Can Dialysis Patients Identify and Diagnose Pulmonary Congestion Using Self-Lung Ultrasound?
Abstract
1. Introduction
2. Materials and Methods
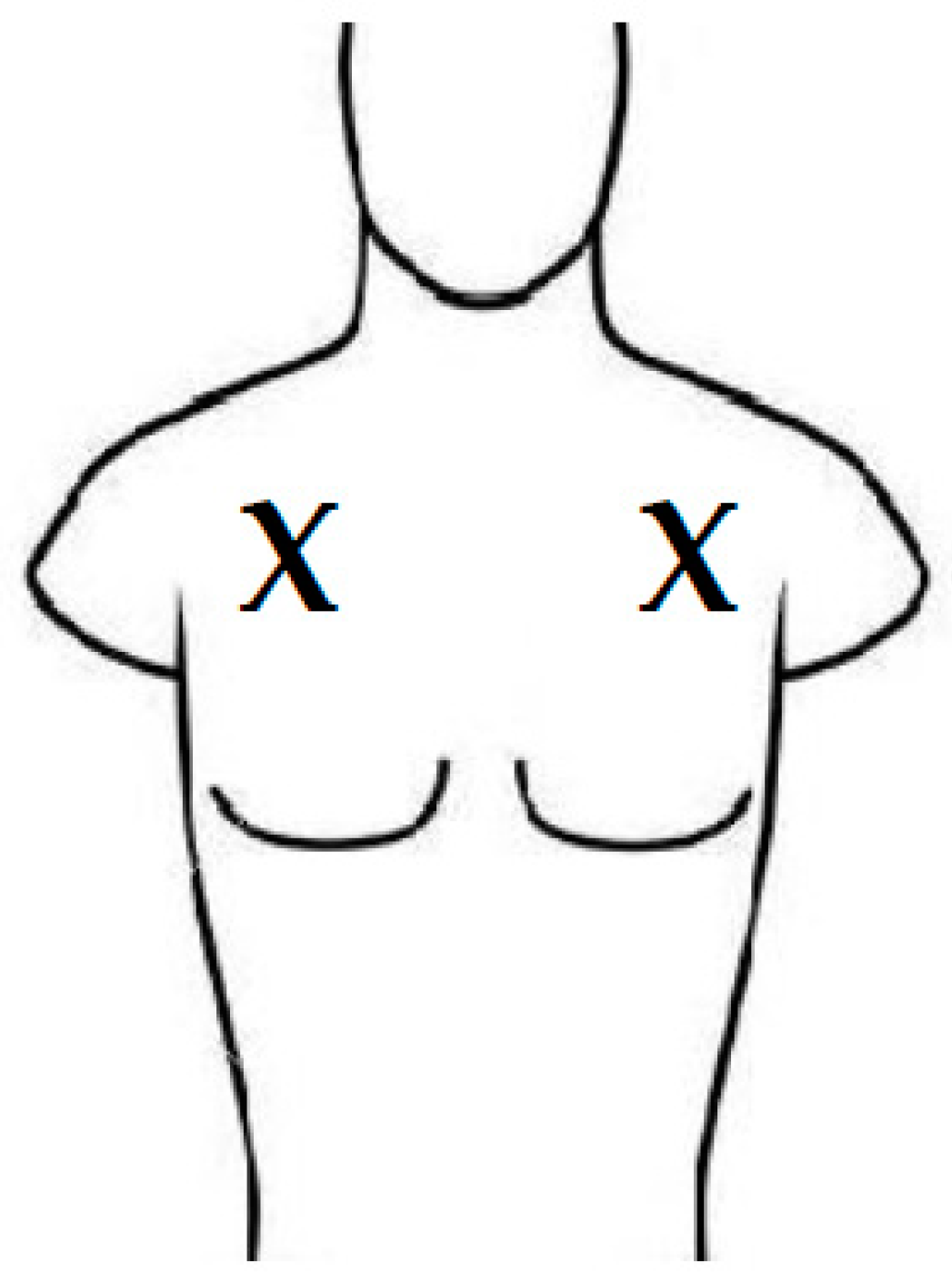
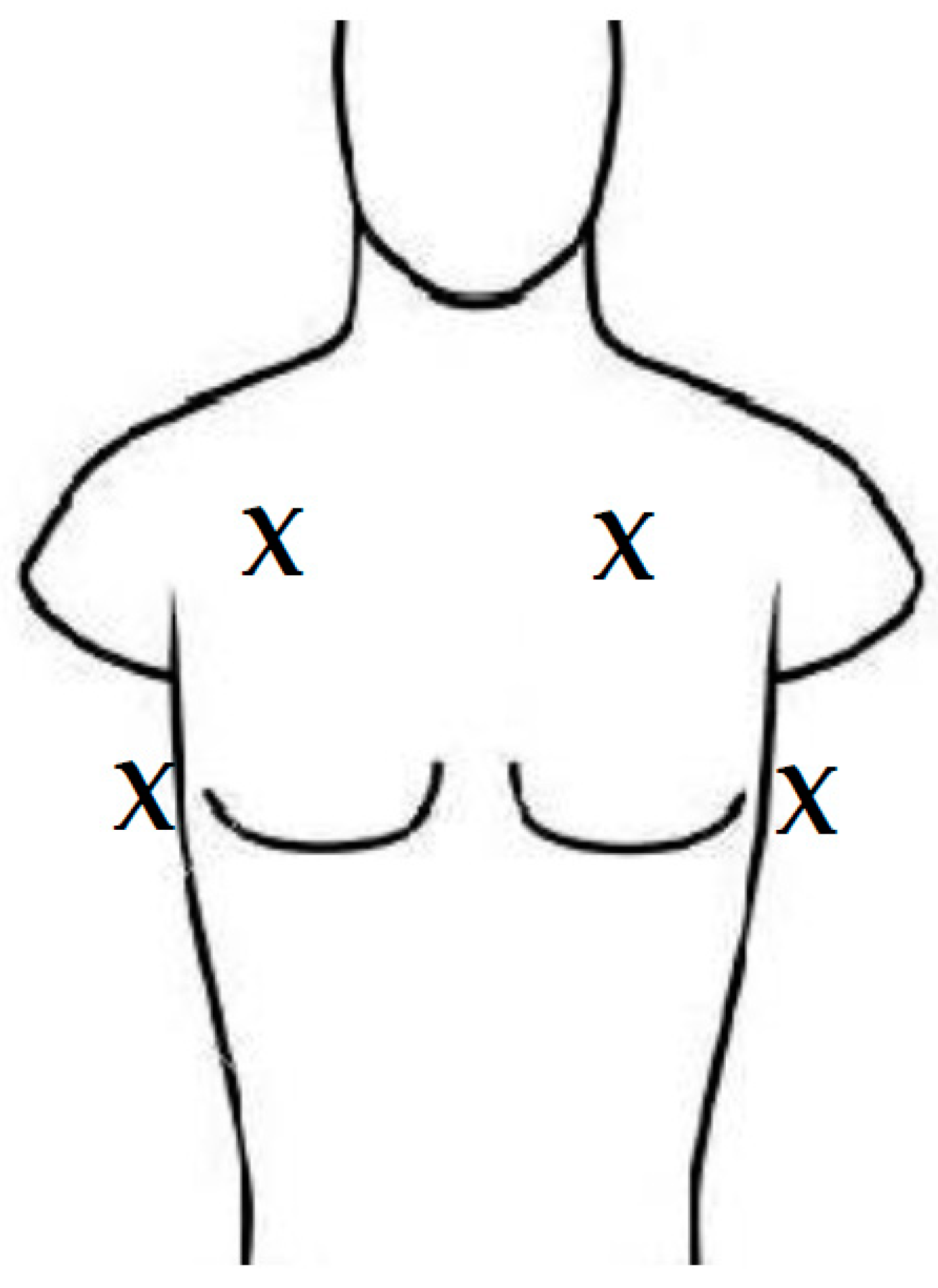
2.1. Participants and Setting
2.2. Study Flow
2.3. Statistical Analysis
3. Results
3.1. General Information
3.2. B-Line Count Agreement
3.3. Correct Lung Image
3.4. Schedules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Barjaktarevic, I.; Kenny, J.S.; Berlin, D.; Cannesson, M. The Evolution of Ultrasound in Critical Care: From Procedural Guidance to Hemodynamic Monitor. J. Ultrasound Med. 2021, 40, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gómez, J.L.; Mayo, P.H.; Koenig, S.J. Point-of-Care Ultrasonography. N. Engl. J. Med. 2021, 385, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Yaoting, W.M.; Huihui, C.M.; Ruizhong, Y.M.; Jingzhi, L.M.P.; Ji-Bin, L.M.; Chen, L.; Chengzhong, P. Point-of-Care Ultrasound: New Concepts and Future Trends. Adv. Ultrasound Diagn. Ther. 2021, 5, 268. [Google Scholar] [CrossRef]
- Arnold, M.J.; Jonas, C.E.; Carter, R.E. Point-of-Care Ultrasonography. Am. Fam. Physician 2020, 101, 275–285. [Google Scholar]
- Ben-Baruch Golan, Y.; Sadeh, R.; Mizrakli, Y.; Shafat, T.; Sagy, I.; Slutsky, T.; Kobal, S.L.; Novack, V.; Fuchs, L. Early Point-of-Care Ultrasound Assessment for Medical Patients Reduces Time to Appropriate Treatment: A Pilot Randomized Controlled Trial. Ultrasound Med. Biol. 2020, 46, 1908–1915. [Google Scholar] [CrossRef]
- Tsaban, G.; Galante, O.; Almog, Y.; Ullman, Y.; Fuchs, L. Feasibility of machine integrated point of care lung ultrasound automatic B-lines tool in the Corona-virus 2019 critical care unit. Crit. Care 2021, 25, 345. [Google Scholar] [CrossRef]
- Gohar, E.; Herling, A.; Mazuz, M.; Tsaban, G.; Gat, T.; Kobal, S.; Fuchs, L. Artificial Intelligence (AI) versus POCUS Expert: A Validation Study of Three Automatic AI-Based, Real-Time, Hemodynamic Echocardiographic Assessment Tools. J. Clin. Med. 2023, 12, 1352. [Google Scholar] [CrossRef]
- Baribeau, Y.; Sharkey, A.; Chaudhary, O.; Krumm, S.; Fatima, H.; Mahmood, F.; Matyal, R. Handheld Point-of-Care Ultrasound Probes: The New Generation of POCUS. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3139–3145. [Google Scholar] [CrossRef]
- Haji-Hassan, M.; Lenghel, L.M.; Bolboacă, S.D. Hand-Held Ultrasound of the Lung: A Systematic Review. Diagnostics 2021, 11, 1381. [Google Scholar] [CrossRef]
