Association between Serum Irisin and Leptin Levels and Risk of Depressive Symptoms in the Diabetic Elderly Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Population
2.3. Measurements
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. General Demographic, Clinical, and Metabolic Parameters
3.2. Irisin and Leptin Levels in Depressive Patients and Controls
3.3. Logistic Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 8 May 2023).
- Stringaris, A. Editorial: What is depression? J. Child Psychol. Psychiatry 2017, 58, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Mezuk, B.; Eaton, W.W.; Albrecht, S.; Golden, S.H. Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care 2008, 31, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Chireh, B.; Li, M.; D’Arcy, C. Diabetes increases the risk of depression: A systematic review, meta-analysis and estimates of population attributable fractions based on prospective studies. Prev. Med. Rep. 2019, 14, 100822. [Google Scholar] [CrossRef]
- Zhang, X.; Norris, S.L.; Gregg, E.W.; Cheng, Y.J.; Beckles, G.; Kahn, H.S. Depressive symptoms and mortality among persons with and without diabetes. Am. J. Epidemiol. 2005, 161, 652–660. [Google Scholar] [CrossRef]
- Bergmans, R.S.; Rapp, A.; Kelly, K.M.; Weiss, D.; Mezuk, B. Understanding the relationship between type 2 diabetes and depression: Lessons from genetically informative study designs. Diabet. Med. 2021, 38, e14399. [Google Scholar] [CrossRef]
- Borgland, S.L. Can treatment of obesity reduce depression or vice versa? J. Psychiatry Neurosci. 2021, 46, E313–E318. [Google Scholar] [CrossRef]
- Subba, R.; Sandhir, R.; Singh, S.P.; Mallick, B.N.; Mondal, A.C. Pathophysiology linking depression and type 2 diabetes: Psychotherapy, physical exercise, and fecal microbiome transplantation as damage control. Eur. J. Neurosci. 2021, 53, 2870–2900. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.H.; Rizvi, M.A.; Fatima, M.; Mondal, A.C. Pathophysiological implications of neuroinflammation mediated HPA axis dysregulation in the prognosis of cancer and depression. Mol. Cell. Endocrinol. 2021, 520, 111093. [Google Scholar] [CrossRef]
- Kim, D.J.; Yu, J.H.; Shin, M.S.; Shin, Y.W.; Kim, M.S. Hyperglycemia Reduces Efficiency of Brain Networks in Subjects with Type 2 Diabetes. PLoS ONE 2016, 11, e0157268. [Google Scholar] [CrossRef] [PubMed]
