Simultaneous Surgical Approach with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients with Concurrent Peritoneal and Liver Metastases of Colon Cancer Origin
Abstract
:1. Introduction
2. Material and Methodology
2.1. Study Design and Patient Selection
2.2. Follow-Up
2.3. Operative Technique
2.4. Endpoints
2.5. Statistical Analysis
2.6. Financial Support
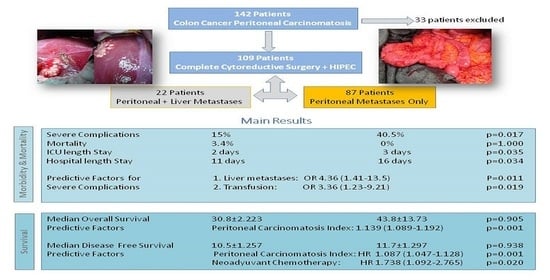
3. Results
3.1. Demographics and Perioperative Characteristics
3.2. Morbidity and Mortality
3.3. Survival and Recurrence
4. Discussion
4.1. Morbidity and Mortality
4.2. Survival and Recurrence
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cloyd, J.M.; Abdel-Misih, S.; Hays, J.; Dillhoff, M.E.; Pawlik, T.M.; Schmidt, C. Impact of Synchronous Liver Resection on the Perioperative Outcomes of Patients Undergoing CRS-HIPEC. J. Gastrointest. Surg. 2018, 22, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Downs-Canner, S.; Shuai, Y.; Ramalingam, L.; Pingpank, J.F.; Holtzman, M.P.; Zeh, H.J.; Bartlett, D.L.; Choudry, H.A. Safety and efficacy of combined resection of colorectal peritoneal and liver metastases. J. Surg. Res. 2017, 219, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Hadden, W.J.; De Reuver, P.R.; Brown, K.; Mittal, A.; Samra, J.S.; Hugh, T.J. Resection of colorectal liver metastases and extra-hepatic disease: A systematic review and proportional meta- analysis of survival outcomes. Int. Hepato-Pancreato-Biliary Assoc. 2016, 18, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Franko, J.; Shi, Q.; Meyers, J. Prognosis of colorectal peritoneal metastases: An analysis of 10,553 patients treated with systemic therapy in prospective randomized trials (ARCAD database) of individual patient data from prospective randomised trials from the Analysis and Research in C. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Cao, C.Q.; Yan, T.D.; Liauw, W.; Morris, D.L. Comparison of Optimally Resected Hepatectomy and Peritonectomy Patients With Colorectal Cancer Metastasis. J. Surg. Oncol. 2009, 100, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Blackham, A.U.; Russell, G.B.; Stewart IV, J.H.; Votanopoulos, K.; Levine, E.A.; Shen, P. Metastatic colorectal cancer: Survival comparison of hepatic resection versus cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2014, 21, 2667–2674. [Google Scholar] [CrossRef] [Green Version]
- Thomassen, I.; van Gestel, Y.R.; Lemmens, V.E.; de Hingh, I.H. Incidence, Prognosis, and Treatment Options for Patients With Synchronous Peritoneal Carcinomatosis and Liver Metastases from Colorectal Origin. Dis. Colon Rectum 2013, 56, 1373–1380. [Google Scholar] [CrossRef]
- Shubert, C.R.; Habermann, E.B.; Bergquist, J.R.; Thiels, C.A.; Thomsen, K.M.; Kremers, W.K.; Kendrick, M.L.; Cima, R.R.; Nagorney, D.M. A NSQIP Review of Major Morbidity and Mortality of Synchronous Liver Resection for Colorectal Metastasis Stratified by Extent of Liver Resection and Type of Colorectal Resection. J. Gastrointest. Surg. 2015, 19, 1982–1994. [Google Scholar] [CrossRef]
