ZAG (Zinc-Alpha 2 Glycoprotein) Serum Levels in Girls with Anorexia Nervosa
Abstract
1. Introduction
2. Materials
2.1. Characteristic Features of the Girls Involved in the Study
2.2. Criteria for Inclusion in the AN Group
- A diagnosis of the restrictive form of anorexia nervosa according to DSM-V criteria;
- Age 11–18 years;
- Written informed consent obtained from the parents/legal guardians of the girls involved to allow them to participate in the study;
- Written informed consent to participation in the study obtained from the study subject aged ≥16 years.
2.3. Criteria for Exclusion from the AN Group
- The purgative form of anorexia nervosa;
- Poor health condition preventing blood sampling, e.g., dehydration or vomiting;
- Abnormal functional parameters and indices of the liver and kidneys;
- Acute infection at the time of the study or during the previous three months before the study onset;
- Pharmacotherapy administered immediately before the study onset;
- The coexistence of another medical condition that may have led to cachexia.
2.4. Criteria for Inclusion in Group H
- Normal body weight and BMI, according to the applicable standards for sex and age in the Polish population (Palczewska et al.).
- Age 11–18 years;
- Written informed consent obtained from the parents/legal guardians of the girls involved to allow them to participate in the study;
- Written informed consent to participation in the study obtained from study subject aged ≥16 years.
2.5. Criteria for Exclusion from Group H
- Chronic medical conditions;
- Menstruation disorders;
- Acute infectious conditions during the three months before the study onset;
- Pharmacotherapy during the month before the study onset;
- Adherence to various dieting methods during the three months before the study onset or a history of eating disorders.
2.6. Criteria for Inclusion in the OT Group
- A diagnosis of simple obesity with reference to the centile grids for BMI, according to Palczewska and Niedźwiedzka (BMI > 97th percentile for sex and age, and BMI SDS > 2 standard deviations);
- Age 11–18 years;
- Written informed consent obtained from the parents/legal guardians of the girls involved to allow them to participate in the study;
- Written informed consent to participation in the study obtained from study subject aged ≥16 years.
2.7. Criteria for Exclusiom from Group OT
- Chronic medical conditions other than obesity;
- Genetic syndromes and hormonal disorders concomitant with obesity;
- Menstruation disorders;
- Acute infectious conditions during the three months before the study onset;
- Pharmacotherapy during the month before the study onset;
- Adherence to various dieting methods during the three months before the study onset or a history of eating disorders.
3. Methods
3.1. Interview, Physical Examination, and Anthropometric Measurements
3.2. Blood Collection Conditions and Methods Used for Laboratory Assays
3.3. Approval by the Bioethics Committee
4. Statistics
5. Results
Analysis of Results and Discussion
6. Conclusions
- Girls with anorexia nervosa differed from healthy and obese girls due to the significantly higher concentrations of ZAG in their blood.
- The high concentrations of ZAG in the blood of the patients with anorexia nervosa seemed to constitute an adaptation mechanism, secondary to undernourishment, facilitating the use of lipolysis products for the patient’s system.
- The ZAG concentrations in the blood of the girls with anorexia nervosa did not depend on the illness duration. They did, however, correlate negatively with the parameters of nutritional status (body mass, BMI, and the Cole’s index), which was collectively observed across the entire study group in our research project.
- The elevated concentrations of ZAG in the blood of the patients with anorexia nervosa may have resulted from an elevated concentration of cortisol in the blood. This is a manifestation of the incorrect functioning of the hypothalamic–pituitary–adrenal axis in such patients that may additionally promote body weight loss.
