In Marfan Syndrome and Related Diseases, STABILISE Technique Should Be Used with Care: Results from a Volumetric Comparative Study of Endovascular Treatment for Aortic Dissection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Perioperative Approach
2.3. TEVAR Technique
2.4. STABILISE Technique
2.4.1. Distal Aortic Bare-Stent Deployment
2.4.2. Management of Visceral Arteries
2.4.3. Balloon Dilatation of the Bare Stent
2.5. Epidemiological Data
2.6. Radiological Data
2.7. Diameter Analysis
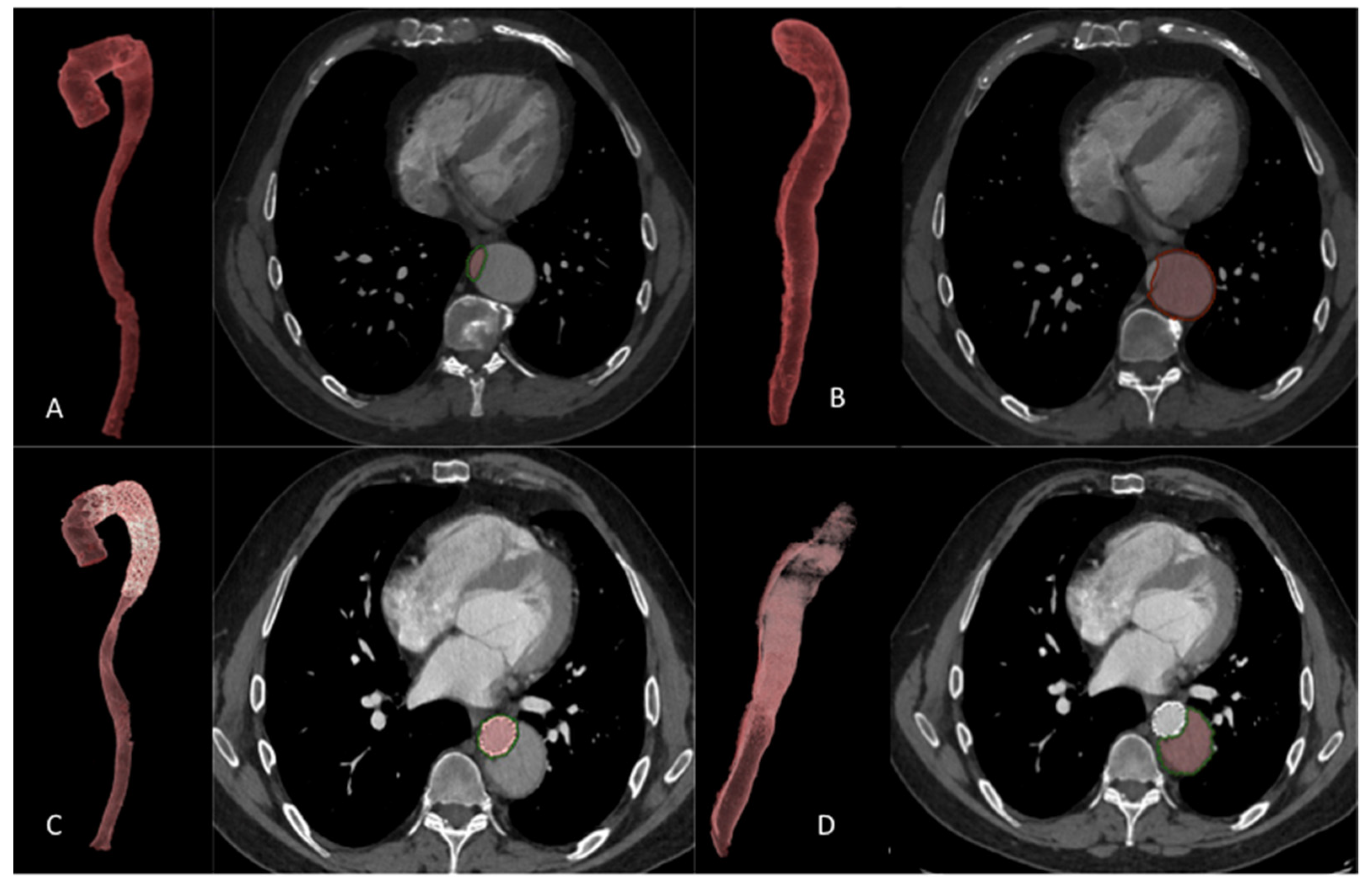
2.8. Volume Analysis
2.9. FL Status
2.10. Entry Tear
2.11. Endpoints
2.12. Statistical Analysis
3. Results
3.1. Population (Figure 2)

3.2. FL Status
3.3. Anatomical Results
3.3.1. Diameter Analysis
3.3.2. Volume Analysis
Results at 1 Year
Results at the End of the Follow-Up
3.3.3. Subgroup Study: TEVAR/STABILISE: Volume Analysis (Table 3)
| At 1 Year | True Lumen | False Lumen | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | non HTAD | HTAD | p-value | Non-HTAD | HTAD | p-value | Non-HTAD | HTAD | p-value |
| STABILISE (−) % mean (SD) | 45.7 (±39.7) | 47.0 (±22.5) | 0.77 | −17.5 (±43.3) | 6.6 (±73.0) | 0.482 | 1.7 (±20.3) | 16.7 (±30.1) | 0.263 |
| STABILISE (+) % mean (SD) | 98.3 (±38.9) | 160.1 (±52.3) | 0.029 | −74.9 (±15.2) | −66.3 (±21.9) | 0.694 | −3.1 (±19.1) | 26.2 (±16.4) | 0.009 |
| p-value | 0.011 | <0.001 | 0.001 | 0.006 | 0.447 | 0.277 | |||
| Last Follow-Up | True Lumen | False Lumen | Total | ||||||
| Group | Non-HTAD | HTAD | p-value | Non-HTAD | HTAD | p-value | Non-HTAD | HTAD | p-value |
| STABILISE (−) % mean (SD) | 71.2 (±59.5) | 58.6 (±38.4) | 0.967 | −17.6 (±52.4) | −13.3 (±39.2) | 0.432 | 12.3 (±40.0) | 17.1 (±17.6) | 0.773 |
| STABILISE (+) % mean (SD) | 89.2 (±29.4) | 189.5 (±92.5) | 0.042 | −71.2 (±22.8) | −63.4 (±24.2) | 0.648 | 2.7 (±22.5) | 35.7 (±17.2) | 0.042 |
| p-value | 0.17 | 0.004 | 0.042 | 0.026 | 1 | 0.128 | |||
Results at 1 Year
Results at the End of the Follow-Up
3.4. Risk Factors for Unfavorable Anatomical Evolution in HTAD Group
3.5. Morbi-Mortality
3.5.1. Perioperative Morbidity and Mortality
3.5.2. Long-Term Morbidity and Mortality
3.6. Reoperations
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MFS | marfan syndrome |
| HTAD | Heritable Thoracic Aortic Disease |
| TEVAR | thoracic endovascular aortic repair |
| AD | aortic dissection |
| TL | True lumen |
| FL | false lumen |
| NET | new entry tears |
References
- Judge, D.P.; Dietz, H.C. Marfan’s syndrome. Lancet 2005, 366, 1965–1976. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [PubMed] [Green Version]
- Grabenwoger, M.; Alfonso, F.; Bachet, J.; Bonser, R.; Czerny, M.; Eggebrecht, H.; Evangelista, A.; Fattori, R.; Jakob, H.; Lonn, L.; et al. Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: A position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. J. Cardiothorac. Surg. 2012, 42, 17–24. [Google Scholar] [PubMed] [Green Version]