- Elias, D.; Benizri, E.; Pocard, M.; Ducreux, M.; Boige, V.; Lasser, P. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. Eur. J. Surg. Oncol. 2006, 32, 632–636. [Google Scholar] [CrossRef]
- Alzahrani, N.; Ung, L.; Valle, S.J.; Liauw, W.; Morris, D.L. Synchronous liver resection with cytoreductive surgery for the treatment of liver and peritoneal metastases from colon cancer: Results from an Australian centre. ANZ J. Surg. 2015, 87, E167–E172. [Google Scholar] [CrossRef]
- Lo Dico, R.; Faron, M.; Yonemura, Y.; Glehen, O.; Pocard, M.; Sardi, A.; Hübner, M.; Baratti, D.; Liberale, G.; Kartheuser, A.; et al. Combined liver resection and cytoreductive surgery with HIPEC for metastatic colorectal cancer: Results of a worldwide analysis of 565 patients from the Peritoneal Surface Oncology Group International (PSOGI). Eur. J. Surg. Oncol. 2021, 47, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kianmanesh, R.; Scaringi, S.; Sabate, J.-M.; Castel, B.; Pons-Kerjean, N.; Coffin, B.; Hay, J.-M.; Flamant, Y.; Msika, S. Iterative Cytoreductive Surgery Associated With Hyperthermic Intraperitoneal Chemotherapy for Treatment of Peritoneal Carcinomatosis of Colorectal Origin With or Without Liver Metastases. Ann. Surg. 2007, 245, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Delhorme, J.B.; Dupont-Kazma, L.; Addeo, P.; Lefebvre, F.; Triki, E.; Romain, B.; Meyer, N.; Bachellier, P.; Rohr, S.; Brigand, C. Peritoneal carcinomatosis with synchronous liver metastases from colorectal cancer: Who will benefit from complete cytoreductive surgery? Int. J. Surg. 2016, 25, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Navez, J.; Remue, C.; Leonard, D.; Bachmann, R.; Kartheuser, A.; Hubert, C.; Coubeau, L.; Komuta, M.; Van den Eynde, M.; Zech, F.; et al. Surgical Treatment of Colorectal Cancer with Peritoneal and Liver Metastases Using Combined Liver and Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Report from a Single-Centre Experience. Ann. Surg. Oncol. 2016, 23, 666–673. [Google Scholar] [CrossRef]
- Mouw, T.J.; Lu, J.; Woody-Fowler, M.; Ashcraft, J.; Valentino, J.; DiPasco, P.; Mammen, J.; Al-Kasspooles, M. Morbidity and mortality of synchronous hepatectomy with cytoreductive surgery/hyperthermic intraperitoneal chemotherapy (CRS/HIPEC). J. Gastrointest. Oncol. 2018, 9, 828–832. [Google Scholar] [CrossRef]
- Flood, M.P.; Das Atalindra, A.; Soucisse, M.L.; Kong, J.; Ramsay, R.G.; Michael, M.; Hons, M.B.B.S.; Hons, B.S.; Loveday, B.P.T.; Warrier, S.K. Synchronous Liver Resection, Cytoreductive Surgery, and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Liver and Peritoneal Metastases: A Systematic Review and Meta-analysis. Dis. Colon Rectum 2021, 64, 754–764. [Google Scholar] [CrossRef]
- Morales Soriano, R.; Morón Canis, J.M.; Molina Romero, X.; Pérez Celada, J.; Tejada Gavela, S.; Segura Sampedro, J.J.; Jiménez Morillas, P.; Díaz Jover, P.; García Pérez, J.M.; Sena Ruiz, F.; et al. Influence of simultaneous liver and peritoneal resection on postoperative morbi-mortality and survival in patients with colon cancer treated with surgical cytoreduction and intraperitoneal hyperthermic chemotherapy. Cirugía Española 2017, 95, 214–221. [Google Scholar] [CrossRef]