- Rom, S.; Zuluaga-Ramirez, V.; Gajghate, S.; Seliga, A.; Winfield, M.; Heldt, N.A.; Kolpakov, M.A.; Bashkirova, Y.V.; Sabri, A.K.; Persidsky, Y. Hyperglycemia-Driven Neuroinflammation Compromises BBB Leading to Memory Loss in Both Diabetes Mellitus (DM) Type 1 and Type 2 Mouse Models. Mol. Neurobiol. 2019, 56, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; van Praag, H.; Gage, F.H. Adult brain neurogenesis and psychiatry: A novel theory of depression. Mol. Psychiatry 2000, 5, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Gil-Mohapel, J.; Brocardo, P.S.; Choquette, W.; Gothard, R.; Simpson, J.M.; Christie, B.R. Hippocampal neurogenesis levels predict WATERMAZE search strategies in the aging brain. PLoS ONE 2013, 8, e75125. [Google Scholar] [CrossRef]
- Triviño-Paredes, J.; Patten, A.R.; Gil-Mohapel, J.; Christie, B.R. The effects of hormones and physical exercise on hippocampal structural plasticity. Front. Neuroendocrinol. 2016, 41, 23–43. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F.; Valaris, S.; Wrann, C.D. A role for FNDC5/Irisin in the beneficial effects of exercise on the brain and in neurodegenerative diseases. Prog. Cardiovasc. Dis. 2019, 62, 172–178. [Google Scholar] [CrossRef]
- Siteneski, A.; Olescowicz, G.; Pazini, F.L.; Camargo, A.; Fraga, D.B.; Brocardo, P.S.; Gil-Mohapel, J.; Cunha, M.P.; Rodrigues, A.L. Antidepressant-like and pro-neurogenic effects of physical exercise: The putative role of FNDC5/irisin pathway. J. Neural Transm. 2020, 127, 355–370. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Sousa, R.A.L.; Improta-Caria, A.C.; Souza, B.S.F. Exercise-Linked Irisin: Consequences on Mental and Cardiovascular Health in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 2199. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrionol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef]
- Zou, X.; Zhong, L.; Zhu, C.; Zhao, H.; Zhao, F.; Cui, R.; Gao, S.; Li, B. Role of Leptin in Mood Disorder and Neurodegenerative Disease. Front. Neurosci. 2019, 13, 378. [Google Scholar] [CrossRef]
- Gorska-Ciebiada, M.; Saryusz-Wolska, M.; Ciebiada, M.; Loba, J. Mild cognitive impairment and depressive symptoms in elderly patients with diabetes: Prevalence, risk factors, and comorbidity. J. Diabetes Res. 2014, 2014, 179648. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Lloyd, C.E. Epidemiology of depression and diabetes: A systematic review. J. Affect. Disord. 2012, 142, S8–S21. [Google Scholar] [CrossRef] [PubMed]
- Wexler, D.J.; Grant, R.W.; Wittenberg, E.; Bosch, J.L.; Cagliero, E.; Delahanty, L.; Blais, M.A.; Meigs, J.B. Correlates of health-related quality of life in type 2 diabetes. Diabetologia 2006, 49, 1489–1497. [Google Scholar] [CrossRef]
- Prigge, R.; Wild, S.H.; Jackson, C.A. Depression, diabetes, comorbid depression and diabetes and risk of all-cause and cause-specific mortality: A prospective cohort study. Diabetologia 2022, 65, 1450–1460. [Google Scholar] [CrossRef]
