Exploring Autonomic Alterations during Seizures in Temporal Lobe Epilepsy: Insights from a Heart-Rate Variability Analysis
Abstract
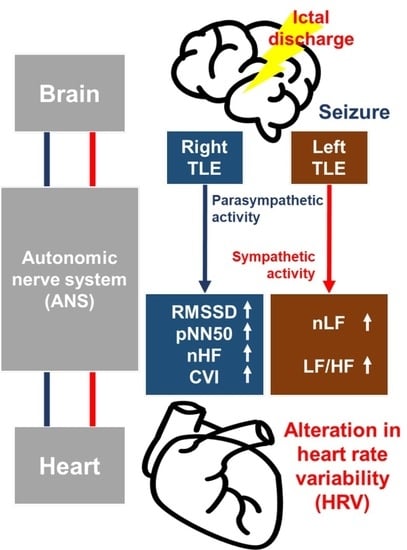
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HRV Data Acquisition and Preprocessing
2.3. Ultra-Short-Term HRV Analysis
2.4. Statistical Analysis
3. Results
3.1. Lateral Difference of HRV Alteration around the Ictal Period
3.2. Effects of Neurological Clinical Factors on HRV Alteration
4. Discussion
4.1. Ictal Cardiac Manifestation along with Hemispheric Lateralization
4.2. Effects of Neurological Clinical Factors on HRV Alteration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Fiest, K.M.; Sauro, K.M.; Wiebe, S.; Patten, S.B.; Kwon, C.S.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.L.; Jetté, N. Prevalence and Incidence of Epilepsy. Neurology 2017, 88, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-Adjusted Life Years (DALYs) for 291 Diseases and Injuries in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Shmuely, S.; van der Lende, M.; Lamberts, R.J.; Sander, J.W.; Thijs, R.D. The Heart of Epilepsy: Current Views and Future Concepts. Seizure 2017, 44, 176–183. [Google Scholar] [CrossRef]
- Ansakorpi, H.; Korpelainen, J.T.; Suominen, K.; Tolonen, U.; Myllylä, V.V.; Isojärvi, J.I.T. Interictal Cardiovascular Autonomic Responses in Patients with Temporal Lobe Epilepsy. Epilepsia 2000, 41, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Druschky, A. Interictal Cardiac Autonomic Dysfunction in Temporal Lobe Epilepsy Demonstrated by [123I]Metaiodobenzylguanidine-SPECT. Brain 2001, 124, 2372–2382. [Google Scholar] [CrossRef]
- Sathyaprabha, T.N.; Satishchandra, P.; Netravathi, K.; Sinha, S.; Thennarasu, K.; Raju, T.R. Cardiac Autonomic Dysfunctions in Chronic Refractory Epilepsy. Epilepsy Res. 2006, 72, 49–56. [Google Scholar] [CrossRef]
- Harnod, T.; Yang, C.C.H.; Hsin, Y.L.; Wang, P.J.; Shieh, K.R.; Kuo, T.B.J. Heart Rate Variability in Patients with Frontal Lobe Epilepsy. Seizure 2009, 18, 21–25. [Google Scholar] [CrossRef]
- Ravindran, K.; Powell, K.L.; Todaro, M.; O’Brien, T.J. The Pathophysiology of Cardiac Dysfunction in Epilepsy. Epilepsy Res. 2016, 127, 19–29. [Google Scholar] [CrossRef]
- Mukherjee, S.; Tripathi, M.; Chandra, P.S.; Yadav, R.; Choudhary, N.; Sagar, R.; Bhore, R.; Pandey, R.M.; Deepak, K.K. Cardiovascular Autonomic Functions in Well-Controlled and Intractable Partial Epilepsies. Epilepsy Res. 2009, 85, 261–269. [Google Scholar] [CrossRef]
- Nashef, L.; Walker, F.; Allen, P.; Sander, J.W.A.S.; Shorvon, S.D.; Fish, D.R. Apnoea and Bradycardia during Epileptic Seizures: Relation to Sudden Death in Epilepsy. J. Neurol. Neurosurg. Psychiatry 1996, 60, 297–300. [Google Scholar] [CrossRef]
- Rocamora, R.; Kurthen, M.; Lickfett, L.; Von Oertzen, J.; Elger, C.E. Cardiac Asystole in Epilepsy: Clinical and Neurophysiologic Features. Epilepsia 2003, 44, 179–185. [Google Scholar] [CrossRef]
- Sevcencu, C.; Struijk, J.J. Autonomic Alterations and Cardiac Changes in Epilepsy. Epilepsia 2010, 51, 725–737. [Google Scholar] [CrossRef]