- Chua, T.C.; Yan, T.D.; Zhao, J.; Morris, D.L. Peritoneal carcinomatosis and liver metastases from colorectal cancer treated with cytoreductive surgery perioperative intraperitoneal chemotherapy and liver resection. Eur. J. Surg. Oncol. 2009, 35, 1299–1305. [Google Scholar] [CrossRef]
- Allard, M.A.; Adam, R.; Ruiz, A.; Vibert, E.; Paule, B.; Levi, F.; Sebagh, M.; Guettier, C.; Azoulay, D.; Castaing, D. Is unexpected peritoneal carcinomatosis still a contraindication for resection of colorectal liver metastases?: Combined resection of colorectal liver metastases with peritoneal deposits discovered intra-operatively. Eur. J. Surg. Oncol. 2013, 39, 981–987. [Google Scholar] [CrossRef]
- Soldevila-Verdeguer, C.; Segura-Sampedro, J.J.; Pineño-Flores, C.; Sanchís-Cortés, P.; González-Argente, X.; Morales-Soriano, R. Hepatic resection and blood transfusion increase morbidity after cytoreductive surgery and HIPEC for colorectal carcinomatosis. Clin. Transl. Oncol. 2020, 22, 2032–2039. [Google Scholar] [CrossRef]
- Chua, T.C.; Yan, T.D.; Saxena, A.; Morris, D.L. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure? A systematic review of morbidity and mortality. Ann. Surg. 2009, 249, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Segura-Sampedro, J.J.; Morales-Soriano, R.; Pineño Flores, C.; Craus-Miguel, A.; Sugarbaker, P.H. Laparoscopy technique in the setting of peritoneal metastases to avoid port site relapse. Surg. Oncol. 2021, 37, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Pérez, N.; Ferrer-Robles, A.; Gómez-Romero, G.; Fabián-Gonzalez, D.; Verd-Rodriguez, M.; Mora-Fernandez, L.C.; Segura-Sampedro, J.J.; Tejada-Gavela, S.; Morales-Soriano, R. Goal-directed therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: A prospective observational study. Clin. Transl. Oncol. 2019, 21, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Bandou, E.; Kawamura, T.; Endou, Y.; Sasaki, T. Quantitative prognostic indicators of peritoneal dissemination of gastric cancer. Eur. J. Surg. Oncol. 2006, 32, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J.; Sticca, R.; Sugarbaker, P.; Levine, E.; Yan, T.D.; Alexander, R.; Baratti, D.; Bartlett, D.; Barone, R.; Barrios, P.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in the Management of Peritoneal Surface Malignancies of Colonic Origin: A Consensus Statement. Ann. Surg. Oncol. 2007, 14, 128–133. [Google Scholar] [CrossRef]
- González-Bayón, L.; González-Moreno, S.; Ortega-Pérez, G. Safety considerations for operating room personnel during hyperthermic intraoperative intraperitoneal chemotherapy perfusion. Eur. J. Surg. Oncol. 2006, 32, 619–624. [Google Scholar] [CrossRef]
- González-Moreno, S.; González-Bayón, L.; Ortega-Pérez, G. Hyperthermic Intraperitoneal Chemotherapy. Methodology and Safety Considerations. Surg. Oncol. Clin. North Am. 2012, 21, 543–557. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The clavien-dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D.J. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: A pooled analysis of North Central Cancer Treatment Group phase III trials N9741 and N9841. J. Clin. Oncol. 2012, 30, 263–267. [Google Scholar] [CrossRef]