- The elevated serum ZAG levels observed in patients with mental anorexia nervosa, as in patients with cancer, may have been a phenomenon secondary to malnutrition caused by an underlying disease.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cabassi, A.; Tedeschi, S. Zinc-α2-glycoprotein as a marker of fat catabolism in humans. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef]
- Grabiec, K.; Burchert, M.; Milewska, M.; Błaszczyk, M.; Grzelkowska-Kowalczyk, K. Ogólnoustrojowe i miejscowe mechanizmy prowadzące do kacheksji w chorobach nowotworowych. Postepy Hig. Med. Dosw. 2013, 67, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, D.; Karpíšek, M.; Reutova, H.; Stejskal, P.; Kotolova, H.; Kollár, P. Determination of serum zinc-alpha-2-glycoprotein in patients with metabolic syndrome by a new ELISA. Clin. Biochem. 2008, 41, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Barraco, G.M.; Luciano, R.; Manco, M. Zinc-α2-glycoprotein is associated with insulin resistance in children. Obesity 2015, 23, 5–6. [Google Scholar] [CrossRef]
- Ziora, K.; Jarzumbek, A.; Świętochowska, E.; Gorczyca, P.; Oświęcimska, J.; Ostrowska, Z.; Kukla, M. Stężenie białka MIC-1 (Macrophage Inhibitory Cytokine-1) w surowicy krwi u dziewcząt z jadłowstrętem psychicznym. Endokrynol. Ped. 2018, 64, 163–174. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder (DSM V); APA: Washington, DC, USA, 2013. [Google Scholar]
- Palczewska, I.; Niedźwiecka, Z. Wskaźniki rozwoju somatycznego dzieci i młodzieży warszawskiej. Med. Wieku Rozw. 2002, 2 (Suppl. I), 17–118. [Google Scholar]
- Holden, R.J.; Pakula, I.S. The role of tumor necrosis factor-alpha in the pathogenesis of anorexia and bulimia nervosa, cancer cachexia and obesity. Med. Hypotheses 1996, 47, 423–438. [Google Scholar] [CrossRef]
- Schattner, A.; Tepper, R.; Steinbock, M.; Hahn, T.; Schoenfeld, A. TNF, Interferon-y and cell-mediated cytotoxicity in anorexia nervosa: Effect of refeeding. J. Clin. Lab. Immunol. 1990, 321, 83–184. [Google Scholar]
- Bessler, H.; Karp, L.; Notti, I.; Apter, A.; Tyano, S.; Djaldetti, M.; Weizman, R. Cytokine production in anorexia nervosa. Clin. Neuropharmacol. 1993, 162, 37–243. [Google Scholar] [CrossRef]
- Miller, G.E.; Rohleder, N.; Stetler, C.; Kirschbaum, C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom. Med. 2005, 676, 79–87. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A metaanalysis of cytokines in major depression. Biol. Psychiatr. 2010, 674, 46–57. [Google Scholar] [CrossRef]
