BILE: A Literature Review Based Novel Clinical Classification and Treatment Algorithm of Iatrogenic Bile Duct Injuries
Abstract
1. Introduction
2. Methods
2.1. Study Protocol
2.2. Eligibility Criteria
2.3. Literature Search
- [Iatrogenic] AND [Bile duct] AND [Injury]
2.4. Study Selection and Data Collection
2.5. Results
3. Intraoperative Diagnosis
4. Clinical Consequences of Bile Duct Injuries
5. Postoperative Imaging Investigation
6. Treatment Strategy
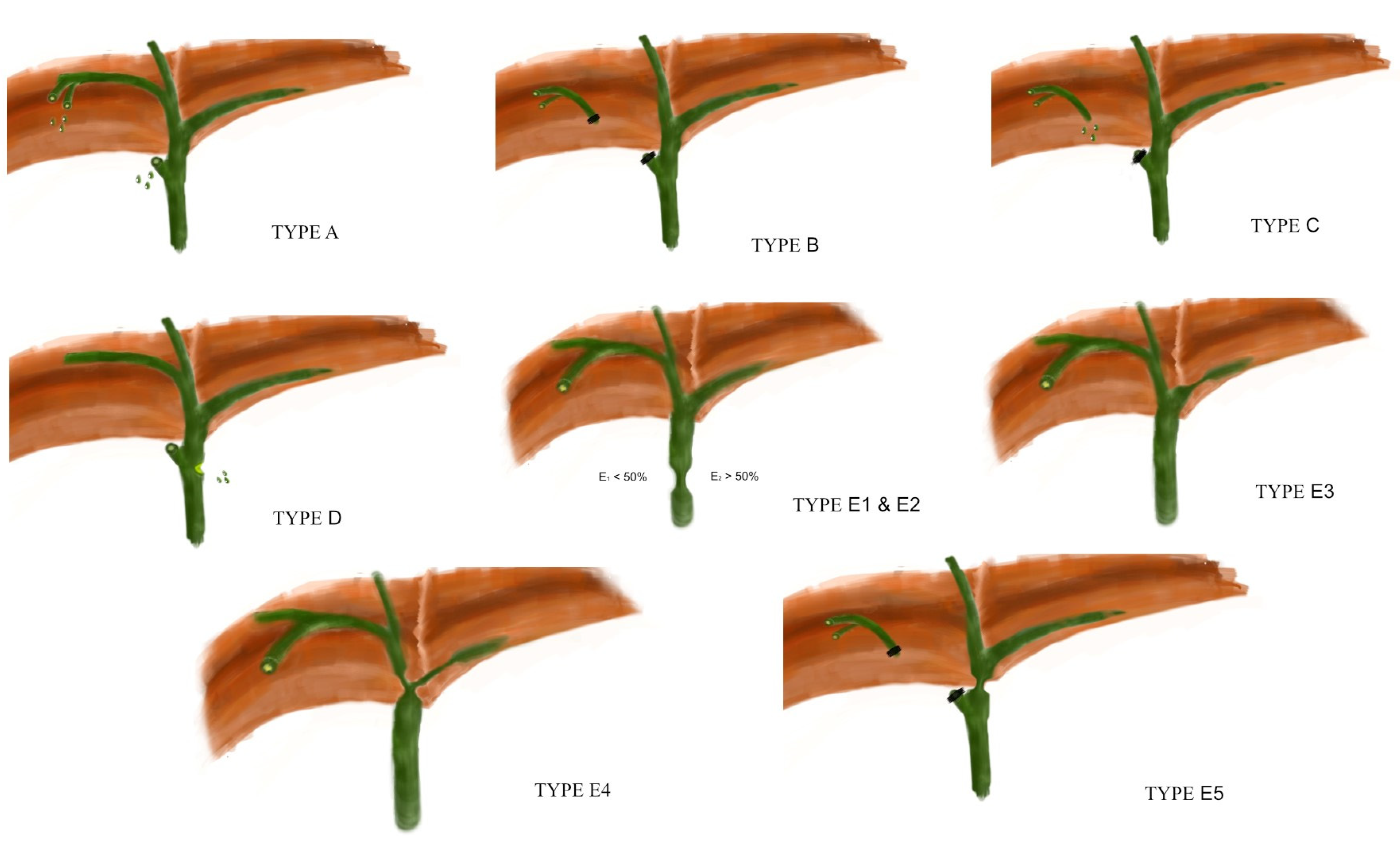
7. The BILE Classification
7.1. Grade A
7.2. Grade B
7.3. Grade C
7.4. Grade D
7.5. Grade E
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Symeonidis, D.; Zacharoulis, D.; Diamantis, A.; Samara, A.A.; Magouliotis, D.E.; Floros, T.; Tepetes, K. Iatrogenic Bile Duct Injuries: A Critical Appraisal of Classification Systems. Chirurgia 2021, 116, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, H. Postoperative strictures of the bile duct. In The Biliary Tract; Blumbart, L.H., Ed.; Clinical Surgery International; Churchill Livingstone: Edinburgh, UK, 1982; pp. 209–2018. [Google Scholar]
- Strasberg, S.M.; Hertl, M.; Soper, N.J. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J. Am. Coll. Surg. 1995, 180, 101–125. [Google Scholar] [PubMed]
- McMahon, A.J.; Fullarton, G.; Baxter, J.N.; O’dwyer, P.J. Bile duct injury and bile leakage in laparoscopic cholecystectomy. Br. J. Surg. 1995, 82, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.J.; Brink, G.R.V.D.; Rauws, E.A.; de Wit, L.; Obertop, H.; Huibregtse, K.; Tytgat, G.N.; Gouma, D.J. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 1996, 38, 141–147. [Google Scholar] [CrossRef]
- Neuhaus, P.; Schmidt, S.C.; Hintze, R.E.; Adler, A.; Veltzke, W.; Raakow, R.; Langrehr, J.M.; Bechstein, W.O. Classification and treatment of bile duct injuries after laparoscopic cholecystectomy. Chirurgry 2000, 71, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Csendes, A.; Navarrete, C.; Burdiles, P.; Yarmuch, J. Treatment of Common Bile Duct Injuries during Laparoscopic Cholecystectomy: Endoscopic and Surgical Management. World J. Surg. 2001, 25, 1346–1351. [Google Scholar] [CrossRef]
- Stewart, L.; Domingez, C.O.; Way, L.W. Way, Bile duct injuries during laparoscopic cholecystectomy: A sensemaking analysis of operative reports. In Proceedings of the Eighth International NDM Conference, Pacific Grove, CA, USA, 21 March 2007. [Google Scholar]
- Bektas, H.; Schrem, H.; Winny, M.; Klempnauer, J. Surgical treatment and outcome of iatrogenic bile duct lesions after cholecystectomy and the impact of different clinical classification systems. Br. J. Surg. 2007, 94, 1119–1127. [Google Scholar] [CrossRef]
- Fingerhut, A.; Dziri, C.; Garden, O.J.; Gouma, D.J.; Millat, B.; Neugebauer, E.; Paganini, A.M.; Targarona, E.M. ATOM, the all-inclusive, nominal EAES classification of bile duct injuries during cholecystectomy. Surg. Endosc. 2013, 27, 4608–4619. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T. Collaboration Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Brunt, L.M.; Deziel, D.J.; Telem, D.A.; Strasberg, S.M.; Aggarwal, R.; Asbun, H.; Bonjer, J.; McDonald, M.; Alseidi, A.; Ujiki, M.; et al. Safe Cholecystectomy Multi-society Practice Guideline and State of the Art Consensus Conference on Prevention of Bile Duct Injury during Cholecystectomy. Ann. Surg. 2020, 272, 3–23. [Google Scholar] [CrossRef]