- Lenos, K.J.; Bach, S.; Ferreira Moreno, L.; ten Hoorn, S.; Sluiter, N.R.; Bootsma, S.; Vieira Braga, F.A.; Nijman, L.E.; van den Bosch, T.; Miedema, D.M.; et al. Molecular characterization of colorectal cancer related peritoneal metastatic disease. Nat. Commun. 2022, 13, 4443. [Google Scholar] [CrossRef]
- de Cuba, E.M.V.; Kwakman, R.; Knol, D.L.; Bonjer, H.J.; Meijer, G.A.; te Velde, E.A. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases. Systematic review of all literature and meta-analysis of observational studies. Cancer Treat. Rev. 2013, 39, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Dube, P.; Bonvalot, S.; Meshaka, P.; Manai, M.; Cavalcanti, A.; Lasser, P. Treatment of liver metastases with moderate peritoneal carcinomatosis by hepatectomy and cytoreductive surgery follow by inmediate post-operative intraperitoneal chemotherapy: Feasibility and preliminary results. Hepato-Gastroenterol. 1999, 46, 360–363. [Google Scholar] [PubMed]
- Esquivel, J.; Elias, D.; Baratti, D.; Kusamura, S.; Deraco, M. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J. Surg. Oncol. 2008, 98, 263–267. [Google Scholar] [CrossRef]
- Maggiori, L.; Goéré, D.; Viana, B.; Tzanis, D.; Dumont, F.; Honoré, C.; Eveno, C.; Elias, D. Should Patients With Peritoneal Carcinomatosis of Colorectal Origin With Synchronous Liver Metastases Be Treated With a Curative Intent? A Case-Control Study. Ann. Surg. 2013, 258, 116–121. [Google Scholar] [CrossRef] [PubMed]
- El-Nakeep, S.; Rashad, N.; Oweira, H.; Schmidt, J.; Helbling, D.; Giryes, A.; Petrausch, U.; Mehrabi, A.; Decker, M.; Abdel-Rahman, O. Intraperitoneal chemotherapy and cytoreductive surgery for peritoneal metastases coupled with curative treatment of colorectal liver metastases: An updated systematic review. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Maggiori, L.; Elias, D. Curative treatment of colorectal peritoneal carcinomatosis: Current status and future trends. Eur. J. Surg. Oncol. 2010, 36, 599–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, A.; Valle, S.J.; Liauw, W.; Morris, D.L. Limited synchronous hepatic resection does not compromise peri-operative outcomes or survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Surg. Oncol. 2017, 115, 417–424. [Google Scholar] [CrossRef]
- Hübner, M.; Kusamura, S.; Villeneuve, L.; Al-niaimi, A.; Alyami, M.; Balonov, K.; Bell, J.; Bristow, R.; Glehen, O.; Fagotti, A.; et al. European Journal of Surgical Oncology Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced recovery after surgery (ERAS®) Society Recommendations d Part I: Pre. Eur. J. Surg. Oncol. 2020, 46, 2292–2310. [Google Scholar] [CrossRef]
- Saxena, A.; Yan, T.D.; Chua, T.C.; Morris, D.L. Critical assessment of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann. Surg. Oncol. 2010, 17, 1291–1301. [Google Scholar] [CrossRef]
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial Therapy with FOLFOXIRI and Bevacizumab for Metastatic Colorectal Cancer. N. Engl. J. Med. 2014, 371, 1609–1618. [Google Scholar] [CrossRef] [Green Version]