- Reale, M.; Patruno, A.; De Lutiis, M.A.; Pesce, M.; Felaco, M.; Di Giannantonio, M.; Di Nicola, M.; Grilli, A. Dysregulation of chemo-cytokine production in schizophrenic patients versus healthy controls. BMC Neurosci. 2011, 12, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Selva, D.M.; Lecube, A.; Hernandez, C.; Baena, J.A.; Fort, J.M.; Simo, R. Lower zinc-α2-glycoprotein production by adipose tissue and liver in obese patients unrelated to insulin resistance. J. Clin. Endocrinol. Metab. 2009, 94, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.Y.; Zhang, S.J.; Deng, J.Y.; Zhu, H.J.; Pan, H.; Li, N.S.; Shi, Y.F. Zinc-alpha2-glycoprotein is involved in regulation of body weight through inhibition of lipogenic enzymes in adipose tissue. Int. J. Obes. 2009, 33, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, R.; Li, S.; Luo, Y.; Zhang, Y.; Zhang, L.; Liu, D.; Wang, Y.; Xiong, Z.; Boden, G.; et al. Zinc-a2-glycoprotein is associated with insulin resistance in humans and is regulated by hyperglycemia, hyperinsulinemia, or liraglutide administration: Cross-sectional and interventional studies in normal subjects, insulin-resistant subjects, and subjects with newly diagnosed diabetes. Diabetes Care 2013, 36, 1074–1082. [Google Scholar] [CrossRef]
- Tian, M.; Liang, Z.; Liu, R.; Li, K.; Tan, X.; Luo, Y.; Yang, M.; Gu, H.F.; Liu, H.; Li, L.; et al. Effects of sitagliptin on circulating zinc-α2-glycoprotein levels in newly diagnosed type 2 diabetes patients: A randomized trial. Eur. J. Endocrinol. 2016, 174, 147–155. [Google Scholar] [CrossRef]
- Balaz, M.; Vician, M.; Janakova, Z.; Kurdiova, T.; Surova, M.; Imrich, R.; Majercikova, Z.; Penesova, A.; Vlcek, M.; Kiss, A.; et al. Subcutaneous adipose tissue zinc-α2-glycoprotein is associated with adipose tissue and whole-body insulin sensitivity. Obesity 2014, 22, 1821–1829. [Google Scholar] [CrossRef]
- Mracek, T.; Ding, Q.; Tzanavari, T.; Kos, K.; Pinkney, J.; Wilding, J.; Trayhurn, P.; Bing, C. The adipokine zinc-α2-glycoprotein (ZAG) is downregulated with fat mass expansion in obesity. Clin. Endocrinol. 2010, 72, 34–341. [Google Scholar] [CrossRef]
- Ballantyne, G.H.; Gumbs, A.; Modlin, I.M. Changes in insulin resistance following bariatric surgery and the adipoinsular axis: Role of the adipocytokines, leptin, adiponectin and resistin. Obes. Surg. 2005, 15, 692–699. [Google Scholar] [CrossRef]