- Tomson, T.; Ericson, M.; Ihrman, C.; Lindblad, L.E. Heart Rate Variability in Patients with Epilepsy. Epilepsy Res. 1998, 30, 77–83. [Google Scholar] [CrossRef]
- Dünser, M.W.; Hasibeder, W.R. Sympathetic Overstimulation during Critical Illness: Adverse Effects of Adrenergic Stress. J. Intensive Care Med. 2009, 24, 293–316. [Google Scholar] [CrossRef]
- Bateman, L.M.; Li, C.S.; Seyal, M. Ictal Hypoxemia in Localization-Related Epilepsy: Analysis of Incidence, Severity and Risk Factors. Brain 2008, 131, 3239–3245. [Google Scholar] [CrossRef]
- Devinsky, O. Sudden, Unexpected Death in Epilepsy. N. Engl. J. Med. 2011, 365, 1801–1811. [Google Scholar] [CrossRef]
- Ficker, D.M.; So, E.L.; Shen, W.K.; Annegers, J.F.; O’Brien, P.C.; Cascino, G.D.; Belau, P.O. Population-Based Study of the Incidence of Sudden Unexplained Death in Epilepsy. Neurology 1998, 51, 1270–1274. [Google Scholar] [CrossRef]
- Langan, Y.; Nashef, L.; Sander, J.W.A.S. Sudden Unexpected Death in Epilepsy: A Series of Witnessed Deaths. J. Neurol. Neurosurg. Psychiatry 2000, 68, 211–213. [Google Scholar] [CrossRef]
- Tomson, T.; Nashef, L.; Ryvlin, P. Sudden Unexpected Death in Epilepsy: Current Knowledge and Future Directions. Lancet Neurol. 2008, 7, 1021–1031. [Google Scholar] [CrossRef]
- So, E.L. What Is Known about the Mechanisms Underlying SUDEP? Epilepsia 2008, 49, 93–98. [Google Scholar] [CrossRef]
- Devinsky, O.; Hesdorffer, D.C.; Thurman, D.J.; Lhatoo, S.; Richerson, G. Sudden Unexpected Death in Epilepsy: Epidemiology, Mechanisms, and Prevention. Lancet Neurol. 2016, 15, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.A.; Bello-Espinosa, L.E.; Symonds, J.D.; Zuberi, S.M.; Clegg, R.; Sadleir, L.G.; Buchhalter, J.; Scheffer, I.E. Heart Rate Variability in Epilepsy: A Potential Biomarker of Sudden Unexpected Death in Epilepsy Risk. Epilepsia 2018, 59, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Suna, N.; Suna, I.; Gutmane, E.; Kande, L.; Karelis, G.; Viksna, L.; Folkmanis, V. Electrocardiographic Abnormalities and Mortality in Epilepsy Patients. Medicina 2021, 57, 504. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, L.; Rheims, S. Ictal and Interictal Cardiac Manifestations in Epilepsy. A Review of Their Relation with an Altered Central Control of Autonomic Functions and With the Risk of SUDEP. Front. Neurol. 2021, 12, 642645. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, S. Cerebrogenic Cardiac Arrhythmias: Cortical Lateralization and Clinical Significance Stephen Oppenheimer. Clin. Auton. Res. 2006, 16, 6–11. [Google Scholar] [CrossRef]
- Oppenheimer, S.M.; Wilson, J.X.; Guiraudon, C.; Cechetto, D.F. Insular Cortex Stimulation Produces Lethal Cardiac Arrhythmias: A Mechanism of Sudden Death? Brain Res. 1991, 550, 115–121. [Google Scholar] [CrossRef]
- Cechetto, D.F.; Wilson, J.X.; Smith, K.E.; Wolski, D.; Silver, M.D.; Hachinski, V.C. Autonomic and Myocardial Changes in Middle Cerebral Artery Occlusion: Stroke Models in the Rat. Brain Res. 1989, 502, 296–305. [Google Scholar] [CrossRef]
- Cechetto, D.F. Cortical Control of the Autonomic Nervous System. Exp. Physiol. 2014, 99, 326–331. [Google Scholar] [CrossRef]