- Engum, A.; Mykletun, A.; Midthjell, K.; Holen, A.; Dahl, A.A. Depression and diabetes: A large population-based study of sociodemographic, lifestyle, and clinical factors associated with depression in type 1 and type 2 diabetes. Diabetes Care 2005, 28, 1904–1909. [Google Scholar] [CrossRef]
- Katon, W.; von Korff, M.; Ciechanowski, P.; Russo, J.; Lin, E.; Simon, G.; Ludman, E.; Walker, E.; Bush, T.; Young, B. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care 2004, 27, 914–920. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Huang, J.H.; Li, R.H.; Tsai, L.C. Relationship between Depression with Physical Activity and Obesity in Older Diabetes Patients: Inflammation as a Mediator. Nutrients 2022, 14, 4200. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D. FNDC5/irisin—their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Br. Plast. 2015, 1, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Zhang, J.; Yu, K.; Song, F. Irisin ameliorates the postoperative depressive-like behavior by reducing the surface expression of epidermal growth factor receptor in mice. Neurochem. Int. 2020, 135, 104705. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pan, J. Irisin ameliorates depressive-like behaviors in rats by regulating energy metabolism. Biochem. Biophys. Res. Commun. 2016, 474, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.O.; Kim, N.; Kim, J.K.; Kim, H.I.; Lee, Y.W.; Kim, S.J.; Choi, J.I.; Oh, Y.; Kim, J.H.; et al. Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK. Mol. Endocrinol. 2015, 29, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Du, F.; Li, X.; Wang, M.; Duan, R.; Zhang, J.; Wu, Y.; Zhang, Q. Effects and underlying mechanisms of irisin on the proliferation and apoptosis of pancreatic β cells. PLoS ONE 2017, 12, e0175498. [Google Scholar] [CrossRef]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belén Crujeiras, A.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Pardo, M.; Crujeiras, A.B.; Amil, M.; Aguera, Z.; Jiménez-Murcia, S.; Baños, R.; Botella, C.; de la Torre, R.; Estivill, X.; Fagundo, A.B.; et al. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. Int. J. Endocrinol. 2014, 2014, 857270. [Google Scholar] [CrossRef]