- Filopei, J.; Siedenburg, H.; Rattner, P.; Fukaya, E.; Kory, P. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of Dyspnea. J. Hosp. Med. 2014, 9, 594–597. [Google Scholar] [CrossRef]
- Gustafsson, M.; Alehagen, U.; Johansson, P. Imaging Congestion with a Pocket Ultrasound Device: Prognostic Implications in Patients with Chronic Heart Failure. J. Card. Fail. 2015, 21, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, C.; Zhao, Y.; Lu, J.; Jiang, H.; Cao, Y.; Xu, Y. Telemedicine application in patients with chronic disease: A systematic review and meta-analysis. BMC Med. Inform. Decis. Mak. 2022, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Timpel, P.; Oswald, S.; Schwarz, P.E.H.; Harst, L. Mapping the Evidence on the Effectiveness of Telemedicine Interventions in Diabetes, Dyslipidemia, and Hypertension: An Umbrella Review of Systematic Reviews and Meta-Analyses. J. Med. Internet Res. 2020, 22, e16791. [Google Scholar] [CrossRef]
- Hadar, E.; Wolff, L.; Tenenbaum-Gavish, K.; Eisner, M.; Shmueli, A.; Barbash-Hazan, S.; Bergel, R.; Shmuel, E.; Houri, O.; Dollinger, S.; et al. Mobile Self-Operated Home Ultrasound System for Remote Fetal Assessment During Pregnancy. Telemed. e-Health 2022, 28, 93–101. [Google Scholar] [CrossRef]
- Duggan, N.M.; Jowkar, N.; Ma, I.W.Y.; Schulwolf, S.; Selame, L.A.; Fischetti, C.E.; Kapur, T.; Goldsmith, A.J. Novice-performed point-of-care ultrasound for home-based imaging. Sci. Rep. 2022, 12, 20461. [Google Scholar] [CrossRef] [PubMed]
- Prinz, C.; Voigt, J.-U. Diagnostic Accuracy of a Hand-Held Ultrasound Scanner in Routine Patients Referred for Echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 111–116. [Google Scholar] [CrossRef]
- Giannese, D.; Puntoni, A.; Cupisti, A.; Morganti, R.; Varricchio, E.; D’alessandro, C.; Mannucci, C.; Serio, P.; Egidi, M.F. Lung ultrasound and BNP to detect hidden pulmonary congestion in euvolemic hemodialysis patients: A single centre experience. BMC Nephrol. 2021, 22, 36. [Google Scholar] [CrossRef]
- Torino, C.; Tripepi, R.; Loutradis, C.; Sarafidis, P.; Tripepi, G.; Mallamaci, F.; Zoccali, C. Can the assessment of ultrasound lung water in haemodialysis patients be simplified? Nephrol. Dial. Transpl. 2021, 36, 2321–2326. [Google Scholar] [CrossRef]
- Reisinger, N.; Lohani, S.; Hagemeier, J.; Panebianco, N.; Baston, C. Lung Ultrasound to Diagnose Pulmonary Congestion Among Patients on Hemodialysis: Comparison of Full Versus Abbreviated Scanning Protocols. Am. J. Kidney Dis. 2022, 79, 193–201.e1. [Google Scholar] [CrossRef]
