Percutaneous Vertebral Reconstruction (PVR) Technique of Pathological Compression Fractures: An Innovative Combined Treatment of Microwave Ablation, Bilateral Expandable Titanium SpineJack Implants Followed by Vertebroplasty
Abstract
1. Introduction
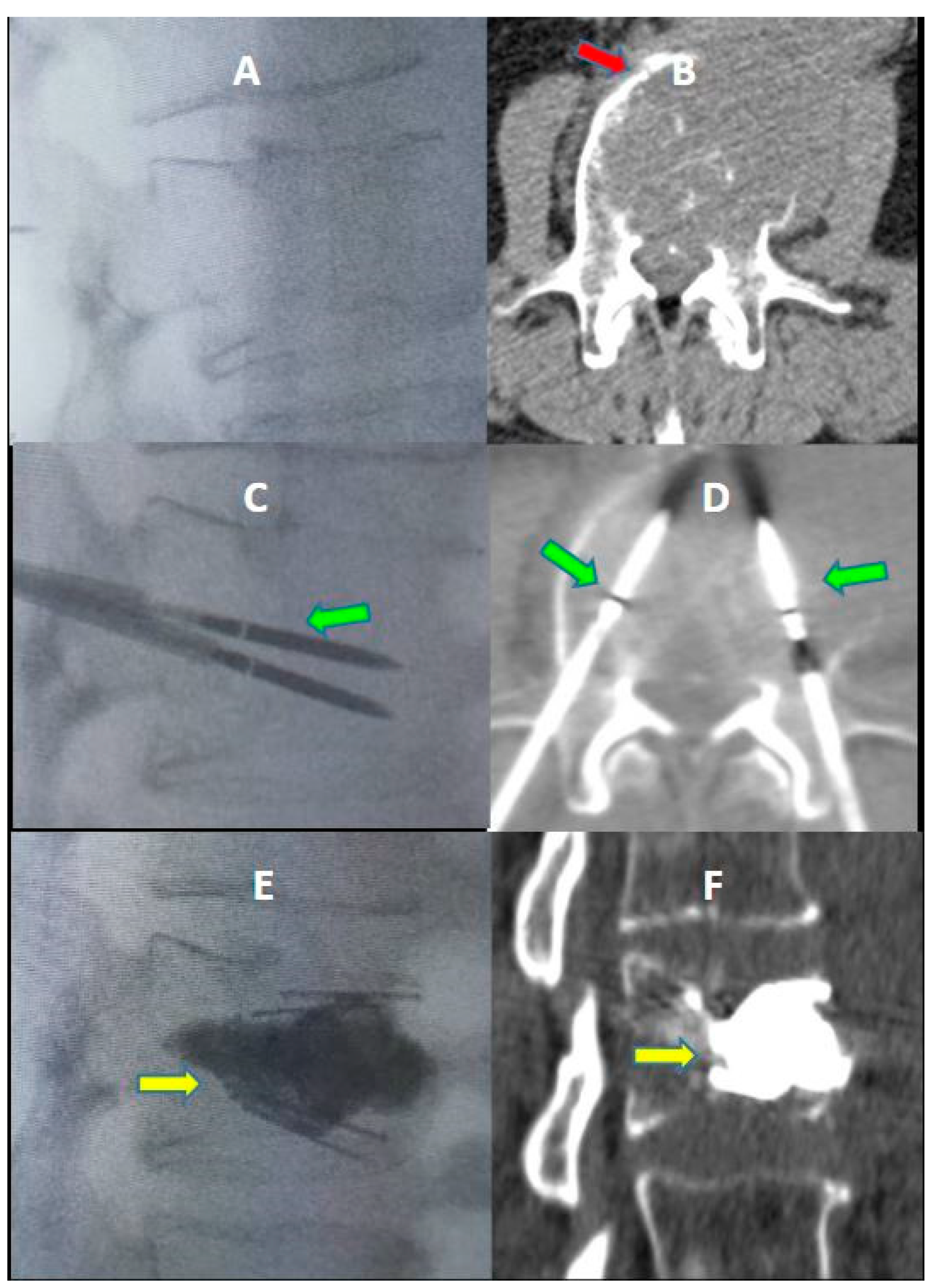
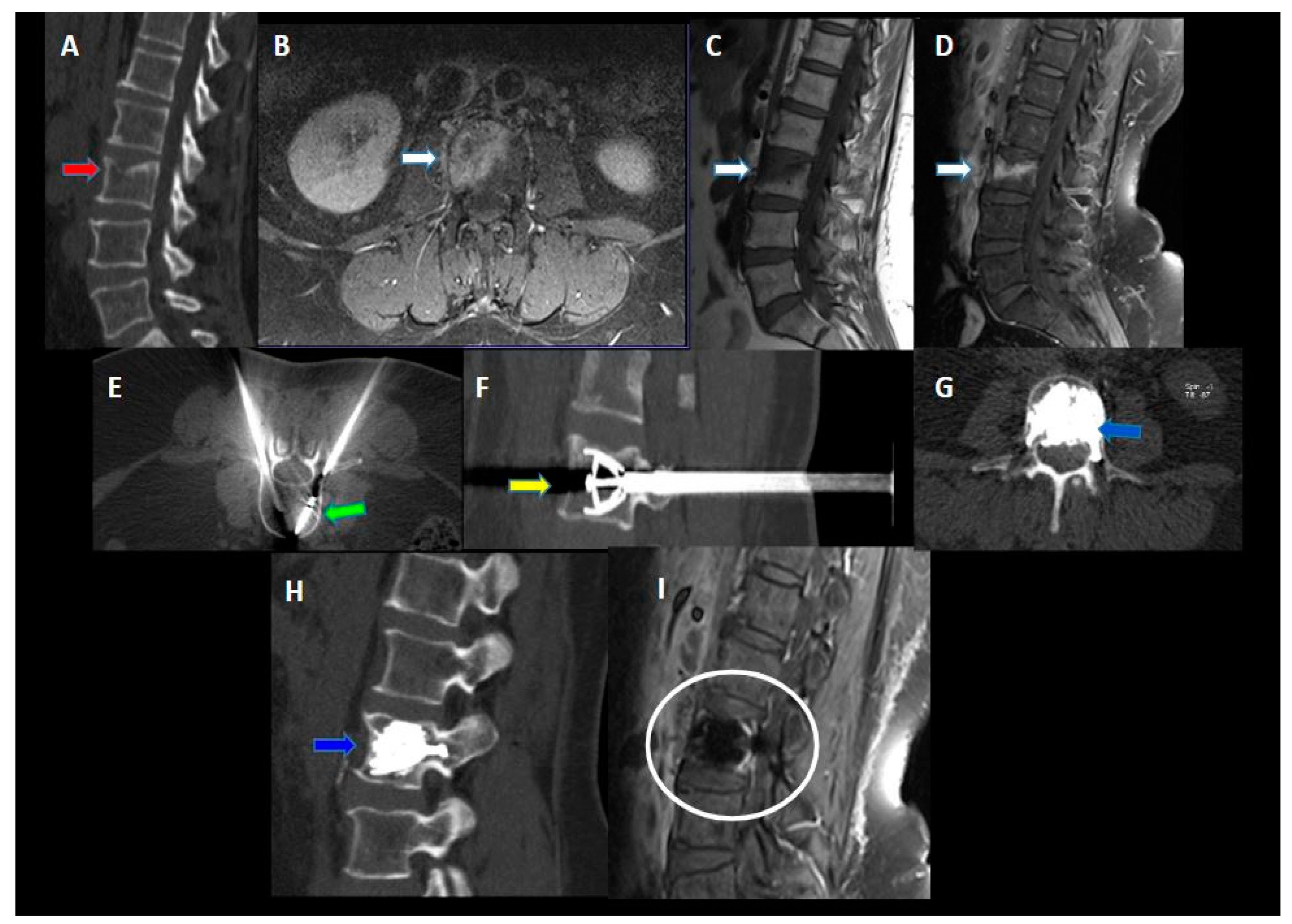
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janjan, N. Bone metastases: Approaches to management. Semin. Oncol. 2001, 28, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Gonzalez, D.E.; Roblesgil-Medrano, A.; Villarreal-Espinosa, J.B.; Tellez-Garcia, E.; Bueno-Gutierrez, L.C.; Rodriguez-Barreda, J.R.; Flores-Villalba, E.; Martinez, H.R.; Benvenutti-Regato, M.; Figueroa-Sanchez, J.A. Minimally Invasive versus Open Surgery for Spinal Metastasis: A Systematic Review and Meta-Analysis. Asian Spine J. 2022, 16, 583. [Google Scholar] [CrossRef] [PubMed]
- Gangi, A.; Kastler, B.; Klinkert, A.; Dietemann, J.L. Injection of alcohol into bone metastases under CT guidance. J. Comput. Assist. Tomogr. 1994, 18, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Cotten, A.; Demondion, X.; Boutry, N.; Cortet, B.; Chastanet, P.; Duquesnoy, B.; Leblond, D. Therapeutic percutaneous injections in the treatment of malignant acetabular osteolyses. Radiographics 1999, 19, 647–653. [Google Scholar] [CrossRef]
- Gangi, A.; Gasser, B.; De Unamuno, S.; Fogarrassy, E.; Fuchs, C.; Siffert, P.; Dietemann, J.-L.; Roy, C. New trends in interstitial laser photocoagulation of bones. Semin. Musculoskelet. Radiol. 1997, 1, 331–338. [Google Scholar] [CrossRef]
- Groënemeyer, D.H.; Schirp, S.; Gevargez, A. Image-guided percutaneous thermal ablation of bone tumors. Acad. Radiol. 2002, 9, 467–477. [Google Scholar] [CrossRef]
- Cooper, I. Cryogenic surgery: A new method of destruction or extirpation of benign or malignant tissues. N. Engl. J. Med. 1963, 268, 743–749. [Google Scholar] [CrossRef]
- Sewell, P.; Jackson, M.; Dhillon, G. Percutaneous MRI guided cryosurgery of bone tumors. Radiology 2002, 225, 514. [Google Scholar]