- Goldstein, S.D.; Lautz, T.B. Fluorescent cholangiography during laparoscopic cholecystectomy: Shedding new light on biliary anatomy. JAMA Surg. 2020, 155, 978. [Google Scholar] [CrossRef] [PubMed]
- Osayi, S.N.; Wendling, M.R.; Drosdeck, J.M.; Chaudhry, U.I.; Perry, K.A.; Noria, S.F.; Mikami, D.J.; Needleman, B.J.; Muscarella, P.; Abdel-Rasoul, M.; et al. Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg. Endosc. 2015, 29, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Tan, H.T.A.; Shelat, V.G. Comparison of indocyanine green dye fluorescent cholangiography with intra-operative cholangiography in laparoscopic cholecystectomy: A meta-analysis. Surg. Endosc. 2021, 35, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Vlek, S.L.; Van Dam, D.A.; Rubinstein, S.; Klerk, E.S.M.D.L.-D.; Schoonmade, L.; Tuynman, J.B.; Meijerink, W.; Ankersmit, M. Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: Results of a systematic review. Surg. Endosc. 2016, 31, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Mesleh, M.G.; Asbun, H.J. Management of common bile duct injury. In The SAGES Manual of Biliary Surgery; Asbun, H.J., Ed.; Springer: Cham, Switzerland, 2020; pp. 213–231. [Google Scholar]
- Wang, X.; Yu, W.-L.; Fu, X.-H.; Zhu, B.; Zhao, T.; Zhang, Y.-J. Early Versus Delayed Surgical Repair and Referral for Patients With Bile Duct Injury: A Systematic Review and Meta-analysis. Ann. Surg. 2020, 271, 449–459. [Google Scholar] [CrossRef]
- Perera, T.; Silva, M.A.; Hegab, B.; Muralidharan, V.; Bramhall, S.R.; Mayer, A.D.; Buckels, J.A.C.; Mirza, D.F. Specialist Early and Immediate Repair of Post-laparoscopic Cholecystectomy Bile Duct Injuries Is Associated with an Improved Long-term Outcome. Ann. Surg. 2011, 253, 553–560. [Google Scholar] [CrossRef]
- Rystedt, J.; Lindell, G.; Montgomery, A. Bile Duct Injuries Associated With 55,134 Cholecystectomies: Treatment and Outcome from a National Perspective. World J. Surg. 2016, 40, 73–80. [Google Scholar] [CrossRef]
- Pekolj, J.; Alvarez, F.A.; Palavecino, M.; Clariá, R.S.; Mazza, O.; de Santibañes, E. Intraoperative Management and Repair of Bile Duct Injuries Sustained during 10,123 Laparoscopic Cholecystectomies in a High-Volume Referral Center. J. Am. Coll. Surg. 2013, 216, 894–901. [Google Scholar] [CrossRef]
- Silva, M.A.; Coldham, C.; Mayer, A.D.; Bramhall, S.R.; Buckels, J.A.; Mirza, D.F. Specialist outreach service for on-table repair of iatrogenic bile duct injuries—A new kind of ‘travelling surgeon’. Ann. R. Coll. Surg. Engl. 2008, 90, 243–246. [Google Scholar] [CrossRef]
- Sahajpal, A.K.; Chow, S.C.; Dixon, E.; Greig, P.D.; Gallinger, S.; Wei, A.C. Bile duct injuries associated with laparoscopic cholecystectomy: Timing of repair and long-term outcomes. Arch. Surg. 2010, 145, 757–763. [Google Scholar] [CrossRef]
- Rystedt, J.M.; Kleeff, J.; Salvia, R.; Besselink, M.G.; Prasad, R.; Lesurtel, M.; Sturesson, C.; Abu Hilal, M.; Aljaiuossi, A.; Antonucci, A.; et al. Post cholecystectomy bile duct injury: Early, intermediate or late repair with hepaticojejunostomy—An E-AHPBA multi-center study. HPB 2019, 21, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.; Cortina, C.S.; Kornfield, H.; Varelas, A.; Li, R.; Veenstra, B.; Bonomo, S. Bile duct injuries: A contemporary survey of surgeon attitudes and experiences. Surg. Endosc. 2019, 34, 3079–3084. [Google Scholar] [CrossRef] [PubMed]