- Robinson, T.G.; James, M.; Youde, J.; Panerai, R.; Potter, J. Cardiac Baroreceptor Sensitivity Is Impaired after Acute Stroke. Stroke 1997, 28, 1671–1676. [Google Scholar] [CrossRef]
- Oppenheimer, S.M.; Kedem, G.; Martin, W.M. Left-Insular Cortex Lesions Perturb Cardiac Autonomic Tone in Humans. Clin. Auton. Res. 1996, 6, 131–140. [Google Scholar] [CrossRef]
- Epstein, M.A.; Sperling, M.R.; O’connor, M.J. Cardiac Rhythm during Temporal Lobe Seizures. Neurology 1992, 42, 50. [Google Scholar] [CrossRef]
- Britton, J.W.; Ghearing, G.R.; Benarroch, E.E.; Cascino, G.D. The Ictal Bradycardia Syndrome: Localization and Lateralization. Epilepsia 2006, 47, 737–744. [Google Scholar] [CrossRef]
- Malik, M.; Camm, A.J.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart Rate Variability. Standards of Measurement, Physiological Interpretation, and Clinical Use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Myers, K.A.; Sivathamboo, S.; Perucca, P. Heart Rate Variability Measurement in Epilepsy: How Can We Move from Research to Clinical Practice? Epilepsia 2018, 59, 2169–2178. [Google Scholar] [CrossRef]
- do Nascimento Vinholes, L.; Sousa da Silva, A.; Marinho Tassi, E.; Corrêa Borges de Lacerda, G. Heart Rate Variability in Frontal Lobe Epilepsy: Association with SUDEP Risk. Acta Neurol. Scand. 2021, 143, 62–70. [Google Scholar] [CrossRef]
- Sivathamboo, S.; Friedman, D.; Laze, J.; Nightscales, R.; Chen, Z.; Kuhlmann, L.; Devore, S.; Macefield, V.; Kwan, P.; D’Souza, W.; et al. Association of Short-Term Heart Rate Variability and Sudden Unexpected Death in Epilepsy. Neurology 2021, 97, e2357–e2367. [Google Scholar] [CrossRef]
- Verrier, R.L.; Pang, T.D.; Nearing, B.D.; Schachter, S.C. Epileptic Heart: A Clinical Syndromic Approach. Epilepsia 2021, 62, 1780–1789. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Malik, M. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Nolan, J.; Batin, P.D.; Andrews, R.; Lindsay, S.J.; Brooksby, P.; Mullen, M.; Baig, W.; Flapan, A.D.; Cowley, A.; Prescott, R.J.; et al. Prospective Study of Heart Rate Variability and Mortality in Chronic Heart Failure: Results of the United Kingdom Heart Failure Evaluation and Assessment of Risk Trial (UK-Heart). Circulation 1998, 98, 1510–1516. [Google Scholar] [CrossRef]
- Berkoff, D.J.; Cairns, C.B.; Sanchez, L.D.; Moorman, C.T. Heart Rate Variability in Elite American Track-and-Field Athletes. J. Strength. Cond. Res. 2007, 21, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Abhishekh, H.A.; Nisarga, P.; Kisan, R.; Meghana, A.; Chandran, S.; Raju, T.; Sathyaprabha, T.N. Influence of Age and Gender on Autonomic Regulation of Heart. J. Clin. Monit. Comput. 2013, 27, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, S.; Laitinen, T.; Tarvainen, M.P.; Tompuri, T.; Veijalainen, A.; Savonen, K.; Lakka, T. Normal Values for Heart Rate Variability Parameters in Children 6–8 Years of Age: The PANIC Study. Clin. Physiol. Funct. Imaging 2014, 34, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public. Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Pernice, R.; Faes, L.; Kotiuchyi, I.; Stivala, S.; Busacca, A.; Popov, A.; Kharytonov, V. Time, Frequency and Information Domain Analysis of Short-Term Heart Rate Variability before and after Focal and Generalized Seizures in Epileptic Children. Physiol. Meas. 2019, 40, 074003. [Google Scholar] [CrossRef]