- Pinto, A.; Hobeika, C.; Philis, A.; Kirzin, S.; Carrère, N.; Ghouti, L. Synchronous liver metastases and peritoneal carcinomatosis from colorectal cancer: Different strategies for curative treatment? Langenbeck’s Arch. Surg. 2019, 404, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.M.; Ferron, G.; Guilloit, J.M.; Meeus, P.; Goéré, D.; et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J. Clin. Oncol. 2009, 27, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Lorimier, G.; Linot, B.; Paillocher, N.; Dupoiron, D.; Verrièle, V.; Wernert, R.; Hamy, A.; Capitain, O. Curative cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis and synchronous resectable liver metastases arising from colorectal cancer. Eur. J. Surg. Oncol. 2017, 43, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Faron, M.; Goéré, D.; Dumont, F.; Honoré, C.; Boige, V.; Malka, D.; Ducreux, M. A simple tumor load-based nomogram for surgery in patients with colorectal liver and peritoneal metastases. Ann. Surg. Oncol. 2014, 21, 2052–2058. [Google Scholar] [CrossRef]
- Goéré, D.; Souadka, A.; Faron, M.; Cloutier, A.S.; Viana, B.; Honoré, C.; Dumont, F.; Elias, D. Extent of Colorectal Peritoneal Carcinomatosis: Attempt to Define a Threshold Above Which HIPEC Does Not Offer Survival Benefit: A Comparative Study. Ann. Surg. Oncol. 2015, 22, 2958–2964. [Google Scholar] [CrossRef]



| Perioperative Data | LR (−) N: 87 | LR (+) N: 22 | p |
|---|---|---|---|
| Age | 61.4 (54.9) | 66.6 (60.5–71.3) | 0.030 |
| Female | 42 (48.3%) | 10 (45.5%) | 0.812 |
| Men | 45 (51.7% | 12 (54.5%) | |
| ASA-I | 8 (9.2%) | 3 (13.6%) | NA |
| ASA-II | 55 (63.2%) | 12 (54.5%) | |
| ASA-III | 24 (26.4%) | 7 (31.8%) | |
| ECOG | NA | ||
| 0 | 52 (59.8%) | 18 (81.8%) | |
| 1 | 32 (36.8%) | 4 (18.2%) | |
| 2 | 2 (2.3%) | 0 | |
| 3 | 1 (1.1%) | 0 | |
| Charlson | 6 (6–7) | 7 (6–8) | 0.035 |
| Preoperative Chemotherapy | 35 (40.2%) | 12 (54%) | 0.226 |
| KRAS mutation | 35 (40.2%) | 13 (59.1%) | 0.111 |
| Surgical PCI | 8 (3–14) | 9 (6–14) | 0.380 |
| Operative Time (minutes) | 467 (390–567) | 512 (456–638) | 0.117 |
| No. resected organs | 4 (2–4) | 4 (3–5) | 0.171 |
| No. of anastomosis | 1 (1–3) | 1 (1–3) | 0.735 |
| CCR-0 | 83 (95.4%) | 21 (95.4%) | 0.581 |
| CCR-1 | 4 (4.6%) | 1 (4.6%) | |
| Transfusion rate Blood packs/patient | 37 (42.5%) 1.4 (0–10) | 11 (50%) 2.7 (0–12) | 0.528 |
| Stoma formation | 4 (4.6%) | 0 | 0.581 |
| Liver Metastases | n: 22 |
|---|---|
| Location | |
| Subcapsular | 5 (22.7%) |
| Intraparenchymal | 15 (68.2%) |
| Both | 2 (9.1%) |
| Number of metastases | |
| 1 | 9 (40.9%) |
| 2 | 11 (50%) |
| 3 | 2 (9.1%) |
| Size (cm) | 2 |
| Type of liver resection | |
| Segmentectomy | 9 (40.9%) |
| Atypical resection | 13 (59.1%) |
| Types of Resected Organ | LR (−) No (%) | LR (+) No (%) | p |
|---|---|---|---|
| Peritoneum | 79 (21.9) | 17 (19.9) | 0.34 |
| Omentectomy | 62 (17.4) | 10 (11.6) | 0.06 |
| Diaphragm resection | 8 (2.2) | 4 (4.6) | 0.20 |
| Gastric resection | 4 (1.1) | 0 | 0.31 |
| Cholecystectomy | 38 (10.7) | 13 (15.1) | 0.15 |
| Splenectomy | 16 (4.5) | 0 | 0.03 |