- Parisi, R.; Secco, G.G.; Di Eusanio, M.; Fattori, R. Endovascular Repair of Aortic Dissection in Marfan Syndrome: Current Status and Future Perspectives. Diseases 2015, 3, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacini, D.; Parolari, A.; Berretta, P.; Di Bartolomeo, R.; Alamanni, F.; Bavaria, J. Endovascular treatment for type B dissection in Marfan syndrome: Is it worthwhile? Ann. Thorac. Surg. 2013, 95, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Eid-Lidt, G.; Gaspar, J.; Meléndez-Ramírez, G.; Cervantes, S.J.; González-Pacheco, H.; Dámas de Los Santos, F.; Meave-González, A.; Ramírez Marroquín, S. Endovascular treatment of type B dissection in patients with Marfan syndrome: Mid-term outcomes and aortic remodeling. Catheter. Cardiovasc. Interv. 2013, 82, E898–E905. [Google Scholar] [CrossRef]
- Czerny, M.; Schmidli, J.; Adler, S.; van den Berg, J.C.; Bertoglio, L.; Carrel, T.; Chiesa, R.; Clough, R.E.; Eberle, B.; Etz, C.; et al. Editor’s Choice—Current Options and Recommendations for the Treatment of Thoracic Aortic Pathologies Involving the Aortic Arch: An Expert Consensus Document of the European Association for Cardio-Thoracic Surgery (EACTS) & the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2019, 57, 165–198. [Google Scholar]
- Odofin, X.; Houbby, N.; Hagana, A.; Nasser, I.; Ahmed, A.; Harky, A. Thoracic aortic aneurysms in patients with heritable connective tissue disease. J. Card. Surg. 2021, 36, 1083–1090. [Google Scholar] [CrossRef]
- Lovato, L.; Cefarelli, M.; Gatta, E.; Di Eusanio, M.; Fattori, R. Devices for thoracic endovascular aortic repair of type B aortic dissection: Is there any chance for Marfan syndrome? Expert Rev. Med. Devices 2020, 17, 683–696. [Google Scholar] [CrossRef]
- Faure, E.M.; El Batti, S.; Abou Rjeili, M.; Ben Abdallah, I.; Julia, P.; Alsac, J.M. Stent-assisted, balloon-induced intimal disruption and relamination of aortic dissection in patients with Marfan syndrome: Midterm outcomes and aortic remodeling. J. Thorac. Cardiovasc. Surg. 2018, 156, 1787–1793. [Google Scholar] [CrossRef]
- Soler, R.; Bartoli, M.A.; Amabile, P.; Sarlon-Bartoli, G.; Magnan, P.E. STABILISE for Complicated Type B Dissection after 15 Months’ Follow Up: A Word of Caution. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Fattori, R.; Montgomery, D.; Lovato, L.; Kische, S.; Di Eusanio, M.; Ince, H.; Eagle, K.A.; Isselbacher, E.M.; Nienaber, C.A. Survival after endovascular therapy in patients with type B aortic dissection: A report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc. Interv. 2013, 6, 876–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faure, E.M.; Canaud, L.; Agostini, C.; Shaub, R.; Böge, G.; Marty-ané, C.; Alric, P. Reintervention after thoracic endovascular aortic repair of complicated aortic dissection. J. Vasc. Surg. 2014, 59, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Fu, W.; Wang, Y.; Wang, C.; Yan, Z.; Guo, D.; Xu, X.; Chen, B. Stent graft-induced new entry after endovascular repair for Stanford type B aortic dissection. J. Vasc. Surg. 2010, 52, 1450–1457. [Google Scholar] [CrossRef] [Green Version]
