Intermittent Surface Oxygenation Results in Similar Mitochondrial Protection and Maintenance of Aerobic Metabolism as Compared to Continuous Oxygenation during Hypothermic Machine Kidney Machine Perfusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Anesthetic and Surgical Protocol
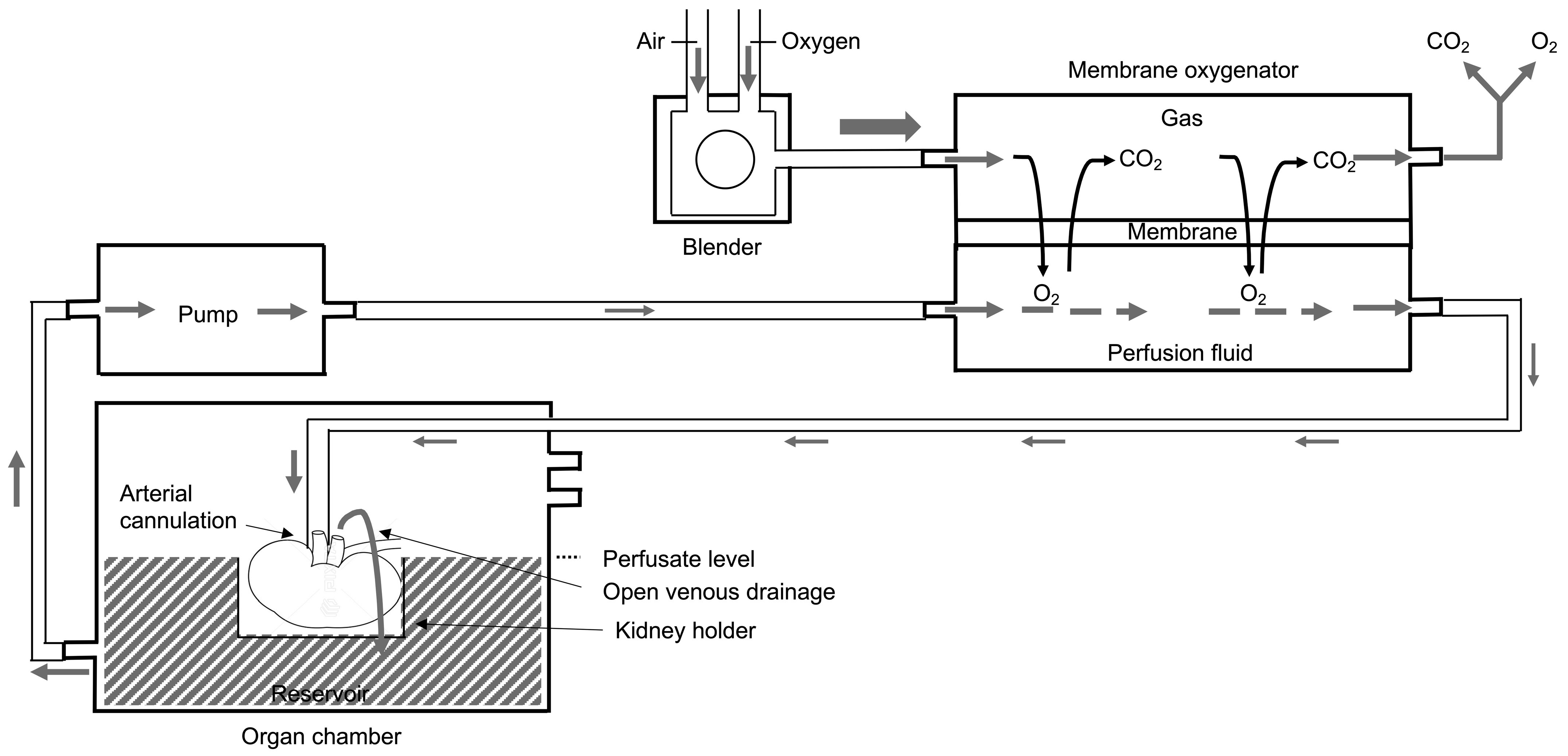
2.4. Machine Perfusion
2.4.1. Membrane Oxygenation
2.4.2. Bubble and Surface Oxygenation
2.5. Outcome Measures
2.6. Samples and Analyses
2.6.1. Oxygen, Renal Resistance, and Flow Measurement during HMP and Perfusate Analyses
2.6.2. Tissue Sampling and Analysis
2.7. Statistical Methods
3. Results
3.1. Operative Data and Adverse Events during HMP
3.2. Efficacy of Oxygen Administration, Oxygen Consumption and Renal Flow during HMP
3.3. Quantification of Metabolite Concentrations in Perfusate
3.4. Quantification of Metabolite Concentrations in Tissue
3.5. Histological Tissue Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hoyer, D.P.; Gallinat, A.; Swoboda, S.; Wohlschlaeger, J.; Rauen, U.; Paul, A.; Minor, T. Influence of oxygen concentration during hypothermic machine perfusion on porcine kidneys from donation after circulatory death. Transplantation 2014, 98, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Gallinat, A.; Paul, A.; Efferz, P.; Luer, B.; Kaiser, G.; Wohlschlaeger, J.; Treckmann, J.; Minor, T. Hypothermic reconditioning of porcine kidney grafts by short-term preimplantation machine perfusion. Transplantation 2012, 93, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Kron, P.; Schlegel, A.; de Rougemont, O.; Oberkofler, C.E.; Clavien, P.A.; Dutkowski, P. Short, Cool, and Well Oxygenated—HOPE for Kidney Transplantation in a Rodent Model. Ann. Surg. 2016, 264, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, R.; Allain, G.; Celhay, O.; Hebrard, W.; Barrou, B.; Badet, L.; Leuvenink, H.; Hauet, T. Benefits of active oxygenation during hypothermic machine perfusion of kidneys in a preclinical model of deceased after cardiac death donors. J. Surg. Res. 2013, 184, 1174–1181. [Google Scholar] [CrossRef]
- Koetting, M.; Frotscher, C.; Minor, T. Hypothermic reconditioning after cold storage improves postischemic graft function in isolated porcine kidneys. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2010, 23, 538–542. [Google Scholar] [CrossRef]
- Darius, T.; Gianello, P.; Vergauwen, M.; Mourad, N.; Buemi, A.; De Meyer, M.; Mourad, M. The effect on early renal function of various dynamic preservation strategies in a preclinical pig ischemia-reperfusion autotransplant model. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2019, 19, 752–762. [Google Scholar] [CrossRef]
- Lazeyras, F.; Buhler, L.; Vallee, J.P.; Hergt, M.; Nastasi, A.; Ruttimann, R.; Morel, P.; Buchs, J.B. Detection of ATP by ‘in line’ 31P magnetic resonance spectroscopy during oxygenated hypothermic pulsatile perfusion of pigs’ kidneys. Magma 2012, 25, 391–399. [Google Scholar] [CrossRef]