- Koratala, A.; Ronco, C.; Kazory, A. The Promising Role of Lung Ultrasound in Assessment of Volume Status for Patients Receiving Maintenance Renal Replacement Therapy. Blood Purif. 2020, 49, 643–646. [Google Scholar] [CrossRef]
- Zoccali, C.; Torino, C.; Tripepi, R.; Tripepi, G.; D’arrigo, G.; Postorino, M.; Gargani, L.; Sicari, R.; Picano, E.; Mallamaci, F. Pulmonary Congestion Predicts Cardiac Events and Mortality in ESRD. J. Am. Soc. Nephrol. 2013, 24, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Loutradis, C.; Sarafidis, P.A.; Ferro, C.J.; Zoccali, C. Volume overload in hemodialysis: Diagnosis, cardiovascular consequences, and management. Nephrol. Dial. Transplant. 2021, 36, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Loutradis, C.; Sarafidis, P.A.; Ekart, R.; Papadopoulos, C.; Sachpekidis, V.; Alexandrou, M.E.; Papadopoulou, D.; Efstratiadis, G.; Papagianni, A.; London, G.; et al. The effect of dry-weight reduction guided by lung ultrasound on ambulatory blood pressure in hemodialysis patients: A randomized controlled trial. Kidney Int. 2019, 95, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Torino, C.; Mallamaci, F.; Sarafidis, P.; Papagianni, A.; Ekart, R.; Hojs, R.; Klinger, M.; Letachowicz, K.; Fliser, D.; et al. A randomized multicenter trial on a lung ultrasound–guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int. 2021, 100, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Loutradis, C.; Papagianni, A.; Ekart, R.; Theodorakopoulou, M.; Minopoulou, I.; Pagourelias, E.; Douma, S.; Karagiannis, A.; Mallamaci, F.; Zoccali, C.; et al. Excess volume removal following lung ultrasound evaluation decreases central blood pressure and pulse wave velocity in hemodialysis patients: A LUST sub-study. J. Nephrol. 2020, 33, 1289–1300. [Google Scholar] [CrossRef]
- Torino, C.; Gargani, L.; Sicari, R.; Letachowicz, K.; Ekart, R.; Fliser, D.; Covic, A.; Siamopoulos, K.; Stavroulopoulos, A.; Massy, Z.A.; et al. The Agreement between Auscultation and Lung Ultrasound in Hemodialysis Patients: The LUST Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 2005–2011. [Google Scholar] [CrossRef]
- Alexandrou, M.-E.; Theodorakopoulou, M.P.; Sarafidis, P.A. Lung Ultrasound as a Tool to Evaluate Fluid Accumulation in Dialysis Patients. Kidney Blood Press. Res. 2022, 47, 163–176. [Google Scholar] [CrossRef]
- Mallamaci, F.; Benedetto, F.A.; Tripepi, R.; Rastelli, S.; Castellino, P.; Tripepi, G.; Picano, E.; Zoccali, C. Detection of Pulmonary Congestion by Chest Ultrasound in Dialysis Patients. JACC Cardiovasc. Imaging 2010, 3, 586–594. [Google Scholar] [CrossRef]