- Sobocinski, J.; Lombardi, J.V.; Dias, N.V.; Berger, L.; Zhou, Q.; Jia, F.; Resch, T.; Haulon, S. Volume analysis of true and false lumens in acute complicated type B aortic dissections after thoracic endovascular aortic repair with stent grafts alone or with a composite device design. J. Vasc. Surg. 2016, 63, 1216–1224. [Google Scholar] [CrossRef] [Green Version]
- Trimarchi, S.; Tolenaar, J.L.; Jonker, F.H.; Murray, B.; Tsai, T.T.; Eagle, K.A.; Rampoldi, V.; Verhagen, H.J.; van Herwaarden, J.A.; Moll, F.L.; et al. Importance of false lumen thrombosis in type B aortic dissection prognosis. J. Thorac. Cardiovasc. Surg. 2013, 145, S208–S212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nienaber, C.A.; Kische, S.; Rousseau, H.; Eggebrecht, H.; Rehders, T.C.; Kundt, G.; Glass, A.; Scheinert, D.; Czerny, M.; Kleinfeldt, T.; et al. Endovascular repair of type B aortic dissection: Long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ. Cardiovasc. Interv. 2013, 6, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Qato, K.; Conway, A.; Lu, E.; Tran, N.N.; Giangola, G.; Carroccio, A. Outcomes of Thoracic Endovascular Aneurysm Repair (TEVAR) in Patients With Connective Tissue Disorders. Vasc. Endovascular. Surg. 2020, 54, 676–680. [Google Scholar] [CrossRef]
- Nordon, I.M.; Hinchliffe, R.J.; Holt, P.J.; Morgan, R.; Jahangiri, M.; Loftus, I.M.; Thompson, M.M. Endovascular management of chronic aortic dissection in patients with Marfan syndrome. J. Vasc. Surg. 2009, 50, 987–991. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.H.; Fu, W.G.; Wang, Y.Q.; Guo, D.Q.; Xu, X.; Ji, Y.; Chen, B.; Jiang, J.H.; Yang, J.; Shi, Z.Y.; et al. Retrograde type A aortic dissection after endovascular stent graft placement for treatment of type B dissection. Circulation 2009, 119, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Martin, J.; Shennib, H.; Dunning, J.; Muneretto, C.; Schueler, S.; Von Segesser, L.; Sergeant, P.; Turina, M. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease a systematic review and meta-analysis of comparative studies. J. Am. Coll. Cardiol. 2010, 55, 986–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhao, Z.; Chen, Y.; Sun, Y.; Bao, J.; Jing, Z.; Zhou, J. Reintervention after endovascular repair for aortic dissection: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2016, 152, 1279–1288.e1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milleron, O.; Arnoult, F.; Delorme, G.; Detaint, D.; Pellenc, Q.; Raffoul, R.; Tchitchinadze, M.; Langeois, M.; Guien, C.; Beroud, C.; et al. Pathogenic FBN1 Genetic Variation and Aortic Dissection in Patients With Marfan Syndrome. J. Am. Coll. Cardiol. 2020, 75, 843–853. [Google Scholar] [CrossRef] [PubMed]