- Stavrinou, M.L.; Sakellaropoulos, G.C.; Trachani, E.; Sirrou, V.; Polychronopoulos, P.; Nikiforidis, G.; Chroni, E. Methodological Issues in the Spectral Analysis of the Heart Rate Variability: Application in Patients with Epilepsy. Biomed. Signal. Process. Control 2014, 13, 1–7. [Google Scholar] [CrossRef]
- Xu, H.; Chen, W.; Zhao, N.; Li, Z.; Bu, J.; Li, Z.; Liu, Y.; Zhao, Y.; Pei, D.; Feng, Y.; et al. Unsupervised Anomaly Detection via Variational Auto-Encoder for Seasonal KPIs in Web Applications. In Proceedings of the World Wide Web Conference, Lyon, France, 23–27 April 2018. [Google Scholar]
- Romigi, A.; Albanese, M.; Placidi, F.; Izzi, F.; Mercuri, N.B.; Marchi, A.; Liguori, C.; Campagna, N.; Duggento, A.; Canichella, A.; et al. Heart Rate Variability in Untreated Newly Diagnosed Temporal Lobe Epilepsy: Evidence for Ictal Sympathetic Dysregulation. Epilepsia 2016, 57, 418–426. [Google Scholar] [CrossRef]
- Salahuddin, L.; Cho, J.; Jeong, M.G.; Kim, D. Ultra Short Term Analysis of Heart Rate Variability for Monitoring Mental Stress in Mobile Settings. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007. [Google Scholar]
- Nussinovitch, U.; Elishkevitz, K.P.; Katz, K.; Nussinovitch, M.; Segev, S.; Volovitz, B.; Nussinovitch, N. Reliability of Ultra-Short ECG Indices for Heart Rate Variability. Ann. Noninvasive Electrocardiol. 2011, 16, 117–122. [Google Scholar] [CrossRef]
- Nussinovitch, U.; Cohen, O.; Kaminer, K.; Ilani, J.; Nussinovitch, N. Evaluating Reliability of Ultra-Short ECG Indices of Heart Rate Variability in Diabetes Mellitus Patients. J. Diabetes Complicat. 2012, 26, 450–453. [Google Scholar] [CrossRef]
- Shaffer, F.; Meehan, Z.M.; Zerr, C.L. A Critical Review of Ultra-Short-Term Heart Rate Variability Norms Research. Front. Neurosci. 2020, 14, 594880. [Google Scholar] [CrossRef]
- Munoz, M.L.; Van Roon, A.; Riese, H.; Thio, C.; Oostenbroek, E.; Westrik, I.; De Geus, E.J.C.; Gansevoort, R.; Lefrandt, J.; Nolte, I.M.; et al. Validity of (Ultra-)Short Recordings for Heart Rate Variability Measurements. PLoS ONE 2015, 10, e0138921. [Google Scholar] [CrossRef]
- You, S.; Jo, H.; Cho, B.; Song, J.; Kim, D.; Hwang, Y.; Shon, Y.; Seo, D.; Kim, I. Comparing Ictal Cardiac Autonomic Changes in Patients with Frontal Lobe Epilepsy and Temporal Lobe Epilepsy by Ultra-Short-Term Heart Rate Variability Analysis. Medicina 2021, 57, 666. [Google Scholar] [CrossRef]
- Zhao, Z.D.; Chen, Y.Q. A New Method for Removal of Baseline Wander and Power Line Interference in ECG Signals. In Proceedings of the 2006 International Conference on Machine Learning and Cybernetics, Dalian, China, 13–16 August 2006. [Google Scholar]
- Elgendi, M.; Jonkman, M.; Deboer, F. Frequency Bands Effects on QRS Detection. In Proceedings of the 3rd International Conference on Bio-Inpsired Systems and Signal Processing, Valencia, Spain, 20–23 January 2010. [Google Scholar]