- Callstrom, M.R.; Atwell, T.D.; Charboneau, J.W.; Farrell, M.A.; Goetz, M.P.; Rubin, J.; Sloan, J.A.; Novotny, P.J.; Welch, T.J.; Maus, T.P.; et al. Painful metastases involving bone: Percutaneous image-guided cryoablation-prospective trial interim analysis. Radiology 2006, 241, 572–580. [Google Scholar] [CrossRef]
- Goetz, M.P.; Callstrom, M.R.; Charboneau, J.W.; Farrell, M.A.; Maus, T.P.; Welch, T.J.; Wong, G.Y.; Sloan, J.A.; Novotny, P.J.; Petersen, I.A.; et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: A multicenter study. J. Clin. Oncol. 2004, 22, 300–306. [Google Scholar] [CrossRef]
- Posteraro, A.; Dupuy, D.; Mayo-Smith, W. Radiofrequency ablation of bony metastatic disease. Clin. Radiol. 2004, 59, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, C.; Sotgia, B.; Fele, R.M.; Melis, L. Treatment of bone metastases with microwave thermal ablation. J. Vasc. Interv. Radiol. 2013, 24, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.; Byrne, C.; Pusceddu, C.; Buy, X.; Tsoumakidou, G.; Filippiadis, D. CIRSE Standards of Practice on Thermal Ablation of Bone Tumours. Cardiovasc. Interv. Radiol. 2022, 45, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Tsoumakidou, G.; Too, C.W.; Koch, G.; Caudrelier, J.; Cazzato, R.L.; Garnon, J.; Gangi, A. CIRSE Guidelines on Percutaneous Vertebral Augmentation. Cardiovasc. Interv. Radiol. 2017, 40, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, C.; Marsico, S.; Derudas, D.; Ballicu, N.; Melis, L.; Zedda, S.; De Felice, C.; Calabrese, A.; Santucci, D.; Faiella, E. Clinical Rationale of Using Steerable Technologies for Radiofrequency Ablation Followed by Cavity Creation and Cement Augmentation in the Treatment of Painful Spinal Metastases. Curr. Oncol. 2023, 30, 4257–4268. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, F.H.; Joly, Q.; Nouri-Neuville, M.; Ben-Ammar, M.; Kastler, B.; Kastler, A.; Amoretti, N.; Hauger, O. Innovative spine implants for improved augmentation and stability in neoplastic vertebral compression fracture. Medicina 2019, 55, 426. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Marcia, S.; Ryan, A.; Beall, D.P.; Masala, S.; Deschamps, F.; Kelekis, A. New implant-based technologies in the spine. Cardiovasc. Interv. Radiol. 2018, 41, 1463–1473. [Google Scholar] [CrossRef]