| Duodenum (lateral resection) | 0 | 3 (3.5) | 0.0004 |
| Pancreatectomy (corporo-caudal) | 3 (0.8) | 0 | 0.38 |
| Adrenalectomy | 0 | 1 (1.2) | 0.004 |
| Small bowel | 33 (9.3) | 8 (9.3) | 1 |
| Right/Transverse colectomy | 30 (8.5) | 7 (8.1) | 0.91 |
| Left/Sigmoid/Rectal resection | 36 (10.1) | 10 (11.6) | 0.63 |
| Subtotal colectomy | 5 (1.4) | 0 | 0.26 |
| Nephrectomy | 0 | 3 (3.5) | 0.0004 |
| Ureter resection | 6 (1.7) | 2 (2.3) | 0.68 |
| Cystectomy | 2 (1.1) | 1 (1.2) | 0.54 |
| Hysterectomy/Ovarian resection | 23 (6.5) | 4 (4.6) | 0.48 |
| Aortic Lymphadenectomy | 10 (2.8) | 3 (3.5) | 0.82 |
| Postoperative Complications | LR (−) N: 87 | LR (+) N: 22 | |
|---|---|---|---|
| Overall Morbidity (90 days) | 22 (25.3%) | 11 (50%) | 0.024 |
| Clavien–Dindo (90 days) | |||
| Grade 0 | 14 (17.7%) | 5 (13.5%) | |
| Grade 1 | 15 (19%) | 2 (5.4%) | 0.059 |
| Grade 2 | 33 (41.8%) | 15 (40.5%) | |
| Grade 3 | 6 (7.6%) | 5 (13.5%) | 0.017 |
| Grade 4 | 9 (11.4%) | 10 (27%) | |
| Reinterventions | 5 (5.7%) | 2 (9.1%) | 0.567 |
| -Evisceration | 2 | 0 | |
| -Colonic fistulae | 1 | 0 | |
| -Abdominal abscess | 1 | 0 | |
| -Anastomotic dehiscence | 1 | 1 | |
| -Ileus | 0 | 1 | |
| Overall Mortality (90 days) | 3 (2.7%) | ||
| Mortality 90 (days) | 3 (3.4%) | 0 (0) | 1.000 |
| Causes of death | |||
| -Bilateral Pneumonia and distress | 2 | 0 | |
| -Hemophagocytic syndrome | 1 | 0 | |
| ICU length of stay (median) | 2 (2–3) | 3 (2–4) | 0.039 |
| Hospital length of stay (median) | 11 (9–16) | 16 (10–35) | 0.035 |
| Abdominal abscess | 13 (14.9%) | 8 (36.4%) | 0.034 |
| Superficial SSI | 10 (11.5%) | 4 (8.2%) | 0.475 |
| Small bowel fistula | 3 (3.4%) | 1 (4.5%) | 1.000 |
| Anastomotic leak | 2 (2.3%) | 1 (4.5%) | 0.495 |
| Hemoperitoneum | 2 (2.3%) | 1 (4.5%) | 0.495 |
| Chylous ascites | 0 | 1 (4.5%) | 0.202 |
| Hemothorax | 1 (1.1%) | 0 | 1.000 |
| Thrombocytopenia | 19 (21.8%) | 9 (40.9%) | 0.067 |
| Ileus | 11 (12.6%) | 6 (27.3%) | 0.106 |
| Leukopenia | 8 (9.2%) | 1 (4.5%) | 0.683 |
| Pneumoniae | 8 (9.2%) | 3 (13.6%) | 0.691 |
| Pleural effusion with drainage | 4 (4.6%) | 2 (9.1%) | 0.599 |
| Pneumonia and respiratory distress | 4 (4.6%) | 8 (36.4%) | 0.001 |
| Central line sepsis | 3 (3.4%) | 0 | 1.000 |
| Urinary infection | 3 (3.4%) | 1 (4.5%) | 1.000 |
| Stroke | 2 (2.3%) | 1 (4.5%) | 0.495 |
| Ulcerative gastritis | 2 (2.3%) | 0 | 1.000 |
| Acute pancreatitis | 1 (1.1%) | 0 | 1.000 |
| Variables | OR Crude | p | OR Adjusted | p |
|---|---|---|---|---|
| Liver metastases | 4.29 (1.61–11.46) | 0.004 | 4.36 (1.41–13.50) | 0.011 |
| Transfusion | 3.25 (1.36–7.74) | 0.008 | 3.36 (1.23–9.21) | 0.019 |
| Age | 1.13 (0.57–2.23) | 0.658 | 0.99 (0.98–1.01) | 0.438 |
| ASA | 1.13 (0.57–2.23) | 0.722 | 1.14 (0.53–2.44) | 0.722 |
| ECOG | 0.51 (0.22–1.20) | 0.124 | 0.72 (0.29–1.82) | 0.494 |
| PCI | 1.02 (0.96–1.09) | 0.533 | 0.99 (0.90–1.08) | 0.747 |
| Neoadjuvant chemotherapy | 1.35 (0.58–3.11) | 0.484 | 1.14 (0.44–2.96) | 0.785 |
| Operative time | 1.00 (1.00–1.01) | 0.112 | 1.00 (1.00–1.01) | 0.644 |
| Survival | LR (−) N: 87 | LR (+) N: 22 | p |
|---|---|---|---|