- Ziora-Jakutowicz, K.N.; Zimowski, J.; Ziora, K.; Bednarska-Makaruk, M.; Świętochowska, E.; Gorczyca, P.; Szczepańska, M.; Machura, E.; Stojewska, M.; Gołąb-Jenerał, K.; et al. Evaluation of the Frequency of ADIPOQ c. 45 T > G and ADIPOQ c. 276 G > T Polymorphisms in the Adiponectin Coding Gene in Girls with Anorexia Nervosa. Endokrynol. Pol. 2021, 72, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Sanchez, L.; García-Fuentes, E.; Fernández-García, D.; Escote, X.; Alcaide, J.; Perez-Martinez, P.; Vendrell, J.; Tinahones, F.J. Zinc-alpha 2-glycoprotein gene expression in adipose tissue is related with insulin resistance and lipolytic genes in morbidly obese patients. PLoS ONE 2012, 7, e33264. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Ohkubo, I.; Niwa, M.; Sasaki, M.; Tateyama, H.; Eimoto, T. Immunohistochemical localization of Zn-alpha 2-glycoprotein in normal human tissues. J. Histochem. Cytochem. 1991, 39, 1221–1226. [Google Scholar] [CrossRef]
- Sanders, P.M.; Tisdale, M.J. Role of lipid-mobilising factor (LMF) in protecting tumour cells from oxidative damage. Br. J. Cancer 2004, 90, 1274–1278. [Google Scholar] [CrossRef]
- Bing, C.; Russell, S.T.; Beckett, E.; Collins, P.; Taylor, S.; Barraclough, R.; Tisdale, M.J.; Williams, G. Expression of uncoupling proteins-1,-2 and-3mRNA is induced by an adenocarcinoma-derived lipid-mobilizing factor. Br. J. Cancer 2002, 86, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Bing, C.; Trayhurn, P. New insights into adipose tissue atrophy in cancer cachexia. Proc. Nutr. Soc. 2009, 68, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.T.; Tisdale, M.J. The role of glucocorticoids in the induction of zinc-α2-glycoprotein expression in adipose tissue in cancer cachexia. Br. J. Cancer 2005, 92, 876–881. [Google Scholar] [CrossRef]
- Bassuk, S.S.; Rifai, N.; Ridker, P.M. High-sensitivity C-reactive protein: Clinical importance. Curr. Probl. Cardiol. 2004, 29, 439–493. [Google Scholar]
- Brooks, G.C.; Blaha, M.J.; Blumenthal, R.S. Relation of C-reactive protein to abdominal adiposity. Am. J. Cardiol. 2010, 106, 56–61. [Google Scholar] [CrossRef]
- Dyaczyński, M.; Drożdż, M.; Wylężek, L.; Scanes, C.G.; Rzepa, A.; Cieśla, A.; Pierzchała-Koziec, K. Opioid-like peptides and ghrelin mitigation of bariatric results depends on obesity level. Endokrynol. Pol. 2020, 71, 27–33. [Google Scholar] [CrossRef]
- Bao, Y.; Bing, C.; Hunter, L.; Jenkins, J.R.; Wabitsch, M.; Trayhurn, P. Zinc-α2-glycoprotein, a lipid mobilizing factor, is expressed and secreted by human (SGBS) adipocytes. FEBS Lett. 2005, 579, 41–47. [Google Scholar] [CrossRef]
- Souza, S.C.; Palmer, H.J.; Kang, Y.H.; Yamamoto, M.T.; Muliro, K.V.; Paulson, K.E.; Greenberg, A.S. TNF-a induction of lipolysis is mediated through activation of the extracellular signal related kinase pathway in 3T3-L1 adipocytes. J. Cell. Biochem. 2003, 89, 1077–1086. [Google Scholar] [CrossRef]