- Patel, K.; Smith, T.B.; Neil, D.A.H.; Thakker, A.; Tsuchiya, Y.; Higgs, E.B.; Hodges, N.J.; Ready, A.R.; Nath, J.; Ludwig, C. The Effects of Oxygenation on Ex Vivo Kidneys Undergoing Hypothermic Machine Perfusion. Transplantation 2019, 103, 314–322. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Smith, T.B.; Patel, K.; Craps, J.; Joris, V.; Aydin, S.; Ury, B.; Buemi, A.; De Meyer, M.; et al. Influence of Different Partial Pressures of Oxygen During Continuous Hypothermic Machine Perfusion in a Pig Kidney Ischemia-reperfusion Autotransplant Model. Transplantation 2020, 104, 731–743. [Google Scholar] [CrossRef]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef]
- Ravaioli, M.; De Pace, V.; Angeletti, A.; Comai, G.; Vasuri, F.; Baldassarre, M.; Maroni, L.; Odaldi, F.; Fallani, G.; Caraceni, P.; et al. Hypothermic Oxygenated New Machine Perfusion System in Liver and Kidney Transplantation of Extended Criteria Donors:First Italian Clinical Trial. Sci. Rep. 2020, 10, 6063. [Google Scholar] [CrossRef]
- Husen, P.; Boffa, C.; Jochmans, I.; Krikke, C.; Davies, L.; Mazilescu, L.; Brat, A.; Knight, S.; Wettstein, D.; Cseprekal, O.; et al. Oxygenated End-Hypothermic Machine Perfusion in Expanded Criteria Donor Kidney Transplant: A Randomized Clinical Trial. JAMA Surg. 2021, 156, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Meister, F.A.; Czigany, Z.; Bednarsch, J.; Boecker, J.; Wiltberger, G.; Rohlfs, W.; Neumann, U.P.; Lurje, G. Hypothermic oxygenated machine perfusion-Preliminary experience with end-ischemic reconditioning of marginal kidney allografts. Clin. Transplant. 2019, 33, e13673. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Vergauwen, M.; Smith, T.; Gerin, I.; Joris, V.; Mueller, M.; Aydin, S.; Muller, X.; Schlegel, A.; Nath, J.; et al. Brief O(2) uploading during continuous hypothermic machine perfusion is simple yet effective oxygenation method to improve initial kidney function in a porcine autotransplant model. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2020, 20, 2030–2043. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Mueller, M.; Aydin, S.; Dutkowski, P.; Gianello, P.; Mourad, M. Brief Bubble and Intermittent Surface Oxygenation Is a Simple and Effective Alternative for Membrane Oxygenation During Hypothermic Machine Perfusion in Kidneys. Transplant. Direct 2020, 6, e571. [Google Scholar] [CrossRef]
- Darius, T.; Devresse, A.; Buemi, A.; Kanaan, N.; De Meyer, M.; Mourad, M. First kidneys transplanted in man after brief bubble and subsequent surface oxygenation as alternative for membrane oxygenation during hypothermic machine perfusion. Artif. Organs 2022, 47, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Nath, J.; Mourad, M. Simply Adding Oxygen during Hypothermic Machine Perfusion to Combat the Negative Effects of Ischemia-Reperfusion Injury: Fundamentals and Current Evidence for Kidneys. Biomedicines 2021, 9, 993. [Google Scholar] [CrossRef]