- Sandha, G.S.; Bourke, M.J.; Haber, G.B.; Kortan, P.P. Endoscopic therapy for bile leak based on a new classification: Results in 207 patients. Gastrointest. Endosc. 2004, 60, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, D.; Psaltis, E.; Scholefield, J.H.; Lobo, D.N. Quality of Life and Medico-Legal Implications Following Iatrogenic Bile Duct Injuries. World J. Surg. 2017, 41, 90–99. [Google Scholar] [CrossRef]
- Mercado, M.A.; Dominguez, I. Classification and management of bile duct injuries. World J. Gastrointest. Surg. 2011, 3, 43–48. [Google Scholar] [CrossRef]
- Jabłońska, B.; Lampe, P. Iatrogenic bile duct injuries: Etiology, diagnosis and management. World J. Gastroenterol. 2009, 15, 4097–4104. [Google Scholar] [CrossRef]
- Laurent, V.; Ayav, A.; Hoeffel, C.; Bruot, O.; Ganne, P.A.; Mathias, J.; Régent, D. Imaging of the postoperative biliary tract. J. Radiol. 2009, 90, 905–917. [Google Scholar] [CrossRef]
- Linhares, B.L.; Magalhaes Ada, G.; Cardoso, P.M.; Linhares Filho, J.P.; Pinho, J.E.; Costa, M.L. Bile duct injury following cholecystectomy. Rev. Col. Bras. Cir. 2011, 38, 95–99. [Google Scholar] [CrossRef]
- Lee, C.M.; Stewart, L.; Way, L.W. Postcholecystectomy Abdominal Bile Collections. Arch. Surg. 2000, 135, 538–542; discussion 542–534. [Google Scholar] [CrossRef]
- Mulé, S.; Colosio, A.; Cazejust, J.; Kianmanesh, R.; Soyer, P.; Hoeffel, C. Imaging of the postoperative liver: Review of normal appearances and common complications. Abdom. Imaging 2015, 40, 2761–2776. [Google Scholar] [CrossRef]
- Mungai, F.; Berti, V.; Colagrande, S. Bile leak after elective laparoscopic cholecystectomy: Role of MR imaging. J. Radiol. Case Rep. 2013, 7, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Chandrashekar, N.; Kumar, R.; Thomas, E.; Agarwal, S.; Bal, C.; Malhotra, A. Hepatobiliary scintigraphy: An effective tool in the management of bile leak following laparoscopic cholecystectomy. Clin. Imaging 2004, 28, 40–43. [Google Scholar] [CrossRef]
- Barkun, A.N.; Rezieg, M.; Mehta, S.N.; Pavone, E.; Landry, S.; Barkun, J.S.; Fried, G.M.; Bret, P.; Cohen, A. Postcholecystectomy biliary leaks in the laparoscopic era: Risk factors, presentation, and management. Gastrointest. Endosc. 1997, 45, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, H.; Fukutomi, A.; Kanemoto, H.; Maeda, A.; Matsunaga, K.; Uesaka, K.; Otake, Y.; Hasuike, N.; Yamaguchi, Y.; Ikehara, H.; et al. Risk of pancreatitis after endoscopic retrograde cholangiopancreatography and endoscopic biliary drainage. HPB 2009, 11, 222–228. [Google Scholar] [CrossRef]
- McGahan, J.P.; Stein, M. Complications of laparoscopic cholecystectomy: Imaging and intervention. AJR Am. J. Roentgenol. 1995, 165, 1089–1097. [Google Scholar] [CrossRef]
- Schreuder, A.M.; Booij, K.A.C.; de Reuver, P.R.; van Delden, O.M.; van Lienden, K.P.; Besselink, M.G.; Busch, O.R.; Gouma, D.J.; Rauws, E.A.J.; van Gulik, T.M. Percutaneous-endoscopic rendezvous procedure for the management of bile duct injuries after cholecystectomy: Short- and long-term outcomes. Endoscopy 2018, 50, 577–587. [Google Scholar] [CrossRef]