- Sahin-Efe, A.; Upadhyay, J.; Ko, B.J.; Dincer, F.; Park, K.H.; Migdal, A.; Vokonas, P.; Mantzoros, C. Irisin and leptin concentrations in relation to obesity, and developing type 2 diabetes: A cross sectional and a prospective case-control study nested in the Normative Aging Study. Metabolism 2018, 79, 24–32. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Zhu, Y.J.; Li, C.G.; Tang, Y.Z.; Jiang, Z.H.; Yang, M.; Ni, C.L.; Chen, L.M.; Niu, W.Y. The relationship between circulating irisin levels and tissues AGE accumulation in type 2 diabetes patients. Biosci. Rep. 2017, 37, BSR20170213. [Google Scholar] [CrossRef] [PubMed]
- Duran, I.D.; Gülçelik, N.E.; Ünal, M.; Topçuoğlu, C.; Sezer, S.; Tuna, M.M.; Berker, D.; Güler, S. Irisin levels in the progression of diabetes in sedentary women. Clin. Biochem. 2015, 48, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Campolo, J.; Corradi, E.; Rizzardi, A.; Parolini, M.; Dellanoce, C.; Di Guglielmo, M.L.; Tarlarini, P.; Cattaneo, M.; Trivella, M.G.; De Maria, R. Irisin and markers of metabolic derangement in non-diabetic Caucasian subjects with stage I-II obesity during early aging. PLoS ONE 2020, 15, e0229152. [Google Scholar] [CrossRef]
- Rana, K.S.; Pararasa, C.; Afzal, I.; Nagel, D.A.; Hill, E.J.; Bailey, C.J.; Griffiths, H.R.; Kyrou, I.; Randeva, H.S.; Bellary, S.; et al. Plasma irisin is elevated in type 2 diabetes and is associated with increased E-selectin levels. Cardiovasc. Diabetol. 2017, 16, 147. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, M.K.; Bae, K.H.; Seo, H.A.; Jeong, J.Y.; Lee, W.K.; Kim, J.G.; Lee, I.K.; Park, K.G. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 100, 96–101. [Google Scholar] [CrossRef]
- Liu, J.J.; Wong, M.D.; Toy, W.C.; Tan, C.S.; Liu, S.; Ng, X.W.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Lower circulating irisin is associated with type 2 diabetes mellitus. J. Diabetes Complicat. 2013, 27, 365–369. [Google Scholar] [CrossRef]
- Hu, W.; Wang, R.; Li, J.; Zhang, J.; Wang, W. Association of irisin concentrations with the presence of diabetic nephropathy and retinopathy. Ann. Clin. Biochem. 2016, 53, 67–74. [Google Scholar] [CrossRef]
- Zheng, Q.; Xu, M.J.; Cheng, J.; Chen, J.M.; Zheng, L.; Li, Z.G. Serum levels of brain-derived neurotrophic factor are associated with depressive symptoms in patients with systemic lupus erythematosus. Psychoneuroendocrinology 2017, 78, 246–252. [Google Scholar] [CrossRef]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Dipaola, L.; Spagnuolo, R.; Cignarelli, A.; Perrini, S.; Laviola, L.; Giorgino, F. Irisin increases the expression of anorexigenic and neurotrophic genes in mouse brain. Diabetes Metab. Res. Rev. 2020, 36, e3238. [Google Scholar] [CrossRef]