- Saad, M.M.; Kamal, J.; Moussaly, E.; Karam, B.; Mansour, W.; Gobran, E.; Abbasi, S.H.; Mahgoub, A.; Singh, P.; Hardy, R.; et al. Relevance of B-Lines on Lung Ultrasound in Volume Overload and Pulmonary Congestion: Clinical Correlations and Outcomes in Patients on Hemodialysis. Cardiorenal Med. 2018, 8, 83–91. [Google Scholar] [CrossRef]
- Ross, D.W.; Abbasi, M.M.; Jhaveri, K.D.; Sachdeva, M.; Miller, I.; Barnett, R.; Narasimhan, M.; Mayo, P.; Merzkani, M.; Mathew, A.T. Lung ultrasonography in end-stage renal disease: Moving from evidence to practice-a narrative review. Clin. Kidney J. 2018, 11, 172–178. [Google Scholar] [CrossRef]
- Martindale, J.L. Resolution of sonographic B-lines as a measure of pulmonary decongestion in acute heart failure. Am. J. Emerg. Med. 2016, 34, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Miglioranza, M.H.; Picano, E.; Badano, L.P.; Sant’Anna, R.; Rover, M.; Zaffaroni, F.; Sicari, R.; Kalil, R.K.; Leiria, T.L.; Gargani, L. Pulmonary congestion evaluated by lung ultrasound predicts decompensation in heart failure outpatients. Int. J. Cardiol. 2017, 240, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Vitturi, N.; Dugo, M.; Soattin, M.; Simoni, F.; Maresca, L.; Zagatti, R.; Maresca, M.C. Lung ultrasound during hemodialysis: The role in the as-sessment of volume status. Int. Urol. Nephrol. 2014, 46, 169–174. [Google Scholar] [CrossRef]
- Pičuljan, A.; Šustić, M.; Brumini, G.; Kuharić, J.; Šustić, A. Reliability of B-line quantification by different-level observers and a software algorithm using point-of-care lung ultrasound. J. Clin. Monit. Comput. 2020, 34, 1259–1264. [Google Scholar] [CrossRef]
- Gargani, L.; Sicari, R.; Raciti, M.; Serasini, L.; Passera, M.; Torino, C.; Letachowicz, K.; Ekart, R.; Fliser, D.; Covic, A.; et al. Efficacy of a remote web-based lung ultrasound training for nephrologists and cardiologists: A LUST trial sub-project. Nephrol. Dial. Transplant. 2016, 31, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Guarracino, F.; Vetrugno, L.; Forfori, F.; Corradi, F.; Orso, D.; Bertini, P.; Ortalda, A.; Federici, N.; Copetti, R.; Bove, T. Lung, Heart, Vascular, and Diaphragm Ultrasound Examination of COVID-19 Patients: A Comprehensive Approach. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1866–1874. [Google Scholar] [CrossRef]