- Freeman, M.L.; DiSario, J.A.; Nelson, D.B.; Fennerty, M.; Lee, J.G.; Bjorkman, D.J.; Overby, C.S.; Aas, J.; Ryan, M.E.; Bochna, G.S.; et al. Risk factors for post-ERCP pancreatitis: A prospective, multicenter study. Gastrointest. Endosc. 2001, 54, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Aduna, M.; Larena, J.A.; Martín, D.; Martínez-Guereñu, B.; Aguirre, I.; Astigarraga, E. Bile duct leaks after laparoscopic cholecystectomy: Value of contrast-enhanced MRCP. Abdom. Imaging 2005, 30, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Cieszanowski, A.; Stadnik, A.; Lezak, A.; Maj, E.; Zieniewicz, K.; Rowinska-Berman, K.; Grudziński, I.; Krawczyk, M.; Rowiński, O. Detection of active bile leak with Gd-EOB-DTPA enhanced MR cholangiography: Comparison of 20–25 min delayed and 60–180 min delayed images. Eur. J. Radiol. 2013, 82, 2176–2182. [Google Scholar] [CrossRef]
- Ribeiro, B.J.; Alves, A.M.A.; De Oliveira, R.S.; Velloni, F.; D’ippolito, G. The role of gadoxetic acid-enhanced magnetic resonance cholangiography in the evaluation of postoperative bile duct injury: Pictorial essay. Radiol. Bras. 2019, 52, 403–407. [Google Scholar] [CrossRef]
- Kantarcı, M.; Pirimoglu, B.; Karabulut, N.; Bayraktutan, U.; Ogul, H.; Ozturk, G.; Aydinli, B.; Kizrak, Y.; Eren, S.; Yilmaz, S. Non-invasive detection of biliary leaks using Gd-EOB-DTPA-enhanced MR cholangiography: Comparison with T2-weighted MR cholangiography. Eur. Radiol. 2013, 23, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.-C.; Wang, L.-J.; Wu, C.-H.; Chen, H.-W.; Fu, C.-J.; Yuan, K.-C.; Lin, B.-C.; Hsu, Y.-P.; Kang, S.-C. Detection and characterization of traumatic bile leaks using Gd-EOB-DTPA enhanced magnetic resonance cholangiography. Sci. Rep. 2018, 8, 1461. [Google Scholar] [CrossRef]
- Krige, J.E.; Bornman, P.C.; Kahn, D. Bile leaks and sepsis: Drain now, fix later. Arch. Surg. 2010, 145, 763. [Google Scholar] [CrossRef]
- Li, J.; Frilling, A.; Nadalin, S.; Paul, A.; Malagò, M.; Broelsch, C.E. Management of concomitant hepatic artery injury in patients with iatrogenic major bile duct injury after laparoscopic cholecystectomy. Br. J. Surg. 2007, 95, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.; Robinson, T.N.; Lee, C.M.; Liu, K.; Whang, K.; Way, L.W. Right hepatic artery injury associated with laparoscopic bile duct injury: Incidence, mechanism, and consequences. J. Gastrointest. Surg. 2004, 8, 523–530; discussion 530–531. [Google Scholar] [CrossRef] [PubMed]
- Yoshidome, H.; Miyazaki, M.; Shimizu, H.; Ito, H.; Nakagawa, K.; Ambiru, S.; Nakajima, N.; Edwards, M.J.; Lentsch, A.B. Obstructivejaundice impairs hepatic sinusoidal endothelial cell functionand renders liver susceptible to hepatic ischemia/reperfusion. J. Hepatol. 2000, 33, 59–67. [Google Scholar] [CrossRef]
- Dilek, O.N.; Atay, A. Dealing with hepatic artery traumas: A clinical literature review. World J. Clin. Cases 2021, 9, 8425–8440. [Google Scholar] [CrossRef]
- Kim, K.M.; Park, J.W.; Lee, J.K.; Lee, K.H.; Lee, K.T.; Shim, S.G. A Comparison of Preoperative Biliary Drainage Methods for Perihilar Cholangiocarcinoma: Endoscopic vs Percutaneous Transhepatic Biliary Drainage. Gut Liver 2015, 9, 791–799. [Google Scholar] [CrossRef]
- Truant, S.; Boleslawski, E.; Lebuffe, G.; Sergent, G.; Pruvot, F.-R. Hepatic resection for post-cholecystectomy bile duct injuries: A literature review. HPB 2010, 12, 334–341. [Google Scholar] [CrossRef]
- Abbas, A.; Sethi, S.; Brady, P.; Taunk, P. Endoscopic management of postcholecystectomy biliary leak: When and how? A nationwide study. Gastrointest. Endosc. 2019, 90, 233–241.e1. [Google Scholar] [CrossRef]
- Adler, D.G.; Papachristou, G.I.; Taylor, L.J.; McVay, T.; Birch, M.; Francis, G.; Zabolotsky, A.; Laique, S.N.; Hayat, U.; Zhan, T.; et al. Clinical outcomes in patients with bile leaks treated via ERCP with regard to the timing of ERCP: A large multicenter study. Gastrointest. Endosc. 2016, 85, 766–772. [Google Scholar] [CrossRef] [PubMed]