- Jacobson, R.E.; Nenov, A.; Duong, H.D. Re-expansion of Osteoporotic Compression Fractures Using Bilateral SpineJack Implants: Early Clinical Experience and Biomechanical Considerations. Cureus 2019, 11, e4572. [Google Scholar] [CrossRef]
- Cornelis, F.H.; Razakamanantsoa, L.; Ben Ammar, M.; Najdawi, M.; Gardavaud, F.; El-Mouhadi, S.; Barral, M. Expandable Intravertebral Implant in Cancer-Related Vertebral Compression Fractures: A Retrospective Review of 36 Implantations. J. Vasc. Interv. Radiol. 2022, 33, 14–18. [Google Scholar] [CrossRef]
- Noriega, D.; Maestretti, G.; Renaud, C.; Francaviglia, N.; Ould-Slimane, M.; Queinnec, S.; Ekkerlein, H.; Hassel, F.; Gumpert, R.; Sabatier, P.; et al. Clinical Performance and Safety of 108 SpineJack Implantations: 1-Year Results of a Prospective Multicentre Single-Arm Registry Study. BioMed Res. Int. 2015, 2015, 173872. [Google Scholar] [CrossRef]
- Hartman, J.; Granville, M.; Jacobson, R.E. Treatment of a High-risk Thoracolumbar Compression Fracture Using Bilateral Expandable Titanium SpineJack Implants. Cureus 2019, 11, e4701. [Google Scholar] [CrossRef]
- Montoya, J.E.M.; Torres, C.; Ferrer, E.R.; Rodríguez, E.E.M. A Colombian experience involving SpineJack®, a consecutive series of patients experiencing spinal fractures, percutaneous approach and anatomical restoration 2016–2017. J. Spine Surg. 2018, 4, 624–629. [Google Scholar] [CrossRef]
- Premat, K.; Perre, S.V.; Cormier, É.; Shotar, E.; Degos, V.; Morardet, L.; Fargeot, C.; Clarençon, F.; Chiras, J. Vertebral augmentation with the SpineJack® in chronic vertebral compression fractures with major kyphosis. Eur. Radiol. 2018, 28, 4985–4991. [Google Scholar] [CrossRef]
- Anselmetti, G.C.; Manca, A.; Tutton, S.; Chiara, G.; Kelekis, A.; Facchini, F.R.; Russo, F.; Regge, D.; Montemurro, F. Percutaneous vertebral augmentation assisted by PEEK implant in painful osteolytic vertebral metastasis involving the vertebral wall: Experience on 40 patients. Pain Physician 2013, 16, E397–E404. [Google Scholar] [CrossRef]