| Median Overall Survival | 30.8 ± 2.223 | 43.8 ± 13.373 | 0.905 |
| 1 year | 92% | 90% | |
| 3 years | 37.7% | 43.8% | |
| 5 years | 21.1% | 14.3% | |
| Median Disease Free Survival | 10.5 ± 1.257 | 11.7 ± 1.297 | 0.938 |
| 1 year | 40% | 38% | |
| 3 years | 22% | 14% | |
| 5 years | 16% | 14% |
| Survival Predictive Factors | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| Survival | Variables | HR | p | HR Cox Regression | p |
| Overall Survival | -PCI | 1.15 (1.10–1.20) | 0.000 | 1.139 (1.089–1.192) | 0.001 |
| -Neoadjuvant Chemo | 1.93 (1.16–3.21) | 0.012 | |||
| -CC score | 7.67 (2.25–26.18) | 0.001 | |||
| -Operative time | 0.48 (0.10–2.34) | 0.002 | |||
| -Severe complications (III–IV) * | 1.32 (1.05–1.65) | 0.016 | |||
| -Transfusion | 1.78 (1.07–2.96) | 0.0927 | |||
| Disease Free Survival | -PCI | 1.09 (1.05–1.13) | 0.000 | 1.087 (1.047–1.128) | 0.001 |
| -Neoadjuvant Chemo | 1.82 (1.18–2.82) | 0.007 | 1.738 (1.092–2.765) | 0.020 | |
| -No. of liver metastases | 25.51 (3.11–209.4) | 0.003 | |||
| -Operative time | 1.00 (1.00–1.00) | 0.029 | |||
| -Severe complications (III–IV) * | 25.51 (1.04–1.50) | 0.016 | |||
| -Native KRAS | 9.70 (1.01–93.23) | 0.049 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Soriano, R.; Pineño-Flores, C.; Morón-Canis, J.M.; Molina-Romero, F.J.; Rodriguez-Pino, J.C.; Loyola-Miró, J.; Gonzalez-Argente, F.X.; Palma-Zamora, E.; Guillot-Morales, M.; Giménez, S.; et al. Simultaneous Surgical Approach with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients with Concurrent Peritoneal and Liver Metastases of Colon Cancer Origin. J. Clin. Med. 2023, 12, 3860. https://doi.org/10.3390/jcm12113860
Morales-Soriano R, Pineño-Flores C, Morón-Canis JM, Molina-Romero FJ, Rodriguez-Pino JC, Loyola-Miró J, Gonzalez-Argente FX, Palma-Zamora E, Guillot-Morales M, Giménez S, et al. Simultaneous Surgical Approach with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients with Concurrent Peritoneal and Liver Metastases of Colon Cancer Origin. Journal of Clinical Medicine. 2023; 12(11):3860. https://doi.org/10.3390/jcm12113860
Chicago/Turabian StyleMorales-Soriano, Rafael, Cristina Pineño-Flores, José Miguel Morón-Canis, Francisco Javier Molina-Romero, José Carlos Rodriguez-Pino, Julia Loyola-Miró, Francisco Xavier Gonzalez-Argente, Elías Palma-Zamora, Mónica Guillot-Morales, Sandra Giménez, and et al. 2023. "Simultaneous Surgical Approach with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients with Concurrent Peritoneal and Liver Metastases of Colon Cancer Origin" Journal of Clinical Medicine 12, no. 11: 3860. https://doi.org/10.3390/jcm12113860
APA StyleMorales-Soriano, R., Pineño-Flores, C., Morón-Canis, J. M., Molina-Romero, F. J., Rodriguez-Pino, J. C., Loyola-Miró, J., Gonzalez-Argente, F. X., Palma-Zamora, E., Guillot-Morales, M., Giménez, S., Alvarez-Mon, M., Ortega, M. A., & Segura-Sampedro, J. J. (2023). Simultaneous Surgical Approach with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients with Concurrent Peritoneal and Liver Metastases of Colon Cancer Origin. Journal of Clinical Medicine, 12(11), 3860. https://doi.org/10.3390/jcm12113860