- De’angelis, N.; Catena, F.; Memeo, R.; Coccolini, F.; Martínez-Pérez, A.; Romeo, O.M.; De Simone, B.; Di Saverio, S.; Brustia, R.; Rhaiem, R.; et al. 2020 WSES guidelines for the detection and management of bile duct injury during cholecystectomy. World J. Emerg. Surg. 2021, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Chichom-Mefire, A.; Labricciosa, F.M.; Hardcastle, T.; Abu-Zidan, F.M.; Adesunkanmi, A.K.; Ansaloni, L.; Bala, M.; Balogh, Z.J.; Beltrán, M.A.; et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J. Emerg. Surg. 2017, 12, 36. [Google Scholar] [CrossRef]
- Sartelli, M.; Coccolini, F.; Kluger, Y.; Agastra, E.; Abu-Zidan, F.M.; Abbas, A.E.S.; Ansaloni, L.; Adesunkanmi, A.K.; Atanasov, B.; Augustin, G.; et al. WSES/GAIS/SIS-E/WSIS/AAST global clinical pathways for patients with intra-abdominal infections. World J. Emerg. Surg. 2021, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Suzuki, K.; Umehara, Y.; Kawabe, A.; Wada, H. Features and management of bile leaks after laparoscopic cholecystectomy. J. Hepatobiliary Pancreatic Surg. 2005, 12, 61–64. [Google Scholar] [CrossRef]
- Huang, Q.; Yao, H.H.; Shao, F.; Wang, C.; Hu, Y.G.; Hu, S.; Qiu, L.J. Analysis of Risk Factors for Postoperative Complication of Repair of Bile Duct Injury After Laparoscopic Cholecystectomy. Dig. Dis. Sci. 2014, 59, 3085–3091. [Google Scholar] [CrossRef] [PubMed]
- Pioche, M.; Ponchon, T. Management of bile duct leaks. J. Visc. Surg. 2013, 150, S33–S38. [Google Scholar] [CrossRef]
- Barton, J.R.; Russell, R.C.; Hatfield, A.R. Management of bile leaks after laparoscopic cholecystectomy. Br. J. Surg. 1995, 82, 980–984. [Google Scholar] [CrossRef]
- Mavrogiannis, C.; Liatsos, C.; Papanikolaou, I.S.; Karagiannis, S.; Galanis, P.; Romanos, A. Biliary stenting alone versus biliary stenting plus sphincterotomy for the treatment of post-laparoscopic cholecystectomy biliary leaks: A prospective randomized study. Eur. J. Gastroenterol. Hepatol. 2006, 18, 405–409. [Google Scholar] [CrossRef]
- Rustagi, T.; Aslanian, H.R. Endoscopic Management of Biliary Leaks After Laparoscopic Cholecystectomy. J. Clin. Gastroenterol. 2014, 48, 674–678. [Google Scholar] [CrossRef]
- De Palma, G.D.; Galloro, G.; Iuliano, G.; Puzziello, A.; Persico, F.; Masone, S.; Persico, G. Leaks from laparoscopic cholecystectomy. Hepatogastroenterology 2002, 49, 924–925. [Google Scholar] [PubMed]
- Bjorkman, D.J.; Carr-Locke, D.L.; Lichtenstein, D.; Ferrari, A.P.; Slivka, A.; Van Dam, J.; Brooks, D.C. Postsurgical bile leaks: Endoscopic obliteration of the transpapillary pressure gradient is enough. Am. J. Gastroenterol. 1995, 90, 2128–2133. [Google Scholar] [PubMed]
- Kaffes, A.J.; Hourigan, L.; De Luca, N.; Byth, K.; Williams, S.J.; Bourke, M.J. Impact of endoscopic intervention in 100 patients with suspected postcholecystectomy bile leak. Gastrointest. Endosc. 2005, 61, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.E.; Geenen, J.E.; Lehman, G.A.; Aliperti, G.; Freeman, M.L.; Silverman, W.B.; Mayeux, G.P.; Frakes, J.T.; Parker, H.W.; Yakshe, P.N.; et al. Endoscopic intervention for biliary leaks after laparoscopic cholecystectomy: A multicenter review. Gastrointest. Endosc. 1998, 47, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Tewani, S.K.; Turner, B.G.; Chuttani, R.; Pleskow, D.; Sawhney, M.S. Location of bile leak predicts the success of ERCP performed for postoperative bile leaks. Gastrointest. Endosc. 2013, 77, 601–608. [Google Scholar] [CrossRef]