- High, K.S.M.; Bashein, G. Principles of Oxygenator Function: Gas Exchange, Heat Transfer, and Blood-artificial surface interaction. In Cardiopulmonary Bypass, Principles and Practice; Gravlee, P.G., Richard, F.D., Alferd, H.S., Rosss, M.U., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 1993; pp. 29–54. [Google Scholar]
- Bodewes, S.B.; van Leeuwen, O.B.; Thorne, A.M.; Lascaris, B.; Ubbink, R.; Lisman, T.; Monbaliu, D.; De Meijer, V.E.; Nijsten, M.W.N.; Porte, R.J. Oxygen Transport during Ex Situ Machine Perfusion of Donor Livers Using Red Blood Cells or Artificial Oxygen Carriers. Int. J. Mol. Sci. 2020, 22, 235. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. Oxygen Supplementation Supports Energy Production During Hypothermic Machine Perfusion in a Model of Donation After Circulatory Death Donors. Transplantation 2019, 103, 1980–1981. [Google Scholar] [CrossRef]
- Avishay, D.M.; Tenny, K.M. Henry’s Law. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Coulier, L.; Bas, R.; Jespersen, S.; Verheij, E.; van der Werf, M.J.; Hankemeier, T. Simultaneous quantitative analysis of metabolites using ion-pair liquid chromatography-electrospray ionization mass spectrometry. Anal. Chem. 2006, 78, 6573–6582. [Google Scholar] [CrossRef]
- Veiga-da-Cunha, M.; Chevalier, N.; Stephenne, X.; Defour, J.P.; Paczia, N.; Ferster, A.; Achouri, Y.; Dewulf, J.P.; Linster, C.L.; Bommer, G.T.; et al. Failure to eliminate a phosphorylated glucose analog leads to neutropenia in patients with G6PT and G6PC3 deficiency. Proc. Natl. Acad. Sci. USA 2019, 116, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Liapis, H.; Gaut, J.P.; Klein, C.; Bagnasco, S.; Kraus, E.; Farris, A.B., 3rd; Honsova, E.; Perkowska-Ptasinska, A.; David, D.; Goldberg, J.; et al. Banff Histopathological Consensus Criteria for Preimplantation Kidney Biopsies. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2017, 17, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Shah, K.; Patel, M.; Nicholson, M.L. The effect of prolonged of warm ischaemic injury on renal function in an experimental ex vivo normothermic perfusion system. J. Transl. Med. 2015, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H. Oxygen and renal metabolism. Kidney Int. 1997, 51, 381–385. [Google Scholar] [CrossRef]
- Lawson, D.S.; Smigla, G.R.; McRobb, C.M.; Walczak, R.; Kaemmer, D.; Shearer, I.R.; Lodge, A.; Jaggers, J. A clinical evaluation of the Dideco Kids D100 neonatal oxygenator. Perfusion 2008, 23, 39–42. [Google Scholar] [CrossRef]