| Demographic Data | HTAD n = 17 | Non-HTAD n = 22 | p-Value |
|---|---|---|---|
| Age, mean (SD) | 40 (±12) | 57 (±8.7) | <0.01 |
| Male sex, n (%) | 13 (77) | 20 (91) | 0.374 |
| Hypertension, n (%) | 10 (58.8) | 21 (95.5) | 0.013 |
| Smokers, n (%) | 10 (58.8) | 11 (50.0) | 0.584 |
| Diabetis mellitus, n (%) | 1 (5.9) | 1 (4.5) | 1.000 |
| Dyslipidemia, n (%) | 3 (17.6) | 5 (22.7) | 1.000 |
| Coronaropathy, n (%) | 0 (0.0) | 1 (4.5) | 1.000 |
| Valvulopathy, n (%) | 4 (23.5) | 1 (4.5) | 0.147 |
| LVEF <55%, n (%) | 2 (11.8) | 1 (4.5) | 0.570 |
| COPD, n (%) | 0 (0.0) | 2 (9.1) | 0.495 |
| Renal failure, n (%) | 0 (0.0) | 1 (4.5) | 1.000 |
| Anticoagulants, n (%) | 5 (29.4) | 11 (50.0) | 0.195 |
| Aortic surgery | |||
| Type A aortic dissection, n (%) | 8 (47.1) | 13 (59.1) | 0.053 |
| Valve replacement, n (%) | 15 (88.2) | 4 (18.2) | 0.140 |
| Aortic replacement, n (%) | 2 (11.8) | 14 (63.6) | 0.570 |
| Treatment phase | |||
| Acute and Sub-Acute phase (14–90 days), n (%) | 9 (52.9) | 7 (31.8) | 0.332 |
| Chronic phase> 90 days, n (%) | 8 (47.1) | 15 (68.2) | 0.053 |
| Indication | |||
| Rupture, n (%) | 0 (0%) | 0(0%) | 1 |
| Malperfusion syndrome, n (%) | 5 (29.4%) | 4(18.2%) | 0.457 |
| Refractory pain, n (%) | 2 (11.8%) | 0 (0%) | 0.457 |
| Refractory hypertension, n (%) | 0 (0%) | 3 (13.6%) | 0.457 |
| Rapid aortic growth > 5 mm/6 month, n (%) | 5 (29.4%) | 7(31.8%) | 0.457 |
| Aneurysmal evolution, n (%) | 5 (29.4%) | 8 (36.4%) | 0.457 |
| HTAD n = 17 | Non-HTAD n = 22 | p-Value | |
|---|---|---|---|
| Peoximal neck management surgery, n (%) | 10 (58.8) | 16 (72.7) | 0.728 |
| IA debranching, n (%) | 3 (17.6) | 12 (54.5) | 0.036 |
| LCCA debranching, n (%) | 4 (23.5) | 14 (63.6) | 0.027 |
| LSA debranching, n (%) | 10 (58.8) | 16 (72.7) | 0.728 |
| 3 supra-aortic trunks debranching, n (%) | 3 (17.6) | 11 (50.0) | 0.065 |
| Proximal landing zone (Ishimaru) | |||
| Z0 n (%) | 2 (11.8) | 11 (50.0) | 0.067 |
| Z1 n (%) | 1 (5.9) | 3 (13.6) | 1.000 |
| Z2 n (%) | 7 (41.2) | 3 (13.6) | 0.022 |
| Z3 n (%) | 7 (41.1) | 5 (22.8) | 0.216 |
| Proximal neck length (mm), mean (SD) | 14.7 (±14.6) | 28.5 (±22.9) | 0.067 |
| Proximal neck diameter (mm), mean (SD) | 29.1 (±9.0) | 32.1 (±7.6) | 0.055 |
| STABILISE, n (%) | 8 (47.1) | 7 (31.8) | 0.332 |
| Length of cover (mm), mean (SD) | 199.4 (±52.8) | 194.1 (±50.5) | 0.989 |
| Number of entry tears, mean (SD) | 5.8 (±3.1) | 4.0 (±2.3) | 0.052 |
| Diameter of the main entry tears, mean (SD) | 13.6(±7.5) | 15.1 (±11.4) | 0.908 |
| Location of the main entry tears | |||
| Segment 2 | 1 (5.9) | 4 (18.2) | 0.267 |
| Segment 3 | 13 (76.5) | 10 (45.5) | 0.267 |
| Segment 4 | 2 (11.8) | 6 (27.3) | 0.267 |