- Kadambe, S.; Murray, R.; Paye Boudreaux-Bartels, G. Wavelet Transform-Based QRS Complex Detector. IEEE Trans. Biomed. Eng. 1999, 46, 838–848. [Google Scholar] [CrossRef]
- Nabil, D.; Bereksi Reguig, F. Ectopic Beats Detection and Correction Methods: A Review. Biomed. Signal. Process. Control 2015, 18, 228–244. [Google Scholar] [CrossRef]
- Bigger, J.T.; Kleiger, R.E.; Fleiss, J.L.; Rolnitzky, L.M.; Steinman, R.C.; Miller, J.P. Components of Heart Rate Variability Measured during Healing of Acute Myocardial Infarction. Am. J. Cardiol. 1988, 61, 208–215. [Google Scholar] [CrossRef]
- Ruf, T. The Lomb-Scargle Periodogram in Biological Rhythm Research: Analysis of Incomplete and Unequally Spaced Time-Series. Biol. Rhythm. Res. 1999, 30, 178–201. [Google Scholar] [CrossRef]
- Clifford, G.D.; Tarassenko, L. Quantifying Errors in Spectral Estimates of HRV Due to Beat Replacement and Resampling. IEEE Trans. Biomed. Eng. 2005, 52, 630–638. [Google Scholar] [CrossRef]
- Fonseca, D.S.; Netto, A.D.A.; Ferreira, R.B.; De Sa, A.M.F.L.M. Lomb-Scargle Periodogram Applied to Heart Rate Variability Study. In Proceedings of the ISSNIP Biosignals and Biorobotics Conference (BRC), Rio de Janeiro, Brazil, 18–20 February 2013. [Google Scholar]
- Reyes del Paso, G.A.; Langewitz, W.; Mulder, L.J.M.; van Roon, A.; Duschek, S. The Utility of Low Frequency Heart Rate Variability as an Index of Sympathetic Cardiac Tone: A Review with Emphasis on a Reanalysis of Previous Studies. Psychophysiology 2013, 50, 477–487. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, H.; Meng, F.; Guan, Y.; Zhao, M.; Qu, W.; Hao, H.; Luan, G.; Zhang, J.; Li, L. The Analysis of Circadian Rhythm of Heart Rate Variability in Patients with Drug-Resistant Epilepsy. Epilepsy Res. 2018, 146, 151–159. [Google Scholar] [CrossRef]
- Hallioglu, O.; Okuyaz, C.; Mert, E.; Makharoblidze, K. Effects of Antiepileptic Drug Therapy on Heart Rate Variability in Children with Epilepsy. Epilepsy Res. 2008, 79, 49–54. [Google Scholar] [CrossRef]
- Stein, P.K.; Bosner, M.S.; Kleiger, R.E.; Conger, B.M. Heart Rate Variability: A Measure of Cardiac Autonomic Tone. Am. Heart J. 1994, 127, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Sztajzel, J. Heart Rate Variability: A Noninvasive Electrocardiographic Method to Measure the Autonomic Nervous System. Swiss Med. Wkly. 2004, 134, 514–522. [Google Scholar] [PubMed]
- Toichi, M.; Sugiura, T.; Murai, T.; Sengoku, A. A New Method of Assessing Cardiac Autonomic Function and Its Comparison with Spectral Analysis and Coefficient of Variation of R-R Interval. J. Auton. Nerv. Syst. 1997, 62, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, A.; Marques, J.L.B.; Reuber, M. Comparison of Heart Rate Variability Parameters during Complex Partial Seizures and Psychogenic Nonepileptic Seizures. Epilepsia 2012, 53, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B.; Belanger, A.; D’Agostino, R.B. A Suggestion for Using Powerful and Informative Tests of Normality. Am. Stat. 1990, 44, 316–321. [Google Scholar] [CrossRef]