- Heylen, L.; Jochmans, I.; Samuel, U.; Tieken, I.; Naesens, M.; Pirenne, J.; Sprangers, B. The duration of asystolic ischemia determines the risk of graft failure after circulatory-dead donor kidney transplantation: A Eurotransplant cohort study. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2018, 18, 881–889. [Google Scholar] [CrossRef]
- Hamed, M.O.; Chen, Y.; Pasea, L.; Watson, C.J.; Torpey, N.; Bradley, J.A.; Pettigrew, G.; Saeb-Parsy, K. Early graft loss after kidney transplantation: Risk factors and consequences. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2015, 15, 1632–1643. [Google Scholar] [CrossRef]
- Saidi, R.F.; Elias, N.; Kawai, T.; Hertl, M.; Farrell, M.L.; Goes, N.; Wong, W.; Hartono, C.; Fishman, J.A.; Kotton, C.N.; et al. Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: Realities and costs. Am. J. Transplant. 2007, 7, 2769–2774. [Google Scholar] [CrossRef]
- O’Neill, S.; Srinivasa, S.; Callaghan, C.J.; Watson, C.J.E.; Dark, J.H.; Fisher, A.J.; Wilson, C.H.; Friend, P.J.; Johnson, R.; Forsythe, J.L.; et al. Novel Organ Perfusion and Preservation Strategies in Transplantation—Where Are We Going in the United Kingdom? Transplantation 2020, 104, 1813–1824. [Google Scholar] [CrossRef]
- Bellini, M.I.; Yiu, J.; Nozdrin, M.; Papalois, V. The Effect of Preservation Temperature on Liver, Kidney, and Pancreas Tissue ATP in Animal and Preclinical Human Models. J. Clin. Med. 2019, 8, 1421. [Google Scholar] [CrossRef]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus. Med. Hemotherapy Off. Organ Der Dtsch. Ges. Fur Transfus. Und Immunhamatol. 2011, 38, 125–142. [Google Scholar] [CrossRef]
- Fuller, B.J.; Lee, C.Y. Hypothermic perfusion preservation: The future of organ preservation revisited? Cryobiology 2007, 54, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 2020, 60, 103014. [Google Scholar] [CrossRef] [PubMed]
- Chatauret, N.; Coudroy, R.; Delpech, P.O.; Vandebrouck, C.; Hosni, S.; Scepi, M.; Hauet, T. Mechanistic analysis of nonoxygenated hypothermic machine perfusion’s protection on warm ischemic kidney uncovers greater eNOS phosphorylation and vasodilation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2014, 14, 2500–2514. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhong, Z.; Lan, J.; Li, M.; Wang, W.; Yang, J.; Tang, C.; Wang, J.; Ye, S.; Xiong, Y.; et al. Mechanisms of Hypothermic Machine Perfusion to Decrease Donation After Cardiac Death Graft Inflammation: Through the Pathway of Upregulating Expression of KLF2 and Inhibiting TGF-beta Signaling. Artif. Organs 2017, 41, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Brown, R.J.; Nicholson, M.L. Advances in kidney preservation techniques and their application in clinical practice. Transplantation 2021, 105, e202. [Google Scholar] [CrossRef]