- Mohamed Ali, A.M.; El-Alali, E.; Weltz, A.S.; Rehrig, S.T. Thoracic Point-of-Care Ultrasound: A SARS-CoV-2 Data Repository for Future Artificial Intelligence and Machine Learning. Surg. Innov. 2021, 28, 214–219. [Google Scholar] [CrossRef]
- Gutsche, H.; Lesser, T.G.; Wolfram, F.; Doenst, T. Significance of Lung Ultrasound in Patients with Suspected COVID-19 Infection at Hospital Admission. Diagnostics 2021, 11, 921. [Google Scholar] [CrossRef]
- Brenner, D.S.; Liu, G.Y.; Omron, R.; Tang, O.; Garibaldi, B.T.; Fong, T.C. Diagnostic accuracy of lung ultrasound for SARS-CoV-2: A retrospective cohort study. Ultrasound J. 2021, 13, 12. [Google Scholar] [CrossRef]
- Demi, L.; Wolfram, F.; Klersy, C.; De Silvestri, A.; Ferretti, V.V.; Muller, M.; Miller, D.; Feletti, F.; Wełnicki, M.; Buda, N.; et al. New International Guidelines and Consensus on the Use of Lung Ultrasound. J. Ultrasound Med. 2022, 42, 309–344. [Google Scholar] [CrossRef]
- Moshavegh, R.; Hansen, K.L.; Moller-Sorensen, H.; Nielsen, M.B.; Jensen, J.A. Automatic Detection of B-Lines in In Vivo Lung Ultra-sound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 309–317. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; He, Q.; Liao, H.; Luo, J. Quantitative Analysis of Pleural Line and B-Lines in Lung Ultrasound Images for Severity Assessment of COVID-19 Pneumonia. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liu, Z.; Dong, Y.; Liu, J.; Bin Huang, B.; Liu, A.; Huang, J.; Pu, X.; Shi, X.; Yu, J.; et al. Evaluation of lung involvement in COVID-19 pneumonia based on ultrasound images. Biomed. Eng. Online 2021, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Brusasco, C.; Santori, G.; Bruzzo, E.; Trò, R.; Robba, C.; Tavazzi, G.; Guarracino, F.; Forfori, F.; Boccacci, P.; Corradi, F. Quantitative lung ultrasonography: A putative new algorithm for automatic detection and quantification of B-lines. Crit. Care 2019, 23, 288. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.F.L.; Du, T.; Liu, J.S.; Chai, C.C.; Nyein, C.M.; Liu, A.Y.L. Automated lung ultrasound image assessment using artificial intelligence to identify fluid overload in dialysis patients. BMC Nephrol. 2022, 23, 410. [Google Scholar] [CrossRef]
- Short, J.; Acebes, C.; Rodriguez-de-Lema, G.; La Paglia, G.M.C.; Pavón, M.; Sánchez-Pernaute, O.; Vazquez, J.C.; Romero-Bueno, F.; Garrido, J.; Esperanza Naredo, E. Visual versus automatic ultra-sound scoring of lung B-lines: Reliability and consistency between systems. Med. Ultrason. 2019, 21, 45. [Google Scholar] [CrossRef]