- Haines, M.S. Endocrine complications of anorexia nervosa. J. Eat Disord. 2023, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Leslie, R.D.; Isaacs, A.J.; Gomez, J.; Raggatt, P.R.; Bayliss, R. Hypothalamo-pituitary-thyroid function in anorexia nervosa: Influence of weight gain. Br. Med. J. 1978, 2, 526–528. [Google Scholar] [CrossRef] [PubMed]
| AN n = 30 | H n = 30 | OB n = 27 | |
|---|---|---|---|
| Mean ± 1.96 SEM (Range) | Mean ± 1.96 SEM (Range) | Mean ± 1.96 SEM (Range) | |
| Age (y) | 15.64 ± 0.41 (13.1–17.3) | 16.17 ± 0.54 (11.2–17.9) | 13.73 ± 0.82 (11.0–17.0) |
| Body weight (kg) | 42.77 ± 2.19 ac (29.2–54.8) | 54.43 ± 3.01 (39–73.6) | 90.15 ± 6.78 b (54.0–118.8) |
| BMI (kg/m2) | 15.78 ± 0.64 ac (11.2–18.1) | 19.92 ± 0.8 (16–24) | 34.47 ± 1.94 b (26.1–43.57) |
| BMI-SDS | −2.22 ± 0.32 ac (−4.35–−0.46) | −0.14 ± 0.45 (−2.72–1.94) | 6.88 ± 1.0 b (3.24–12.87) |
| Cole index (%) | 78 ± 3 ac (54–95) | 98 ± 4 (78–116) | 181 ± 10 b (127–253) |
| Body height (cm) | 164.22 ± 2.64 (151–182.3) | 164.98 ± 2.33 (149.9–177) | 161.21 ± 3.24 (135–173) |
| Maximum body weight prior to the disease (kg) | 58.98 ± 4.35 (36.0–82.0) | _ | _ |
| Illness duration (months) | 17.23 ± 6.65 (3–81) | _ | _ |
| Body weight loss (kg) | 15.92 ± 2.77 (5.3–33.7) | _ | _ |
| AN | H | OB | |
|---|---|---|---|
| Mean ± 1.96 SEM (Range) | Mean ± 1.96 SEM (Range) | Mean ± 1.96 SEM (Range) | |
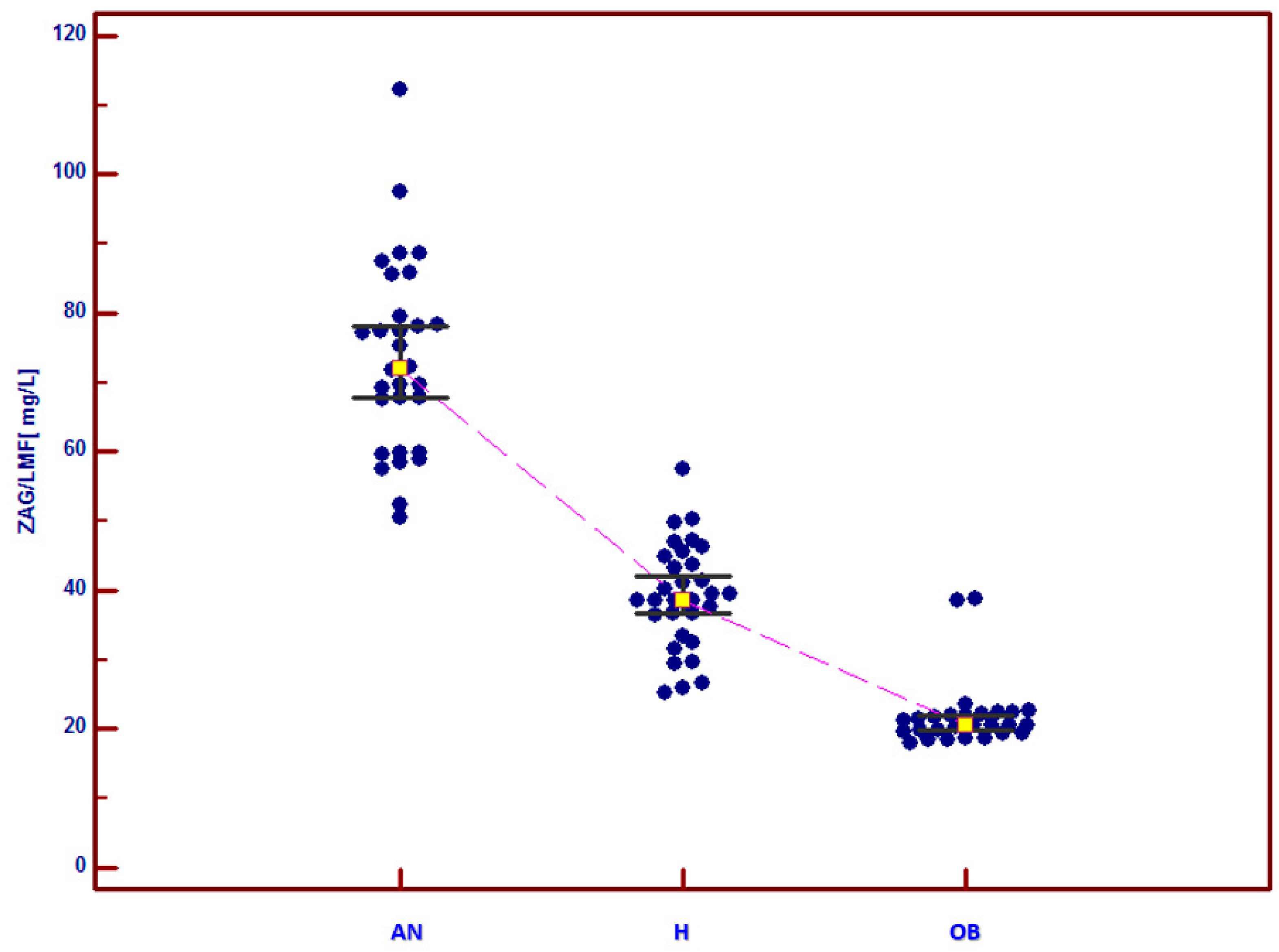
| ZAG (mg/L) | 73.43 ± 4.96 ac (50.5–112.3) | 39.18 ± 2.77 (25.3–57.6) | 21.96 ± 1.91 b (18.1–38.9) |
| Whole Study Group | |
|---|---|
| ZAG (mg/L) | |
| Body weight (kg) | r = −0.802 p < 0.0001 |
| BMI (kg/m2) | r = −0.848 p < 0.0001 |
| BMI-SDS | r = −0.828 p < 0.0001 |
| Cole’s index (%) | r = −0.836 p < 0.0001 |
| AN | H | OB | |
|---|---|---|---|
| Mean ± 1.96 SEM (Range) | Mean ± 1.96 SEM (Range) | Mean ± 1.96 SEM (Range) | |
| Glucose (mg/dL) (norm (N): 60–100 mg/dL) | 78.59 ± 2.97 ac (66.7–104) | 88.74 ± 2.8 (74–106.5) | 84.10 ± 3.09 b (72–105) |
| Insulin (uU/mL) (N: 2.6–24.9 uU/mL) | 5.32 ± 1.92 (0.39–25.9) | 11.28 ± 2.28 (3.04–33.46) | 27.08 ± 5.62 d (4.84–66.1) |
| HOMA-IR (N: < 2) | 0.82 ± 0.25 ac (0.07–3.77) | 2.51 ± 0.52 (0.599–7.421) | 4.49 ± 0.64 b (0.954–8.389) |
| Whole Study Group | |
|---|---|
| ZAG (mg/L) | |
| Glucose (mg/dL) | r = −0.188 p = 0.085 |
| Insulin (uU/mL) | r = −0.731 p < 0.0001 |
| HOMA-IR | r = −0.74 p < 0.0001 |
| AN | H | OB | |