- Moon, H.S.; Dincer, F.; Mantzoros, C.S. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism 2013, 62, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 2017, 68, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I. Superiority of the Non-Glycosylated Form Over the Glycosylated Form of Irisin in the Attenuation of Adipocytic Meta-Inflammation: A Potential Factor in the Fight Against Insulin Resistance. Biomolecules 2019, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Lamers, F.; Bot, M.; Drent, M.L.; Penninx, B.W. Leptin Dysregulation Is Specifically Associated With Major Depression With Atypical Features: Evidence for a Mechanism Connecting Obesity and Depression. Biol. Psychiatry 2017, 81, 807–814. [Google Scholar] [CrossRef]
- Valleau, J.C.; Sullivan, E.L. The impact of leptin on perinatal development and psychopathology. J. Chem. Neuroanat. 2014, 61–62, 221–232. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.H.; Kim, E.Y.; Lee, N.Y.; Yu, H.Y.; Kim, Y.S.; Ahn, Y.M. Leptin is associated with mood status and metabolic homeostasis in patients with bipolar disorder. Neuropsychobiology 2014, 70, 203–209. [Google Scholar] [CrossRef]
- Ge, T.; Fan, J.; Yang, W.; Cui, R.; Li, B. Leptin in depression: A potential therapeutic target. Cell Death Dis. 2018, 9, 1096. [Google Scholar] [CrossRef]
- Pan, H.; Guo, J.; Su, Z. Advances in understanding the interrelations between leptin resistance and obesity. Physiol. Behav. 2014, 130, 157–169. [Google Scholar] [CrossRef]
- Cernea, S.; Both, E.; Huţanu, A.; Şular, F.L.; Roiban, A.L. Correlations of serum leptin and leptin resistance with depression and anxiety in patients with type 2 diabetes. Psychiatry Clin. Neurosci. 2019, 73, 745–753. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metabol. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]




| Parameter | All Subjects (n = 189) | Patients with Depressive Symptoms (n = 57) | Controls (n = 132) | t/Z/χ2 | p |
|---|---|---|---|---|---|
| Gender (M/F) * | 61/128 | 11/46 | 50/82 | 29.2 | <0.001 |
| Age (years) | 72.6 ± 4.7 | 72.9 ± 4.0 | 72.5 ± 4.9 | −0.27 | 0.78 |
| Had ever smoked (%) * | 69 (36.5%) | 33 (57.8%) | 34 (25.7%) | 17.9 | <0.001 |
| Single/married * | 82 (43.4%) | 37/20 | 45/87 | 15.4 | <0.001 |
| No physical activity (%) * | 79 (41.8%) | 43 (75.43%) | 36 (27.3%) | 37.9 | <0.001 |
| T2DM duration (years) * | 7.51 ± 4.7 | 9.96 ± 6.8 | 6.45 ± 5.07 | 3.92 | <0.001 |
| Neuropathy (%) * | 36 (19%) | 23 (40.35%) | 13 (9.8%) | 24.0 | <0.001 |
| Nephropathy (%) | 54 (28.6%) | 16 (28.07%) | 38 (28.8%) | 0.01 | 0.92 |
| Retinopathy (%) | 60 (31.7%) | 17 (29.8%) | 43 (32.6%) | 0.14 | 0.71 |
| Previous CVD (%) | 38 (20.1%) | 10 (17.54%) | 28 (21.2%) | 0.33 | 0.56 |
| Stroke (%) | 7 (3.7%) | 2 (3.5%) | 5 (3.78%) | 0.01 | 0.93 |