- Renaud, C. Treatment of vertebral compression fractures with the craniocaudal expandable implant SpineJack®: Technical note and outcomes in 77 consecutive patients. Orthop. Traumatol. Surg. Res. 2015, 101, 857–859. [Google Scholar] [CrossRef]
- Noriega, D.; Marcia, S.; Theumann, N.; Blondel, B.; Simon, A.; Hassel, F.; Maestretti, G.; Petit, A.; Weidle, P.A.; Mandly, A.G.; et al. A prospective, international, randomized, noninferiority study comparing an implantable titanium vertebral augmentation device versus balloon kyphoplasty in the reduction of vertebral compression fractures (SAKOS study). Spine J. 2019, 19, 1782–1795. [Google Scholar] [CrossRef]
- Noriega, D.C.; Rodrίguez-Monsalve, F.; Ramajo, R.; Sánchez-Lite, I.; Toribio, B.; Ardura, F. Long-term safety and clinical performance of kyphoplasty and SpineJack® procedures in the treatment of osteoporotic vertebral compression fractures: A pilot, monocentric, investigator-initiated study. Osteoporos. Int. 2019, 30, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, C.; Marsico, S.; Derudas, D.; Ballicu, N.; Melis, L.; de Felice, C.; Calabrese, A.; Santucci, D.; Faiella, E. Percutaneous CT-Guided Microwave Ablation Combined with Pedicle Screw Fixation Followed by Vertebroplasty (MASFVA): Initial Experience of a Minimally Invasive Treatment of Vertebral Metastases with Extension to the Vertebral Pedicle. Curr. Oncol. 2023, 30, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Mohme, M.; Riethdorf, S.; Dreimann, M.; Werner, S.; Maire, C.L.; Joosse, S.A.; Bludau, F.; Mueller, V.; Neves, R.P.L.; Stoecklein, N.H.; et al. Circulating Tumour Cell Release after Cement Augmentation of Vertebral Metastases. Sci. Rep. 2017, 7, 7196. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, N.S.; Haider, A.S.; Rowe, S.E.; Haider, M.; Sharma, R.; Neeley, O.J.; Dahdaleh, N.S.; Adogwa, O.; Bagley, C.A.; El Ahmadieh, T.Y.; et al. Microwave Ablation as a Treatment for Spinal Metastatic Tumors: A Systematic Review. World Neurosurg. 2021, 148, 15–23. [Google Scholar] [CrossRef]