- Canena, J.; Horta, D.; Coimbra, J.; Meireles, L.; Russo, P.; Marques, I.; Ricardo, L.; Rodrigues, C.; Capela, T.; Carvalho, D.; et al. Outcomes of endoscopic management of primary and refractory postcholecystectomy biliary leaks in a multicentre review of 178 patients. BMC Gastroenterol. 2015, 15, 105. [Google Scholar] [CrossRef]
- Lalezari, D.; Singh, I.; Reicher, S.; Eysselein, V.E. Evaluation of fully covered self-expanding metal stents in benign biliary strictures and bile leaks. World J. Gastrointest. Endosc. 2013, 5, 332–339. [Google Scholar] [CrossRef]
- Baron, T.H.; Poterucha, J.J. Insertion and Removal of Covered Expandable Metal Stents for Closure of Complex Biliary Leaks. Clin. Gastroenterol. Hepatol. 2006, 4, 381–386. [Google Scholar] [CrossRef]
- Rio-Tinto, R.; Canena, J. Endoscopic Treatment of Post-Cholecystectomy Biliary Leaks. GE-Port. J. Gastroenterol. 2020, 28, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Dumonceau, J.M.; Tringali, A.; Papanikolaou, I.S.; Blero, D.; Mangiavillano, B.; Schmidt, A.; Vanbiervliet, G.; Costamagna, G.; Devière, J.; García-Cano, J.; et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline–Updated October 2017. Endoscopy 2018, 50, 910–930. [Google Scholar] [CrossRef]
- Perri, V.; Familiari, P.; Tringali, A.; Boskoski, I.; Costamagna, G. Plastic Biliary Stents for Benign Biliary Diseases. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 405–433. [Google Scholar] [CrossRef]
- Costamagna, G.; Pandolfi, M.; Mutignani, M.; Spada, C.; Perri, V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest. Endosc. 2001, 54, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Jearth, V.; Sundaram, S. Covered Self-Expanding Metal Stents Versus Multiple Plastic Stents for Benign Biliary Strictures: An Updated Meta-Analysis of Randomized Controlled Trials. Cureus 2022, 14, e24588. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.J.; Burgemeister, L.; Bruno, M.J.; Rauws, E.A.; Gouma, D.J.; Tytgat, G.N.; Huibregtse, K. Long-term follow-up after biliary stent placement for postoperative bile duct stenosis. Gastrointest. Endosc. 2001, 54, 154–161. [Google Scholar] [CrossRef]
- Kassab, C.; Prat, F.; Liguory, C.; Meduri, B.; Ducot, B.; Fritsch, J.; Choury, A.D.; Pelletier, G. Endoscopic management of post-laparoscopic cholecystectomy biliary strictures: Long-term outcome in a multicenter study. Gastroenterol. Clin. Biol. 2006, 30, 124–129. [Google Scholar] [CrossRef]
- de Jong, E.; Moelker, A.; Leertouwer, T.; Spronk, S.; Van Dijk, M.; van Eijck, C. Percutaneous Transhepatic Biliary Drainage in Patients with Postsurgical Bile Leakage and Nondilated Intrahepatic Bile Ducts. Dig. Surg. 2013, 30, 444–450. [Google Scholar] [CrossRef]
- Eum, Y.O.; Park, J.K.; Chun, J.; Lee, S.H.; Ryu, J.K.; Kim, Y.T.; Yoon, Y.B.; Yoon, C.J.; Han, H.S.; Hwang, J.H. Non-surgical treatment of post-surgical bile duct injury: Clinical implications and outcomes. World J. Gastroenterol. 2014, 20, 6924–6931. [Google Scholar] [CrossRef]
- Nakai, Y.; Kogure, H.; Isayama, H.; Koike, K. Endoscopic Ultrasound-Guided Biliary Drainage for Benign Biliary Diseases. Clin. Endosc. 2019, 52, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Sharaiha, R.Z.; Khan, M.A.; Kamal, F.; Tyberg, A.; Tombazzi, C.R.; Ali, B.; Kahaleh, M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 904–914. [Google Scholar] [CrossRef]