- Hayter, A.J. A Proof of the Conjecture That the Tukey-Kramer Multiple Comparisons Procedure Is Conservative. Ann. Stat. 1984, 12, 61–75. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a Test of Whether One of Two Random Variables Is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Evrengül, H.; Tanriverdi, H.; Dursunoglu, D.; Kaftan, A.; Kuru, O.; Unlu, U.; Kilic, M. Time and Frequency Domain Analyses of Heart Rate Variability in Patients with Epilepsy. Epilepsy Res. 2005, 63, 131–139. [Google Scholar] [CrossRef]
- Son, W.H.; Hwang, W.S.; Koo, D.L.; Hwang, K.J.; Kim, D.Y.; Seo, J.-H.; Na, G.-Y.; Joo, E.Y.; Hong, S.B.; Seo, D.-W. The Difference in Heart Rate Change between Temporal and Frontal Lobe Seizures during Peri-Ictal Period. J. Epilepsy Res. 2016, 6, 16–21. [Google Scholar] [CrossRef][Green Version]
- Beissner, F.; Meissner, K.; Bär, K.J.; Napadow, V. The Autonomic Brain: An Activation Likelihood Estimation Meta-Analysis for Central Processing of Autonomic Function. J. Neurosci. 2013, 33, 10503–10511. [Google Scholar] [CrossRef]
- Jansen, K.; Lagae, L. Cardiac Changes in Epilepsy. Seizure 2010, 19, 455–460. [Google Scholar] [CrossRef]
- Kobayashi, E.; Lopes-Cendes, I.; Guerreiro, C.A.M.; Sousa, S.C.; Guerreiro, M.M.; Cendes, F. Seizure Outcome and Hippocampal Atrophy in Familial Mesial Temporal Lobe Epilepsy. Neurology 2001, 56, 166–172. [Google Scholar] [CrossRef]
- Mente, K.; Kim, S.A.; Grunseich, C.; Hefti, M.M.; Crary, J.F.; Danek, A.; Karp, B.I.; Walker, R.H. Hippocampal Sclerosis and Mesial Temporal Lobe Epilepsy in Chorea-Acanthocytosis: A Case with Clinical, Pathologic and Genetic Evaluation. Neuropathol. Appl. Neurobiol. 2017, 43, 542–546. [Google Scholar] [CrossRef]
- Beh, S.M.J.; Cook, M.J.; D’Souza, W.J. Isolated Amygdala Enlargement in Temporal Lobe Epilepsy: A Systematic Review. Epilepsy Behav. 2016, 60, 33–41. [Google Scholar] [CrossRef]
- Lv, R.J.; Sun, Z.R.; Cui, T.; Guan, H.Z.; Ren, H.T.; Shao, X.Q. Temporal Lobe Epilepsy with Amygdala Enlargement: A Subtype of Temporal Lobe Epilepsy. BMC Neurol. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Singh, V.; Ryan, J.M.; Auerbach, D.S. It Is Premature for a Unified Hypothesis of SUDEP A Great Amount of Research Is Still Needed to Understand the Multi-System Cascade. Epilepsia 2023. [Google Scholar] [CrossRef]
- Suorsa, E.; Korpelainen, J.T.; Ansakorpi, H.; Huikuri, H.V.; Suorsa, V.; Myllylä, V.V.; Isojärvi, J.I.T. Heart Rate Dynamics in Temporal Lobe Epilepsy-A Long-Term Follow-up Study. Epilepsy Res. 2011, 93, 80–83. [Google Scholar] [CrossRef]
- Verrotti, A.; Greco, M.; Varriale, G.; Tamborino, A.; Savasta, S.; Carotenuto, M.; Elia, M.; Operto, F.; Margari, L.; Belcastro, V.; et al. Electroclinical Features of Epilepsy Monosomy 1p36 Syndrome and Their Implications. Acta Neurol. Scand. 2018, 138, 523–530. [Google Scholar] [CrossRef]
- Po’, C.; Nosadini, M.; Zedde, M.; Pascarella, R.; Mirone, G.; Cicala, D.; Rosati, A.; Cosi, A.; Toldo, I.; Colombatti, R.; et al. Pediatric Moyamoya Disease and Syndrome in Italy: A Multicenter Cohort. Front. Pediatr. 2022, 10, 892445. [Google Scholar] [CrossRef]