- Kastler, A.; Alnassan, H.; Aubry, S.; Kastler, B. Microwave thermal ablation of spinal metastatic bone tumors. J. Vasc. Interv. Radiol. 2014, 25, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Deib, G.; Deldar, B.; Patel, A.M.; Barr, J.S. Efficacy and safety of percutaneous microwave ablation and cementoplasty in the treatment of painful spinal metastases and myeloma. Am. J. Neuroradiol. 2018, 39, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Luna, L.P.; Sankaran, N.; Ehresman, J.; Sciubba, D.M.; Khan, M. Successful percutaneous treatment of bone tumors using microwave ablation in combination with Zoledronic acid infused PMMA cementoplasty. J. Clin. Neurosci. 2020, 76, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, C.; Faiella, E.; Derudas, D.; Ballicu, N.; Melis, L.; Zedda, S.; Marsico, S. Re-expansion of vertebral compression fractures in patients with multiple myeloma with percutaneous vertebroplasty using spinejack implants: A preliminary and retrospective study. Front. Surg. 2023, 10, 1121981. [Google Scholar] [CrossRef]





| Patient | Age | Gender | Primary Cancer | Treated Vertebrae | Total n° SJ Impalnts | Total n° MWA | Complications |
|---|---|---|---|---|---|---|---|
| 1 | 71 | F | breast | L5 | 2 | 1 | |
| 2 | 69 | F | breast | L1 | 2 | 1 | |
| 3 | 56 | M | thyroid | L1 | 2 | 2 | |
| 4 | 64 | M | lung | L3, T12 | 4 | 1 | T12 vertebra fracture 7 days after L3 treatment |
| 5 | 55 | M | oral cavity | T10 | 2 | 1 | |
| 6 | 58 | M | colon | L3, T12 | 2 | 2 | |
| 7 | 67 | M | lung | L2 | 2 | 1 | |
| 8 | 66 | M | NET | L3, T12 | 2 | 2 | |
| 9 | 49 | M | kidney | L2 | 2 | 2 | intradiscal leakage |
| 10 | 72 | F | breast | L1 | 2 | 1 | |
| 11 | 56 | M | testicle | T12 | 2 | 1 | |
| 12 | 80 | F | pancreas | L1, T12 | 4 | 4 | |
| 13 | 71 | M | lung | T7 | 2 | 2 | |
| 14 | 76 | F | kidney | T10, T12 | 3 | 4 | |
| 15 | 69 | M | lung | T9, T10, T11 | 6 | 2 | T11 vertebra fracture 7 days after T9–T10 treatment |
| 16 | 66 | F | breast | L5 | 2 | 1 | |
| 17 | 64 | F | oral cavity | L1 | 2 | 1 | |
| 18 | 74 | M | lung | L1, T12 | 4 | 4 | |
| 19 | 68 | F | breast | L1, L2, L3 | 6 | 3 | |
| 20 | 75 | F | parotid | L32 | 2 | 1 | |
| 21 | 62 | F | breast | L1 | 2 | 2 | anterolateral cement leakage |
| 22 | 51 | F | breast | L1 | 2 | 1 | |
| 23 | 72 | F | thyroid | L1 | 2 | 1 | |