- Jabłońska, B.; Lampe, P.; Olakowski, M.; Górka, Z.; Lekstan, A.; Gruszka, T. Hepaticojejunostomy vs. End-to-end Biliary Reconstructions in the Treatment of Iatrogenic Bile Duct Injuries. J. Gastrointest. Surg. 2009, 13, 1084–1093. [Google Scholar] [CrossRef]
- Gazzaniga, G.M.; Filauro, M.; Mori, L. Surgical treatment of iatrogenic lesions of the proximal common bile duct. World J. Surg. 2001, 25, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Dageforde, L.A.; Landman, M.P.; Feurer, I.D.; Poulose, B.; Pinson, W.C.; Moore, D.E. A Cost-Effectiveness Analysis of Early vs Late Reconstruction of Iatrogenic Bile Duct Injuries. J. Am. Coll. Surg. 2012, 214, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Thomson, B.N.J.; Parks, R.W.; Madhavan, K.K.; Wigmore, S.J.; Garden, O.J. Early specialist repair of biliary injury. Br. J. Surg. 2006, 93, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, A.; Paineau, J.; Hamy, A.; Schneck, A.; Schaaf, C.; Gugenheim, J. Primary versus delayed repair for bile duct injuries sustained during cholecystectomy: Results of a survey of the Association Francaise de Chirurgie. HPB 2013, 15, 611–616. [Google Scholar] [CrossRef]
- Goykhman, Y.; Kory, I.; Small, R.; Kessler, A.; Klausner, J.M.; Nakache, R.; Ben-Haim, M. Long-term Outcome and Risk Factors of Failure after Bile Duct Injury Repair. J. Gastrointest. Surg. 2008, 12, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.R.; Vogt, D.P.; Ponsky, J.L.; Brown, N.; Mascha, E.; Henderson, M.J. Management of failed biliary repairs for major bile duct injuries after laparoscopic cholecystectomy. J. Am. Coll. Surg. 2004, 199, 192–197. [Google Scholar] [CrossRef]
- Maddah, G.; Mashhadi, M.T.R.; Mashhadi, M.P.; Nooghabi, M.J.; Hassanpour, M.; Abdollahi, A. Iatrogenic injuries of the extrahepatic biliary system. J. Surg. Res. 2015, 213, 215–221. [Google Scholar] [CrossRef]
- Schreuder, A.M.; Busch, O.R.; Besselink, M.G.; Ignatavicius, P.; Gulbinas, A.; Barauskas, G.; Gouma, D.J.; Van Gulik, T.M. Long-Term Impact of Iatrogenic Bile Duct Injury. Dig. Surg. 2020, 37, 10–21. [Google Scholar] [CrossRef]
- Moraca, R.J.; Lee, F.T.; Ryan, J.A.; Traverso, L.W. Long-term Biliary Function After Reconstruction of Major Bile Duct Injuries With Hepaticoduodenostomy or Hepaticojejunostomy. Arch. Surg. 2002, 137, 889–893; discussion 893–894. [Google Scholar] [CrossRef]
- Ai, C.; Wu, Y.; Xie, X.; Wang, Q.; Xiang, B. Roux-en-Y hepaticojejunostomy or hepaticoduodenostomy for biliary reconstruction after resection of congenital biliary dilatation: A systematic review and meta-analysis. Surg. Today 2022, 53, 1–11. [Google Scholar] [CrossRef]
- Donatelli, G.; Cereatti, F.; Dhumane, P.; Antonelli, G.; Dumont, J.-L.; De Palma, G.D.; Dagher, I.; Derhy, S. Long-term Outcomes of Combined Endoscopic-Radiological Approach for the Management of Complete Transection of the Biliary Tract. J. Gastrointest. Surg. 2022, 26, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Fiocca, F.; Salvatori, F.M.; Fanelli, F.; Bruni, A.; Ceci, V.; Corona, M.; Donatelli, G. Complete transection of the main bile duct: Minimally invasive treatment with an endoscopic-radiologic rendezvous. Gastrointest. Endosc. 2011, 74, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rosado, I.; Sanford, D.E.; Liu, J.; Hawkins, W.G.; Mercado, M.A. Timing of Surgical Repair After Bile Duct Injury Impacts Postoperative Complications but Not Anastomotic Patency. Ann. Surg. 2016, 264, 544–553. [Google Scholar] [CrossRef]