- Chiem, A.T.; Lim, G.W.; Tabibnia, A.P.; Takemoto, A.S.; Weingrow, D.M.; Shibata, J.E. Feasibility of patient-performed lung ultrasound self-exams (Patient-PLUS) as a potential approach to telemedicine in heart failure. ESC Heart Fail. 2021, 8, 3997–4006. [Google Scholar] [CrossRef]
- Liang, X.K.; Li, L.J.; Wang, X.H.; Wang, X.X.; Wang, Y.D.; Xu, Z.F. Role of Lung Ultrasound in Adjusting Ultrafiltration Volume in He-modialysis Patients. Ultrasound Med. Biol. 2019, 45, 732–740. [Google Scholar] [CrossRef]
- Miglioranza, M.H.; Gargani, L.; Sant’Anna, R.T.; Rover, M.M.; Martins, V.M.; Mantovani, A.; Weber, C.; Moraes, M.A.; Feldman, C.J.; Kalil, R.A.K.; et al. Lung Ultrasound for the Evaluation of Pulmonary Congestion in Outpatients. JACC Cardiovasc. Imaging 2013, 6, 1141–1151. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Annamalai, I.; Fernando, M.E.; Srinivasaprasad, N.D.; Suren, S.; Thirumalvalavan, K.; Veerakumar, A.M. Volume assessment in hemodialysis: A comparison of present methods in clinical practice with sonographic lung comets. Indian J. Nephrol. 2019, 29, 102–110. [Google Scholar] [CrossRef]
- Roshandel, J.; Alahyari, S.; Khazaei, M.; Asgari, R.; Moharamzad, Y.; Zarei, E.; Taheri, M.S. Diagnostic performance of lung ultrasound com-pared to CT scan in the diagnosis of pulmonary lesions of COVID-19 induced pneumonia: A preliminary study. Virusdisease 2021, 32, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, L.; Galante, O.; Almog, Y.; Dayan, R.R.; Smoliakov, A.; Ullman, Y.; Shamia, D.; Ohayon, R.B.D.; Golbets, E.; El Haj, K.; et al. Point of Care Lung Ultrasound Injury Score—A simple and reliable assessment tool in COVID-19 patients (PLIS I): A retrospective study. PLoS ONE 2022, 17, e0267506. [Google Scholar] [CrossRef]
- Bonnel, A.R.; Baston, C.M.; Wallace, P.; Panebianco, N.; Kinosian, B. Using Point-of-Care Ultrasound on Home Visits: The Home-Oriented Ultrasound Examination (HOUSE). J. Am. Geriatr. Soc. 2019, 67, 2662–2663. [Google Scholar] [CrossRef] [PubMed]
- Nouvenne, A.; Ticinesi, A.; Parise, A.; Prati, B.; Esposito, M.; Cocchi, V.; Crisafulli, E.; Volpi, A.; Rossi, S.; Bignami, E.G.; et al. Point-of-Care Chest Ultrasonography as a Diagnostic Resource for COVID-19 Outbreak in Nursing Homes. J. Am. Med. Dir. Assoc. 2020, 21, 919–923. [Google Scholar] [CrossRef]
- Barnikel, M.; Alig, A.H.S.; Anton, S.; Arenz, L.; Bendz, H.; Fraccaroli, A.; Götschke, J.; Vornhülz, M.; Plohmann, P.; Weiglein, T.; et al. Follow-up lung ultrasound to monitor lung failure in COVID-19 ICU patients. PLoS ONE 2022, 17, e0271411. [Google Scholar] [CrossRef]
