browne, J.P. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin. Otolaryngol. 2009, 34, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Galitz, Y.S.; Halperin, D.; Bavnik, Y.; Warman, M. Sino-Nasal Outcome Test–22. Otolaryngol.—Head Neck Surg. 2016, 154, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Lourijsen, E.S.; de Borgie, C.A.J.M.; Vleming, M.; Fokkens, W.J. Endoscopic sinus surgery in adult patients with chronic rhinosinusitis with nasal polyps (PolypESS): Study protocol for a randomised controlled trial. Trials 2017, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.I.; Mace, J.C.; Bodner, T.E.; Alt, J.A.; Deconde, A.S.; Levy, J.M.; Smith, T.L. Investigating the Minimal Clinically Important Difference for SNOT-22 Symptom Domains in Surgically Managed Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2017, 7, 1149. [Google Scholar] [CrossRef]
- Hellings, P.W.; Akdis, C.A.; Bachert, C.; Bousquet, J.; Pugin, B.; Adriaensen, G.; Advani, R.; Agache, I.; Anjo, C.; Anmolsingh, R.; et al. EUFOREA Rhinology Research Forum 2016: Report of the brainstorming sessions on needs and priorities in rhinitis and rhinosinusitis. Rhinol. J. 2017, 55, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, A.; Wormald, P.J. Role of frontal sinus surgery in nasal polyp recurrence. Laryngoscope 2013, 123, 36–41. [Google Scholar] [CrossRef]
- Cai, S.; Xu, S.; Lou, H.; Zhang, L. Comparison of Different Biologics for Treating Chronic Rhinosinusitis with Nasal Polyps: A Network Analysis. J. Allergy Clin. Immunol. Pract. 2022, 10, 1876–1886.e7. [Google Scholar] [CrossRef]
- Haxel, B.R.; Hummel, T.; Fruth, K.; Lorenz, K.; Gunder, N.; Nahrath, P.; Cuevas, M. Real-world-effectiveness of biological treatment for severe chronic rhinosinusitis with nasal polyps. Rhinology 2022, 60, 435–443. [Google Scholar] [CrossRef]
- Dharmarajan, H.; Falade, O.; Lee, S.E.; Wang, E.W. Outcomes of dupilumab treatment versus endoscopic sinus surgery for chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2022, 12, 986–995. [Google Scholar] [CrossRef]



| Demographics, Laboratory Findings, Clinical Features and Background | Whole Sample (n = 38) | Attended the Follow-Up Visits (n = 25) | Did Not Attend Follow-Up Visits (n = 13) |
|---|---|---|---|
| Age, mean (range) | 51.55 (22–81) | 55.28 (22–81) | 44.38 (30–73) |
| Gender (male:female) | 20:18 | 13:12 | 7:6 |
| Samter’s triad | 9 (23%) | 8 (32%) | 1 (7%) |
| Asthma | 34 (89%) | 24 (96%) | 10 (76%) |
| Allergic status | 25 (65%) | 18 (72%) | 7 (53%) |
| Antihistamine/Montelukast usage | 18 (47%) | 13 (52%) | 5 (38%) |
| Steroid nasal spray usage | 27 (71%) | 20 (80%) | 7 (53%) |
| Steroid inhaler usage | 31 (82%) | 23 (92%) | 8 (61%) |
| Blood Eosinophils%, mean ± SD | 8.26 ± 4.53 | 8.33 ± 5.26 | 8.12 ± 2.92 |
| Blood Eosinophils#, mean ± SD | 0.76 ± 0.92 | 0.57 ± 0.45 | 1.14 ± 1.46 |
| Total IgE, mean ± SD | 310 ± 426 | 400 ± 545 | 158 ± 214 |
| Systemic steroid treatment | 38 (100%) | 25 (100%) | 13 (100%) |
| Number of prior Endoscopic Sinus Surgeries, Mean ± SD | 2.13 ± 1.33 | 2.12 ± 1.30 | 2.15 ± 1.46 |
| Months since last surgery, Mean ± SD | 29.6 ± 29.7 | 25.45 ± 20.50 | 37.9 ± 42.6 |
| Endoscopic nasal polyp score, Mean ± SD | 2.1 ± 1.3 | 1.87 ± 1.42 | 2.5 ± 0.9 |
| SNOT-22 score, Mean ± SD | 62.96 ± 25.42 | 56.21 ± 28.33 | 71.54 ± 19.04 |
| Variable | 95% Confidence Interval | Odds Ratio | p Value |
|---|---|---|---|
| Age | −0.3–0.16 | 0.93 | 0.32 |
| Gender | −2.26–4.4 | 2.9 | 0.3 |
| Samter’s triad | −2.71–2.69 | 0.98 | 0.98 |
| Asthma | −9.05–12.0 | 4.38 | 0.6 |
| Antihistamine/Montelukast usage | −3.3–2.89 | 0.81 | 0.8 |
| Steroid nasal spray usage | −2.24–3.84 | 2.21 | 0.37 |
| Steroid inhaler usage | −4.53–4.75 | 1.12 | 0.92 |
| Blood Eosinophils# | −1.39–2.27 | 1.55 | 0.4 |
| Number of prior endoscopic sinus surgeries | −1.54–1.86 | 1.17 | 0.72 |
| Variable | 95% Confidence Interval | Odds Ratio | p Value |
|---|---|---|---|
| Age | −0.13–0.02 | 0.94 | 0.09 |
| Gender | −0.42–1.97 | 2.16 | 0.1 |
| Samter’s triad | −1.07–0.98 | 0.95 | 0.87 |
| Asthma | −2.68–5.42 | 3.93 | 0.28 |
| Antihistamine/Montelukast usage | −1.28–0.99 | 0.86 | 0.64 |
| Steroid nasal spray usage | −0.46–1.71 | 1.86 | 0.13 |
| Steroid inhaler usage | −1.75–1.72 | 0.98 | 0.97 |
| Blood Eosinophils% | −0.01–0.09 | 1.04 | 0.09 |
| Number of prior endoscopic sinus surgeries | −0.55–0.77 | 1.11 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Book, R.; Eligal, S.; Tal, Y.; Eliashar, R. Biological Treatment for Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: Preliminary Real-World Results from a Tertiary Medical Center. J. Clin. Med. 2023, 12, 3671. https://doi.org/10.3390/jcm12113671
Book R, Eligal S, Tal Y, Eliashar R. Biological Treatment for Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: Preliminary Real-World Results from a Tertiary Medical Center. Journal of Clinical Medicine. 2023; 12(11):3671. https://doi.org/10.3390/jcm12113671
Chicago/Turabian StyleBook, Reut, Shalom Eligal, Yuval Tal, and Ron Eliashar. 2023. "Biological Treatment for Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: Preliminary Real-World Results from a Tertiary Medical Center" Journal of Clinical Medicine 12, no. 11: 3671. https://doi.org/10.3390/jcm12113671
APA StyleBook, R., Eligal, S., Tal, Y., & Eliashar, R. (2023). Biological Treatment for Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: Preliminary Real-World Results from a Tertiary Medical Center. Journal of Clinical Medicine, 12(11), 3671. https://doi.org/10.3390/jcm12113671