- Bellini, M.I.; Tortorici, F.; Amabile, M.I.; D’Andrea, V. Assessing Kidney Graft Viability and Its Cells Metabolism during Machine Perfusion. Int. J. Mol. Sci. 2021, 22, 1121. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Afford, S.C. Machine perfusion of the liver: Which is the best technique to mitigate ischaemia-reperfusion injury? World J. Transplant. 2019, 9, 14–20. [Google Scholar] [CrossRef]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11, 2939. [Google Scholar] [CrossRef] [PubMed]
- Moers, C.; Varnav, O.C.; van Heurn, E.; Jochmans, I.; Kirste, G.R.; Rahmel, A.; Leuvenink, H.G.; Squifflet, J.P.; Paul, A.; Pirenne, J.; et al. The value of machine perfusion perfusate biomarkers for predicting kidney transplant outcome. Transplantation 2010, 90, 966–973. [Google Scholar] [CrossRef]
- Guzzi, F.; Knight, S.R.; Ploeg, R.J.; Hunter, J.P. A systematic review to identify whether perfusate biomarkers produced during hypothermic machine perfusion can predict graft outcomes in kidney transplantation. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2020, 33, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Wurdinger, M.; Meierhofer, D.; Clavien, P.A.; Dutkowski, P. Novel Real-time Prediction of Liver Graft Function During Hypothermic Oxygenated Machine Perfusion Before Liver Transplantation. Ann. Surg. 2019, 270, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Sousa Da Silva, R.X.; Darius, T.; Mancina, L.; Eden, J.; Wernlé, K.; Ghoneima, A.S.; Barlow, A.D.; Clavien, P.-A.; Dutkowski, P.; Kron, P. Real-time assessment of kidney allografts during HOPE using flavin mononucleotide (FMN)—A preclinical study. Front. Transplant. 2023, 2, 3. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darius, T.; Vergauwen, M.; Maistriaux, L.; Evrard, R.; Schlegel, A.; Mueller, M.; O’Neil, D.; Southam, A.; Aydin, S.; Devresse, A.; et al. Intermittent Surface Oxygenation Results in Similar Mitochondrial Protection and Maintenance of Aerobic Metabolism as Compared to Continuous Oxygenation during Hypothermic Machine Kidney Machine Perfusion. J. Clin. Med. 2023, 12, 3731. https://doi.org/10.3390/jcm12113731
Darius T, Vergauwen M, Maistriaux L, Evrard R, Schlegel A, Mueller M, O’Neil D, Southam A, Aydin S, Devresse A, et al. Intermittent Surface Oxygenation Results in Similar Mitochondrial Protection and Maintenance of Aerobic Metabolism as Compared to Continuous Oxygenation during Hypothermic Machine Kidney Machine Perfusion. Journal of Clinical Medicine. 2023; 12(11):3731. https://doi.org/10.3390/jcm12113731
Chicago/Turabian StyleDarius, Tom, Martial Vergauwen, Louis Maistriaux, Robin Evrard, Andrea Schlegel, Matteo Mueller, Donna O’Neil, Andrew Southam, Selda Aydin, Arnaud Devresse, and et al. 2023. "Intermittent Surface Oxygenation Results in Similar Mitochondrial Protection and Maintenance of Aerobic Metabolism as Compared to Continuous Oxygenation during Hypothermic Machine Kidney Machine Perfusion" Journal of Clinical Medicine 12, no. 11: 3731. https://doi.org/10.3390/jcm12113731