- Gurbani, N.; Acosta-Sorensen, M.; Díaz-Pérez, D.; Figueira-Goncalves, J.M.; Ramallo-Fariña, Y.; Trujillo-Castilla, J.L. Clinical outcomes and lung ultrasound findings in COVID-19 follow up: Calm comes after the storm? Respir. Med. Res. 2022, 82, 100907. [Google Scholar] [CrossRef]
- Allegrante, J.P.; Wells, M.T.; Peterson, J.C. Interventions to Support Behavioral Self-Management of Chronic Diseases. Annu. Rev. Public Health 2019, 40, 127–146. [Google Scholar] [CrossRef]
- Lambrinou, E.; Hansen, T.B.; Beulens, J.W. Lifestyle factors, self-management and patient empowerment in diabetes care. Eur. J. Prev. Cardiol. 2019, 26 (Suppl. S2), 55–63. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.M.; Kneale, D.; Lasserson, T.J.; McDonald, V.M.; Grigg, J.; Thomas, J. School-based self management interventions for asthma in children and adolescents: A mixed methods systematic review. Cochrane Database Syst. Rev. 2015, 1, CD011651. [Google Scholar] [CrossRef]
- Nakamura, N.; Koga, T.; Iseki, H. A meta-analysis of remote patient monitoring for chronic heart failure patients. J. Telemed. Telecare 2014, 20, 11–17. [Google Scholar] [CrossRef]
- Shaddock, L.; Smith, T. Potential for Use of Portable Ultrasound Devices in Rural and Remote Settings in Australia and Other Developed Countries: A Systematic Review. J. Multidiscip. Health 2022, 15, 605–625. [Google Scholar] [CrossRef] [PubMed]

| Total (N = 19) | |
|---|---|
| Gender | |
| Female | 7 (36.8%) |
| Male | 12 (63.2%) |
| Age, Mean (SD) | 65.7 (14.3) |
| Years of education, Median [Min, Max] | 12.0 [6.00, 20.0] |
| Missing | 4 (21.1%) |
| Weekly dialysis treatments, Median [Min, Max] | 3.00 [3.00, 4.00] |
| Native language | |
| Amharic | 1 (5.3%) |
| Arabic | 2 (10.5%) |
| Hebrew | 16 (84.2%) |
| Religion | |
| Jewish | 17 (89.5%) |
| Muslim | 2 (10.5%) |
| First wet weight, Mean (SD) | 77.3 (15.3) |
| DM | 7 (36.8%) |
| Missing | 2 (10.5%) |
| HTN | 16 (84.2%) |
| Missing | 2 (10.5%) |
| IHD | 7 (36.8 |
| Missing | 2 (10.5%) |
| Kidney transplantation | 2 (10.5%) |
| Patients’ Count | Expert’s Count on Patients’ Clip | Expert’s Count on Researcher’s Clip | Patients’ Auto-B-Line Count | Researcher’s Auto-B-Line Count | |
|---|---|---|---|---|---|
| Patients’ count | 1 | ||||
| Expert’s count, patient’s clip | 0.08 [−1.0–1.0] (N = 58) | 1 | |||
| Expert’s count, researcher’s clip | 0.20 [−0.89–1.0] (N = 129) | 0.30 [−0.76–1.0] (N = 69) | 1 | ||