- Ismael, H.N.; Cox, S.; Cooper, A.; Narula, N.; Aloia, T. The morbidity and mortality of hepaticojejunostomies for complex bile duct injuries: A multi-institutional analysis of risk factors and outcomes using NSQIP. HPB 2017, 19, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Pulitanò, C.; Parks, R.W.; Ireland, H.; Wigmore, S.J.; Garden, O.J. Impact of concomitant arterial injury on the outcome of laparoscopic bile duct injury. Am. J. Surg. 2011, 201, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Wójcicki, M.; Lubikowski, J.; Chmurowicz, T.; Post, M.; Jarosz, K.; Białek, A.; Milkiewicz, P. Liver transplantation as an ultimate step in the management of iatrogenic bile duct injury complicated by secondary biliary cirrhosis. Ann. Transplant. 2012, 17, 38–44. [Google Scholar] [CrossRef] [PubMed]
- de Santibañes, E.; Ardiles, V.; Gadano, A.; Palavecino, M.; Pekolj, J.; Ciardullo, M. Liver transplantation: The last measure in the treatment of bile duct injuries. World J. Surg. 2008, 32, 1714–1721. [Google Scholar] [CrossRef]

| Clinical Presentation | Anatomical Description and Site of the Injury (Strasberg Classification) | Treatment | |
|---|---|---|---|
| Grade A | Biliary fistula (drain in situ) | A, C, D | Conservative management |
| Grade B | Biloma Biliary Peritonitis | A, C, D | Percutaneous drainage or surgical lavage and drainage |
| Grade C | Biliary fistula not responding to conservative management CBD Stricture with obstructive jaundice or recurrent episodes of acute cholangitis | A, B (Symptomatic), C, D, E1–E2 | ERCP/PTC |
| Grade D | CBD stricture not sufficiently addressed by ERCP/PTC Complete transection of the CBD presenting as bile leak or obstructive jaundice | D, E1–E5 | End to end ductal anastomosis Roux en Y Hepaticojejunostomy (Gold Standard) |
| Grade E | Liver atrophy, abscess due to concomitant vascular injury or delayed bile duct injury diagnosis Liver failure | - | Liver Resection Liver Transplantation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Symeonidis, D.; Tepetes, K.; Tzovaras, G.; Samara, A.A.; Zacharoulis, D. BILE: A Literature Review Based Novel Clinical Classification and Treatment Algorithm of Iatrogenic Bile Duct Injuries. J. Clin. Med. 2023, 12, 3786. https://doi.org/10.3390/jcm12113786
Symeonidis D, Tepetes K, Tzovaras G, Samara AA, Zacharoulis D. BILE: A Literature Review Based Novel Clinical Classification and Treatment Algorithm of Iatrogenic Bile Duct Injuries. Journal of Clinical Medicine. 2023; 12(11):3786. https://doi.org/10.3390/jcm12113786
Chicago/Turabian StyleSymeonidis, Dimitrios, Konstantinos Tepetes, George Tzovaras, Athina A. Samara, and Dimitrios Zacharoulis. 2023. "BILE: A Literature Review Based Novel Clinical Classification and Treatment Algorithm of Iatrogenic Bile Duct Injuries" Journal of Clinical Medicine 12, no. 11: 3786. https://doi.org/10.3390/jcm12113786
APA StyleSymeonidis, D., Tepetes, K., Tzovaras, G., Samara, A. A., & Zacharoulis, D. (2023). BILE: A Literature Review Based Novel Clinical Classification and Treatment Algorithm of Iatrogenic Bile Duct Injuries. Journal of Clinical Medicine, 12(11), 3786. https://doi.org/10.3390/jcm12113786