| 24 | 75 | F | breast | T12 | 2 | 1 | posterolateral cement leakage |
| 25 | 41 | M | melanoma | T10 | 2 | 2 | |
| 26 | 73 | M | bladder | T8, T9 | 4 | 2 | |
| 27 | 76 | F | breast | T11 | 2 | 1 | |
| 28 | 72 | F | lung | T6, T7 | 4 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pusceddu, C.; Marsico, S.; Derudas, D.; Ballicu, N.; Melis, L.; Zedda, S.; de Felice, C.; Calabrese, A.; De Francesco, D.; Venturini, M.; et al. Percutaneous Vertebral Reconstruction (PVR) Technique of Pathological Compression Fractures: An Innovative Combined Treatment of Microwave Ablation, Bilateral Expandable Titanium SpineJack Implants Followed by Vertebroplasty. J. Clin. Med. 2023, 12, 4178. https://doi.org/10.3390/jcm12134178
Pusceddu C, Marsico S, Derudas D, Ballicu N, Melis L, Zedda S, de Felice C, Calabrese A, De Francesco D, Venturini M, et al. Percutaneous Vertebral Reconstruction (PVR) Technique of Pathological Compression Fractures: An Innovative Combined Treatment of Microwave Ablation, Bilateral Expandable Titanium SpineJack Implants Followed by Vertebroplasty. Journal of Clinical Medicine. 2023; 12(13):4178. https://doi.org/10.3390/jcm12134178
Chicago/Turabian StylePusceddu, Claudio, Salvatore Marsico, Daniele Derudas, Nicola Ballicu, Luca Melis, Stefano Zedda, Carlo de Felice, Alessandro Calabrese, Davide De Francesco, Massimo Venturini, and et al. 2023. "Percutaneous Vertebral Reconstruction (PVR) Technique of Pathological Compression Fractures: An Innovative Combined Treatment of Microwave Ablation, Bilateral Expandable Titanium SpineJack Implants Followed by Vertebroplasty" Journal of Clinical Medicine 12, no. 13: 4178. https://doi.org/10.3390/jcm12134178
APA StylePusceddu, C., Marsico, S., Derudas, D., Ballicu, N., Melis, L., Zedda, S., de Felice, C., Calabrese, A., De Francesco, D., Venturini, M., Santucci, D., & Faiella, E. (2023). Percutaneous Vertebral Reconstruction (PVR) Technique of Pathological Compression Fractures: An Innovative Combined Treatment of Microwave Ablation, Bilateral Expandable Titanium SpineJack Implants Followed by Vertebroplasty. Journal of Clinical Medicine, 12(13), 4178. https://doi.org/10.3390/jcm12134178