|---|---|---|---|
| Mean ± 1.96 SEM (Range) | Mean ± 1.96 SEM (Range) | Mean ± 1.96 SEM (Range) | |
| Total cholesterol (mmol/L) (N: 0–5.2 mmol/L) | 4.96 ± 0.46 (2.43–7.78) | 4.16 ± 0.32 (1.92–6.13) | 4.45 ± 0.28 (2.91–5.68) |
| LDL (mmol/L) (N: < 2.59 mmol/L) | 2.76 ± 0.37 a (0.77–5.3) | 2.21 ± 0.22 (0.81–3.54) | 2.82 ± 0.27 b (1.56–4.15) |
| HDL (mmol/L) (N: 1.15–1.68 mmol/L) | 1.73 ± 0.16 c (0.9–2.86) | 1.54 ± 0.15 (0.71–2.61) | 1.05 ± 0.12 d (0.59–1.94) |
| Triglycerides (mmol/L) (N: 0.4–1.8 mmol/L) | 1.06 ± 0.34 (0.35–5.85) | 0.9 ± 0.18 (0.4–3.07) | 1.29 ± 0.18 d (0.33–2.72) |
| Whole Study Group | |
|---|---|
| ZAG (mg/L) | |
| Total cholesterol (mmol/L) | r = 0.148 p = 0.169 |
| LDL (mmol/L) | r = −0.023 p = 0.835 |
| HDL (mmol/L) | r = 0.514 p < 0.0001 |
| TG (mmol/L) | r = −0.278 p = 0.009 |
| AN | H | OB | |
|---|---|---|---|
| Mean ± 1.96 SEM (Range) | Mean ± 1.96 SEM (Range) | Mean ± 1.96 SEM (Range) | |
| TSH (ulU/mL) (N: 0.27–4.2 uIU/mL) | 2.08 ± 0.43 (0.96–5.5) | 2.15 ± 0.31 (0.806–4.42) | 2.28 ± 0.32 (1.11–4.18) |
| FT4 (ng/dL) (N: 0.93–1.7 ng/dL) | 1.06 ± 0.07 a (0.728–1.5) | 1.21 ± 0.06 (0.954–1.73) | 1.15 ± 0.07 (0.964–1.63) |
| Cortisol (ug/dL) (N: 2.3–19.4 ug/dL) | 20.6 ± 2.07 b (10.28–39.63) | 15.09 ± 2.22 (3.81–28.99) | 13.88 ± 1.48 (7.56–19.37) |
| Whole Study Group | |
|---|---|
| ZAG (mg/L) | |
| TSH (ulU/mL) | r = −0.15 p = 0.176 |
| FT4 (ng/dL) | r = −0.115 p = 0.296 |
| Cortisol (ug/dL) | r = 0.482 p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anna, J.; Elżbieta, Ś.; Elżbieta, M.-I.; Katarzyna, G.-J.; Katarzyna, B.-D.; Katarzyna, Z. ZAG (Zinc-Alpha 2 Glycoprotein) Serum Levels in Girls with Anorexia Nervosa. J. Clin. Med. 2023, 12, 4245. https://doi.org/10.3390/jcm12134245
Anna J, Elżbieta Ś, Elżbieta M-I, Katarzyna G-J, Katarzyna B-D, Katarzyna Z. ZAG (Zinc-Alpha 2 Glycoprotein) Serum Levels in Girls with Anorexia Nervosa. Journal of Clinical Medicine. 2023; 12(13):4245. https://doi.org/10.3390/jcm12134245
Chicago/Turabian StyleAnna, Jarzumbek, Świętochowska Elżbieta, Mizgała-Izworska Elżbieta, Gołąb-Jenerał Katarzyna, Bąk-Drabik Katarzyna, and Ziora Katarzyna. 2023. "ZAG (Zinc-Alpha 2 Glycoprotein) Serum Levels in Girls with Anorexia Nervosa" Journal of Clinical Medicine 12, no. 13: 4245. https://doi.org/10.3390/jcm12134245
APA StyleAnna, J., Elżbieta, Ś., Elżbieta, M.-I., Katarzyna, G.-J., Katarzyna, B.-D., & Katarzyna, Z. (2023). ZAG (Zinc-Alpha 2 Glycoprotein) Serum Levels in Girls with Anorexia Nervosa. Journal of Clinical Medicine, 12(13), 4245. https://doi.org/10.3390/jcm12134245