| Hyperlipidemia (%) * | 138 (73%) | 52 (91.22%) | 86 (65.15%) | 13.7 | <0.001 |
| Hypertension (%) | 132 (69.8%) | 39 (68.42%) | 93 (70.4%) | 0.08 | 0.78 |
| Co-morbidity (n) * | 3.6 ± 2.3 | 5.3 ± 2.5 | 2.8 ± 1.8 | 7.74 | <0.001 |
| OAD (%) * | 172 (91%) | 46 (80.7%) | 126 (95.4%) | 65.9 | <0.001 |
| Insulin (%) * | 88 (46.5%) | 41 (71.9%) | 47 (35.6%) | 21.1 | <0.001 |
| HbA1c (%) | 7.02 ± 0.5 | 7.06 ± 0.6 | 7 ± 0.5 | 0.67 | 0.49 |
| Total cholesterol (mmol/L) * | 4.79 ± 1.0 | 5.57 ± 0.9 | 4.46 ± 0.8 | 7.97 | <0.001 |
| Triglycerides (mmol/L) | 1.87 ± 0.4 | 1.86 ± 0.4 | 1.88 ± 0.3 | 0.01 | 1.01 |
| LDL (mmol/L) * | 2.83 ± 0.8 | 3.3 ± 0.8 | 2.63 ± 0.7 | 5.75 | <0.001 |
| HDL (mmol/L) | 1.24 ± 0.2 | 1.25 ± 0.2 | 1.24 ± 0.2 | −0.74 | 0.45 |
| BMI (kg/m2) * | 29.6 ± 3.7 | 32.1 ± 3.7 | 28.6 ± 3.1 | 6.62 | <0.001 |
| GDS-30 score | 6.7 ± 6.6 | 15.9 ± 2.8 | 2.7 ± 2.6 | 30.9 | <0.001 |
| Irisin (ng/mL) | 17.7 ± 7.4 | 9.17 ± 4.4 | 21.4 ± 4.9 | −16.1 | <0.001 |
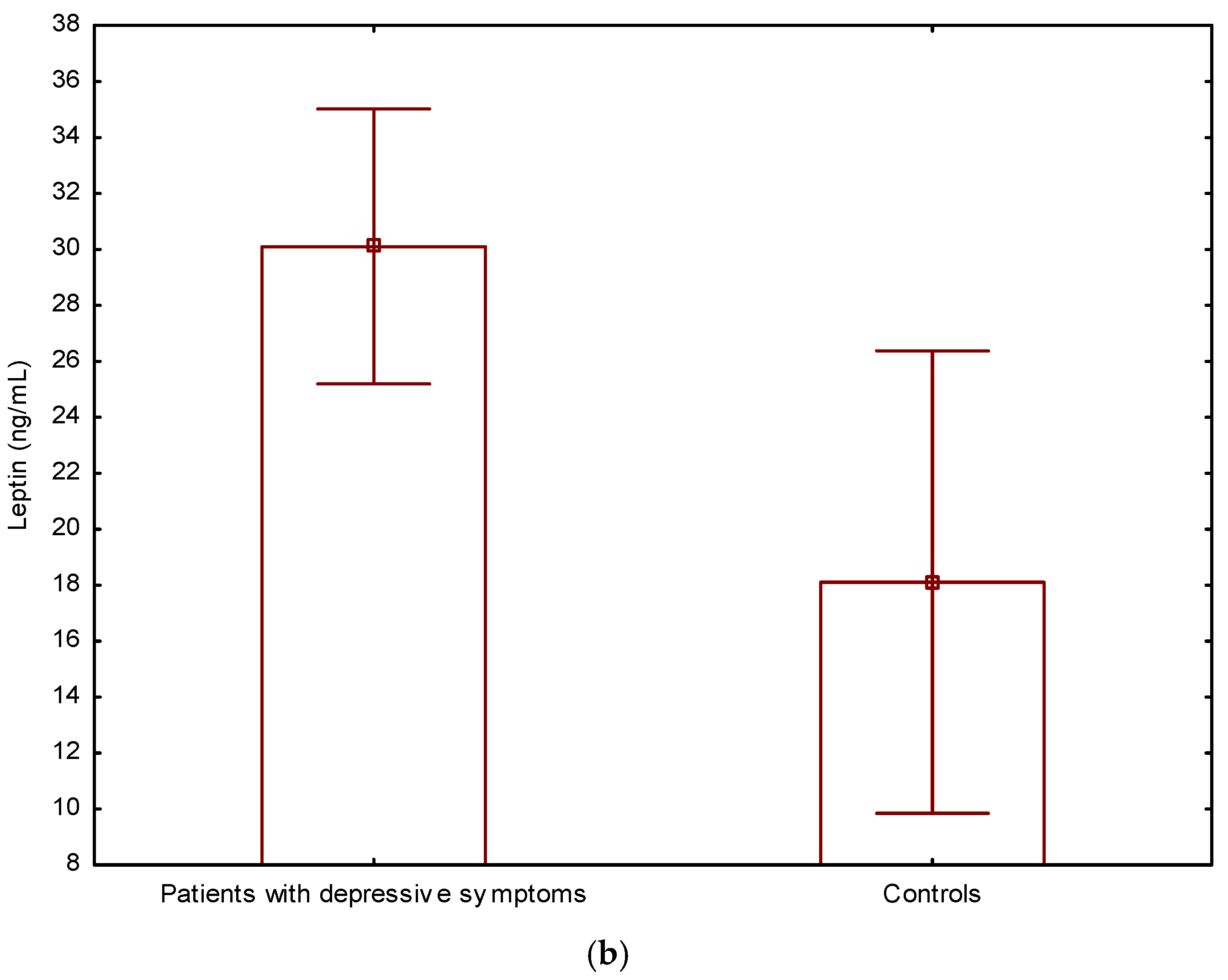
| Leptin (ng/mL) | 21.7 ± 9.2 | 30.1 ± 4.9 | 18.1 ± 8.3 | 10.2 | <0.001 |
| All Subjects (n = 189) | Patients with Depressive Symptoms (n = 57) | Controls (n = 132) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Irisin r | p | Leptin r | p | Irisin r | p | Leptin r | p | Irisin r | p | Leptin r | p |
| Age (years) | −0.06 | 0.38 | 0.15 * | 0.03 | −0.3 * | 0.02 | −0.05 | 0.69 | 0.02 | 0.79 | 0.21 * | 0.02 |
| T2DM duration (years) | −0.32 * | <0.001 | 0.22 * | 0.002 | −0.06 | 0.62 | 0.01 | 0.95 | −0.23 * | 0.008 | 0.1 | 0.24 |
| Co-morbidity (n) | −0.40 * | <0.001 | 0.34 * | <0.001 | −0.27 * | 0.04 | 0.07 | 0.57 | 0.06 | 0.46 | 0.07 | 0.42 |
| HbA1c (%) | −0.08 | 0.26 | 0.03 | 0.66 | −0.2 | 0.134 | 0.18 | 0.16 | −0.007 | 0.93 | 0.05 | 0.53 |
| Total cholesterol (mmol/L) | −0.78 * | <0.001 | 0.36 * | <0.001 | −0.64 * | <0.001 | 0.21 | 0.12 | −0.77 * | <0.001 | 0.05 | 0.56 |
| Triglycerides (mmol/L) | −0.04 | 0.58 | 0.001 | 0.93 | −0.35 * | 0.007 | 0.03 | 0.85 | 0.02 | 0.74 | 0.01 | 0.9 |
| LDL (mmol/L) | −0.63 * | <0.001 | 0.27 * | <0.001 | −0.44 * | 0.001 | 0.27 * | 0.04 | −0.62 * | <0.001 | 0.001 | 0.93 |
| HDL (mmol/L) | −0.04 | 0.57 | −0.16 | 0.25 | 0.02 | 0.89 | −0.03 | 0.78 | −0.04 | 0.59 | −0.26 | 0.25 |
| BMI (kg/m2) | −0.4 * | <0.001 | 0.83 * | <0.001 | −0.20 | 0.12 | 0.94 * | <0.001 | −0.08 | 0.36 | 0.80 * | <0.001 |
| Irisin (ng/mL) | 1 | −0.53 * | <0.001 | 1 | 0.19 | 0.14 | 1 | −0.13 | 0.12 | |||
| Leptin (ng/mL) | −0.53 * | <0.001 | 1 | 0.19 | 0.14 | 1 | −0.13 | 0.12 | 1 | |||
| GDS-30 score | −0.67 * | <0.001 | 0.58 * | <0.001 | −0.04 | 0.78 | 0.42 * | 0.01 | −0.19 * | 0.02 | 0.05 | 0.58 |