| Segment 5 | 1 (5.9) | 2 (9.1) | 0.267 |
| HTAD Group | Secondary Procedure | Time to Reintervention (Months) |
|---|---|---|
| Patient 1 | Hybrid treatment of the throraco abdominal aorta for aneurysmal progression | 18 |
| Patient 2 | Type A Aortic dissection and distal TEVAR for aortic rupture | 57 |
| Patient 3 | Type A Aortic dissection and TEVAR for aneurysmal evolution | 1 |
| Patient 4 | CT embolization, TEVAR, Iliac branch stent graft for type Ib endoleak and aneurysmal progression | 47 |
| Patient 5 | TEVAR + CT embolisation for aneurysmal progression | 37 |
| Patient 6 | TEVAR for aneurysmal progression | 16 |
| Patient 7 | TEVAR for aneurysmal progression | 5 |
| Non HTAD group | Secondary procedure | Time to reintervention (months) |
| Patient 1 | Proximal neck embolisation for type Ia endoleak | 2 |
| Patient 2 | Hybrid treatment of the aortic arch for aneurysmal progression | 31 |
| Patient 3 | EVAR leg angioplasty for lower limb claudication | 41 |
| Patient 4 | Intercarotid bypass for cerebral malperfusion | 49 |
| Patient 5 | Hybrid aortic arch treatment for aorto-bronchial fistula | 7 |
| Patient 6 | TEVAR for aneurysmal evolution | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azogui, R.; Porto, A.; Castelli, M.; Omnes, V.; De Masi, M.; Bartoli, M.; Piquet, P.; Gariboldi, V.; Busa, T.; Jacquier, A.; et al. In Marfan Syndrome and Related Diseases, STABILISE Technique Should Be Used with Care: Results from a Volumetric Comparative Study of Endovascular Treatment for Aortic Dissection. J. Clin. Med. 2023, 12, 4378. https://doi.org/10.3390/jcm12134378
Azogui R, Porto A, Castelli M, Omnes V, De Masi M, Bartoli M, Piquet P, Gariboldi V, Busa T, Jacquier A, et al. In Marfan Syndrome and Related Diseases, STABILISE Technique Should Be Used with Care: Results from a Volumetric Comparative Study of Endovascular Treatment for Aortic Dissection. Journal of Clinical Medicine. 2023; 12(13):4378. https://doi.org/10.3390/jcm12134378
Chicago/Turabian StyleAzogui, Ron, Alizee Porto, Maxime Castelli, Virgile Omnes, Mariangela De Masi, Michel Bartoli, Philippe Piquet, Vlad Gariboldi, Tiffany Busa, Alexis Jacquier, and et al. 2023. "In Marfan Syndrome and Related Diseases, STABILISE Technique Should Be Used with Care: Results from a Volumetric Comparative Study of Endovascular Treatment for Aortic Dissection" Journal of Clinical Medicine 12, no. 13: 4378. https://doi.org/10.3390/jcm12134378
APA StyleAzogui, R., Porto, A., Castelli, M., Omnes, V., De Masi, M., Bartoli, M., Piquet, P., Gariboldi, V., Busa, T., Jacquier, A., Bal, L., & Gaudry, M. (2023). In Marfan Syndrome and Related Diseases, STABILISE Technique Should Be Used with Care: Results from a Volumetric Comparative Study of Endovascular Treatment for Aortic Dissection. Journal of Clinical Medicine, 12(13), 4378. https://doi.org/10.3390/jcm12134378