| Total (n = 75) | Left TLE (n = 41) | Right TLE (n = 34) | p-Value * | |
|---|---|---|---|---|
| Sex (M/F) | 36/39 | 16/25 | 20/14 | |
| Age at evaluation | 36.3 ± 13.5 | 35.9 ± 13.8 | 36.9 ± 13.2 | 0.965 |
| Age at seizure onset | 21.4 ± 15.5 | 21.2 ± 15.4 | 14.7 ± 12.3 | 0.724 |
| Disease duration (year) | 14.9 ± 11.1 | 21.6 ± 15.9 | 15.2 ± 9.5 | 0.071 |
| Etiology of epilepsy | 0.730 ** | |||
| Cryptogenic | 17 | 11 | 6 | |
| Hippocampal sclerosis | 38 | 21 | 17 | |
| Cortical malformation | 4 | 1 | 3 | |
| Tumor | 6 | 2 | 4 | |
| Vascular malformation | 6 | 4 | 2 | |
| History of encephalitis | 2 | 1 | 1 | |
| Destructive lesion | 2 | 1 | 1 | |
| Frequency of seizure | 0.187 ** | |||
| Daily | 11 | 8 | 3 | |
| Weekly | 16 | 11 | 5 | |
| Monthly | 45 | 20 | 25 | |
| Yearly | 3 | 2 | 1 | |
| Number of seizures | 7.9 ± 6.2 | 9.1 ± 7.5 | 6.5 ± 3.6 | 0.020 |
| Number of ASMs used | 2.73 ± 1.4 | 2.7 ± 1.3 | 2.8 ± 1.5 | 0.443 |
| Laterality (Rt/Lt/bilateral) | 0.000 ** | |||
| Rt | 30 | 0 | 30 | |
| Lt | 33 | 33 | 0 | |
| Bilateral or nonlateralized | 12 | 8 | 4 |
| HRV INDEX | PREICTAL | ICTAL | POSTICTAL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Left | Right | p | Left | Right | p | Left | Right | p | |
| RRI (ms) | 751.0 ± 136.2 | 847.3 ± 202.2 | 0.02 * | 581.0 ± 90.5 | 595.4 ± 118.7 | 0.55 | 685.4 ± 131.0 | 703.0 ± 143.8 | 0.58 |
| SDNN (ms) | 108.6 ± 70.7 | 95.7 ± 37.7 | 0.34 | 104.7 ± 51.4 | 128.6 ± 72.0 | 0.10 | 87.2 ± 50.7 | 101.6 ± 65.9 | 0.29 |
| RMSSD (ms) | 33.3 ± 8.9 | 34.8 ± 6.0 | 0.41 | 35.7 ± 7.4 | 45.6 ± 15.7 | <0.01 * | 30.8 ± 7.4 | 32.2 ± 7.0 | 0.41 |
| pNN50 | 6.5 ± 0.5 | 5.1 ± 1.7 | 0.14 | 7.2 ± 3.7 | 13.5 ± 7.7 | <0.01 * | 4.7 ± 3.7 | 5.5 ± 3.3 | 0.32 |
| nLF (%) | 40.3 ± 16.2 | 44.3 ± 15.9 | 0.28 | 56.4 ± 15.5 | 45.4 ± 15.4 | <0.01 * | 43.2 ± 18.7 | 43.7 ± 18.2 | 0.90 |
| nHF (%) | 22.6 ± 16.6 | 21.6 ± 13.2 | 0.78 | 12.2 ± 11.8 | 37.6 ± 22.5 | <0.01 * | 17.0 ± 13.0 | 21.8 ± 15.7 | 0.15 |
| LF/HF | 4.2 ± 5.8 | 3.0 ± 2.0 | 0.25 | 10.6 ± 11.2 | 2.6 ± 3.5 | <0.01 * | 4.5 ± 5.5 | 3.3 ± 2.6 | 0.24 |
| CSI | 3.4 ± 0.4 | 3.4 ± 0.3 | 0.94 | 3.5 ± 0.3 | 3.6 ± 0.3 | 0.01 * | 3.3 ± 0.3 | 3.4 ± 0.4 | 0.33 |
| CVI | 6.0 ± 4.5 | 4.9 ± 2.2 | 0.19 | 5.7 ± 3.6 | 5.3 ± 2.9 | 0.60 | 5.0 ± 2.4 | 5.6 ± 3.0 | 0.35 |
| HRV Index | Source | F | p | |
|---|---|---|---|---|
| Mean RRi (ms) | Laterality | 0.023 | 5.25 | 0.023 * |
| Interval | 0.280 | 42.59 | <0.001 * | |
| Laterality × interval | 0.019 | 2.06 | 0.130 | |
| SDNN (ms) | Laterality | 0.005 | 1.14 | 0.288 |
| Interval | 0.024 | 2.69 | 0.070 * | |
| Laterality × interval | 0.017 | 1.93 | 0.148 | |
| RMSSD (ms) | Laterality | 0.052 | 12.06 | <0.001 * |
| Interval | 0.151 | 19.48 | <0.001 * | |
| Laterality × interval | 0.046 | 5.32 | 0.006 * | |
| pNN 50 (%) | Laterality | 0.435 | 9.97 | 0.002 * |
| Interval | 0.208 | 28.71 | <0.001 * | |
| Laterality × interval | 0.115 | 14.25 | <0.001 * | |
| Normalized LF (%) | Laterality | 0.004 | 0.93 | 0.336 |
| Interval | 0.050 | 5.76 | 0.004 * | |
| Laterality × interval | 0.037 | 4.15 | 0.017 * | |
| Normalized HF (%) | Laterality | 0.090 | 21.54 | <0.001 * |