| Parameter | ß | SE of ß | p Value | OR | 95% CI |
|---|---|---|---|---|---|
| Gender (M/F) * | 1.9 | 0.3 | p < 0.001 | 6.8 | 3.3–14.4 |
| Age (years) | 0.2 | 0.03 | 0.6 | 1.0 | 0.9–1.0 |
| Had ever smoked (%) * | 1.4 | 0.3 | p < 0.001 | 3.9 | 2.1–7.6 |
| Single/married * | 1.3 | 0.3 | p < 0.001 | 3.6 | 1.8–6.8 |
| No physical activity (%) * | 2.1 | 0.3 | p < 0.001 | 8.1 | 4.0–16.7 |
| T2DM duration (years) * | 0.1 | 0.03 | p < 0.001 | 1.1 | 1.0–1.2 |
| Neuropathy (%) * | 1.8 | 0.3 | p < 0.001 | 6.2 | 2.8–13.5 |
| Nephropathy (%) | 0.3 | 0.03 | 0.92 | 0.9 | 0.4–1.9 |
| Retinopathy (%) | 0.3 | 0.01 | 0.71 | 0.9 | 0.4–1.7 |
| Previous CVD (%) | 0.2 | 0.4 | 0.56 | 0.8 | 0.4–1.7 |
| Stroke (%) | 0.1 | 0.8 | 0.93 | 0.9 | 0.2–4.9 |
| Hyperlipidemia (%) * | 1.7 | 0.5 | p < 0.001 | 5.6 | 2.1–14.8 |
| Hypertension (%) | 0.1 | 0.3 | 0.78 | 0.9 | 0.4–1.7 |
| Co-morbidity (n) * | 0.5 | 0.08 | p < 0.001 | 1.7 | 1.4–1.9 |
| OAD (%) * | −3.2 | 0.4 | p < 0.001 | 0.03 | 0.01–0.09 |
| Insulin (%) * | 1.53 | 0.3 | p < 0.001 | 4.6 | 2.4–9.1 |
| HbA1c (%) | 0.2 | 0.2 | 0.5 | 1.2 | 0.6–2.1 |
| Total cholesterol (mmol/L) * | 0.03 | 0.001 | p < 0.001 | 1.03 | 1.02–1.04 |
| Triglycerides (mmol/L) | 0.01 | 0.005 | 0.79 | 0.99 | 0.98–1.01 |
| LDL (mmol/L) * | 0.02 | 0.006 | p < 0.001 | 1.03 | 1.01–1.04 |
| HDL (mmol/L) | 0.08 | 0.02 | 0.69 | 1.01 | 0.9–1.1 |
| BMI (kg/m2) * | 0.3 | 0.05 | p < 0.001 | 1.3 | 1.2–1.5 |
| Irisin (ng/mL) * | −0.4 | 0.05 | p < 0.001 | 0.69 | 0.62–0.76 |
| Leptin (ng/mL) * | 0.2 | 0.03 | p < 0.001 | 1.2 | 1.2–1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorska-Ciebiada, M.; Ciebiada, M. Association between Serum Irisin and Leptin Levels and Risk of Depressive Symptoms in the Diabetic Elderly Population. J. Clin. Med. 2023, 12, 4283. https://doi.org/10.3390/jcm12134283
Gorska-Ciebiada M, Ciebiada M. Association between Serum Irisin and Leptin Levels and Risk of Depressive Symptoms in the Diabetic Elderly Population. Journal of Clinical Medicine. 2023; 12(13):4283. https://doi.org/10.3390/jcm12134283
Chicago/Turabian StyleGorska-Ciebiada, Malgorzata, and Maciej Ciebiada. 2023. "Association between Serum Irisin and Leptin Levels and Risk of Depressive Symptoms in the Diabetic Elderly Population" Journal of Clinical Medicine 12, no. 13: 4283. https://doi.org/10.3390/jcm12134283
APA StyleGorska-Ciebiada, M., & Ciebiada, M. (2023). Association between Serum Irisin and Leptin Levels and Risk of Depressive Symptoms in the Diabetic Elderly Population. Journal of Clinical Medicine, 12(13), 4283. https://doi.org/10.3390/jcm12134283