| Patients’ auto-B-line count | 0.22 [−0.75–1.0] (N = 71) | 0.49 [−0.16–1.0] (N = 74) | 0.49 * [0.05–0.93] (N = 91) | 1 | |
| Researcher’s auto-B-line count | 0.07 [−1.0–1.0] (N = 110) | 0.26 [−0.74–1.0] (N = 69) | 0.67 * [0.67–0.67] (N = 241) | 0.46 [−0.09–1.0] (N = 100) | 1 |
| Enrollment | Second Visit | Third Visit | ||||
|---|---|---|---|---|---|---|
| Patient (N = 55) | Researcher (N = 62) | Patient (N = 47) | Researcher (N = 49) | Patient (N = 35) | Researcher (N = 35) | |
| Quality category | ||||||
| Poor | 12 (21.8%) | 3 (4.8%) | 18 (38.3%) | 3 (6.1%) | 15 (42.9%) | 0 (0%) |
| Good | 12 (21.8%) | 10 (16.1%) | 16 (34.0%) | 10 (20.4%) | 8 (22.9%) | 6 (17.1%) |
| Excellent | 31 (56.4%) | 49 (79.0%) | 13 (27.7%) | 36 (73.5%) | 12 (34.3%) | 29 (82.9%) |
| Descriptive parameters | ||||||
| Mean (SD) | 1.35 (0.821) | 1.74 (0.541) | 0.894 (0.814) | 1.67 (0.591) | 0.914 (0.887) | 1.83 (0.382) |
| Median [Q1, Q3] | 2.00 [1.00, 2.00] | 2.00 [2.00, 2.00] | 1.00 [0, 2.00] | 2.00 [1.00, 2.00] | 1.00 [0, 2.00] | 2.00 [2.00, 2.00] |
| EVENT_NAME | N | Mean | SD | 25th | 50th | 75th | Min | Max |
|---|---|---|---|---|---|---|---|---|
| Enrollment and 2nd visit | 16 | 16.4 | 12.9 | 4.25 | 15.5 | 28 | 2 | 40 |
| 2nd and 3rd visits | 10 | 16.4 | 10.3 | 9.75 | 13.0 | 26 | 3 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, E.; Maimon, N.; Hasidim, A.; Shnaider, A.; Migliozzi, G.; Haviv, Y.S.; Halpern, D.; Abu Ganem, B.; Fuchs, L. Can Dialysis Patients Identify and Diagnose Pulmonary Congestion Using Self-Lung Ultrasound? J. Clin. Med. 2023, 12, 3829. https://doi.org/10.3390/jcm12113829
Schneider E, Maimon N, Hasidim A, Shnaider A, Migliozzi G, Haviv YS, Halpern D, Abu Ganem B, Fuchs L. Can Dialysis Patients Identify and Diagnose Pulmonary Congestion Using Self-Lung Ultrasound? Journal of Clinical Medicine. 2023; 12(11):3829. https://doi.org/10.3390/jcm12113829
Chicago/Turabian StyleSchneider, Eyal, Netta Maimon, Ariel Hasidim, Alla Shnaider, Gabrielle Migliozzi, Yosef S. Haviv, Dor Halpern, Basel Abu Ganem, and Lior Fuchs. 2023. "Can Dialysis Patients Identify and Diagnose Pulmonary Congestion Using Self-Lung Ultrasound?" Journal of Clinical Medicine 12, no. 11: 3829. https://doi.org/10.3390/jcm12113829
APA StyleSchneider, E., Maimon, N., Hasidim, A., Shnaider, A., Migliozzi, G., Haviv, Y. S., Halpern, D., Abu Ganem, B., & Fuchs, L. (2023). Can Dialysis Patients Identify and Diagnose Pulmonary Congestion Using Self-Lung Ultrasound? Journal of Clinical Medicine, 12(11), 3829. https://doi.org/10.3390/jcm12113829