| Interval | 0.020 | 2.27 | 0.106 | |
| Laterality × interval | 0.117 | 14.54 | <0.001 * | |
| LF/HF ratio | Laterality | 0.074 | 17.60 | <0.001 * |
| Interval | 0.048 | 5.52 | 0.005 * | |
| Laterality × interval | 0.063 | 7.37 | <0.001 * | |
| CVI | Laterality | 0.012 | 4.46 | 0.036 * |
| Interval | 0.068 | 7.96 | 0.001 * | |
| Laterality × interval | 0.014 | 1.52 | 0.221 | |
| CSI | Laterality | 0.002 | 0.51 | 0.474 |
| Interval | 0.001 | 0.11 | 0.896 | |
| Laterality × interval | 0.012 | 1.28 | 0.280 |
| HRV INDEX | PREICTAL | ICTAL | POSTICTAL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| – | Dominant (n = 26) | Nondominant (n = 32) | p | Dominant (n = 26) | Nondominant (n = 32) | p | Dominant (n = 26) | Nondominant (n = 32) | p |
| RRI (ms) | 773.46 ± 99.4 | 829.9 ± 222.1 | 0.235 | 593.6 ± 87.7 | 570.2 ± 107.3 | 0.375 | 691.2 ± 95.2 | 683.9 ± 148.1 | 0.830 |
| SDNN (ms) | 120.0 ± 74.5 | 95.0 ± 41.4 | 0.112 | 107.6 ± 52.5 | 131.0 ± 75.3 | 0.184 | 97.8 ± 54.6 | 101.9 ± 68.5 | 0.803 |
| RMSSD (ms) | 34.5 ± 9.3 | 34.1 ± 6.7 | 0.876 | 35.3 ± 7.8 | 44.1 ± 16.5 | 0.015 * | 31.7 ± 7.2 | 31.9 ± 7.5 | 0.914 |
| pNN50 | 6.6 ± 4.6 | 5.0 ± 1.9 | 0.071 | 7.7 ± 4.3 | 13.8 ± 7.9 | <0.001 * | 5.3 ± 4.3 | 5.6 ± 3.5 | 0.802 |
| nLF (%) | 37.9 ± 14.6 | 42.4 ± 16.2 | 0.279 | 57.0 ± 15.9 | 48.0 ± 17.3 | 0.046 * | 43.9 ± 17.5 | 44.5 ± 18.3 | 0.895 |
| nHF (%) | 24.9 ± 14.3 | 19.7 ± 14.0 | 0.169 | 14.0 ± 14.0 | 36.5 ± 22.8 | <0.001 * | 19.7 ± 14.4 | 22.2 ± 15.4 | 0.528 |
| LF/HF | 3.5 ± 6.3 | 3.9 ± 3.6 | 0.618 | 9.7 ± 10.3 | 3.0 ± 4.0 | 0.001 * | 3.8 ± 3.2 | 3.2 ± 2.6 | 0.456 |
| CSI | 3.5 ± 0.3 | 3.4 ± 0.3 | 0.203 | 3.5 ± 0.3 | 3.6 ± 0.4 | 0.071 | 3.4 ± 0.3 | 3.4 ± 0.4 | 0.795 |
| CVI | 6.8 ± 5.1 | 5.0 ± 2.4 | 0.075 | 6.0 ± 3.8 | 5.6 ± 3.1 | 0.659 | 5.6 ± 2.5 | 5.6 ± 3.1 | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, S.-M.; Cho, B.-H.; Bae, H.-E.; Kim, Y.-K.; Kim, J.-R.; Park, S.-R.; Shon, Y.-M.; Seo, D.-W.; Kim, I.-Y. Exploring Autonomic Alterations during Seizures in Temporal Lobe Epilepsy: Insights from a Heart-Rate Variability Analysis. J. Clin. Med. 2023, 12, 4284. https://doi.org/10.3390/jcm12134284
You S-M, Cho B-H, Bae H-E, Kim Y-K, Kim J-R, Park S-R, Shon Y-M, Seo D-W, Kim I-Y. Exploring Autonomic Alterations during Seizures in Temporal Lobe Epilepsy: Insights from a Heart-Rate Variability Analysis. Journal of Clinical Medicine. 2023; 12(13):4284. https://doi.org/10.3390/jcm12134284
Chicago/Turabian StyleYou, Sung-Min, Baek-Hwan Cho, Hyo-Eun Bae, Young-Kyun Kim, Jae-Rim Kim, Soo-Ryun Park, Young-Min Shon, Dae-Won Seo, and In-Young Kim. 2023. "Exploring Autonomic Alterations during Seizures in Temporal Lobe Epilepsy: Insights from a Heart-Rate Variability Analysis" Journal of Clinical Medicine 12, no. 13: 4284. https://doi.org/10.3390/jcm12134284
APA StyleYou, S.-M., Cho, B.-H., Bae, H.-E., Kim, Y.-K., Kim, J.-R., Park, S.-R., Shon, Y.-M., Seo, D.-W., & Kim, I.-Y. (2023). Exploring Autonomic Alterations during Seizures in Temporal Lobe Epilepsy: Insights from a Heart-Rate Variability Analysis. Journal of Clinical Medicine, 12(13), 4284. https://doi.org/10.3390/jcm12134284