Current Insights into Cellular Determinants of Peritoneal Fibrosis in Peritoneal Dialysis: A Narrative Review
Abstract
:1. Introduction
2. The Fundamentals of Peritoneum
3. Introduction to Peritoneal Dialysis
4. Peritoneal Fibrosis as a Long-Term Complication of PD
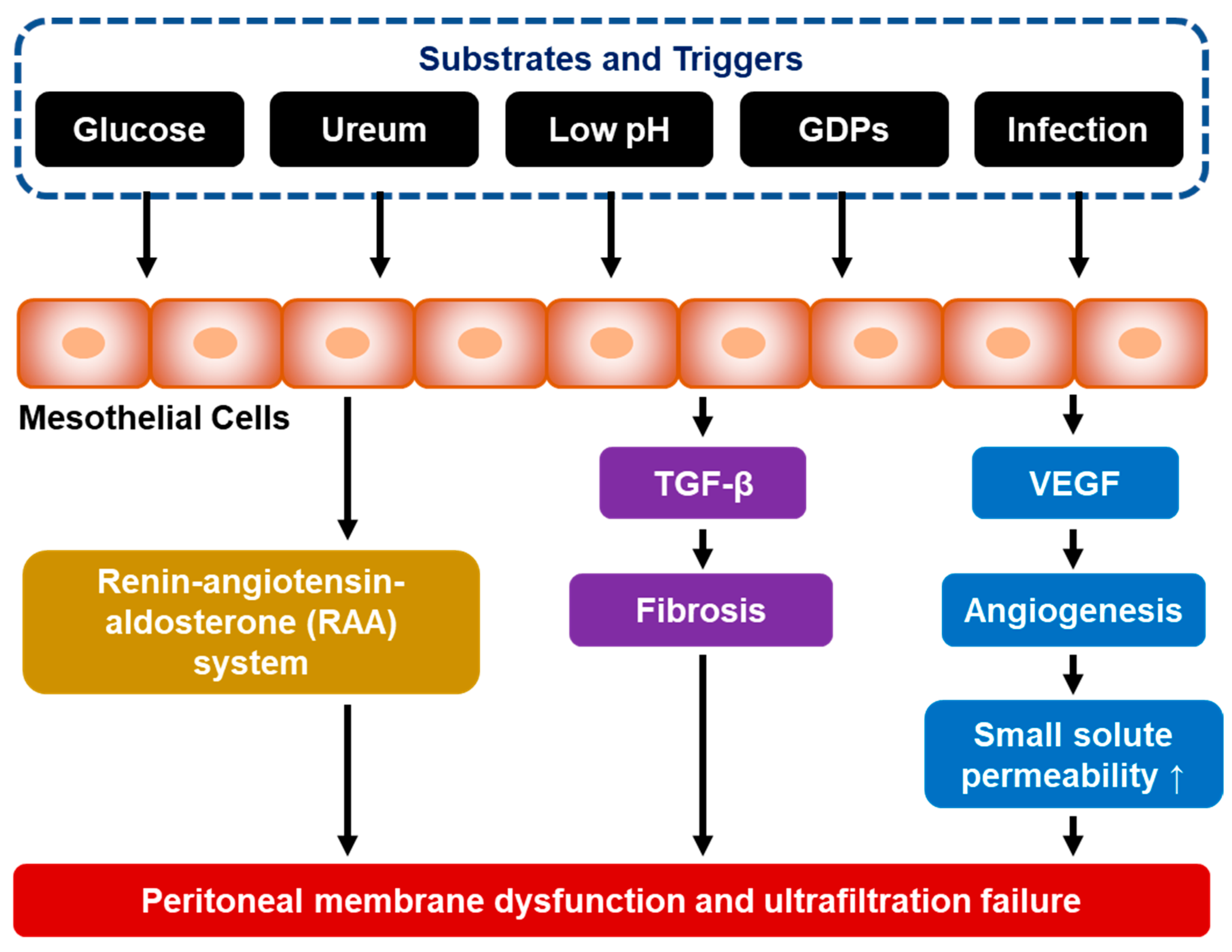
5. The Pathophysiology of Peritoneal Fibrosis
5.1. Mesothelial-to-Mesenchymal Transition (MMT)
5.2. VEGF-Mediated Neoangiogenesis
5.3. The Role of TGF-β and Smad/Non-SMAD Signaling Pathways
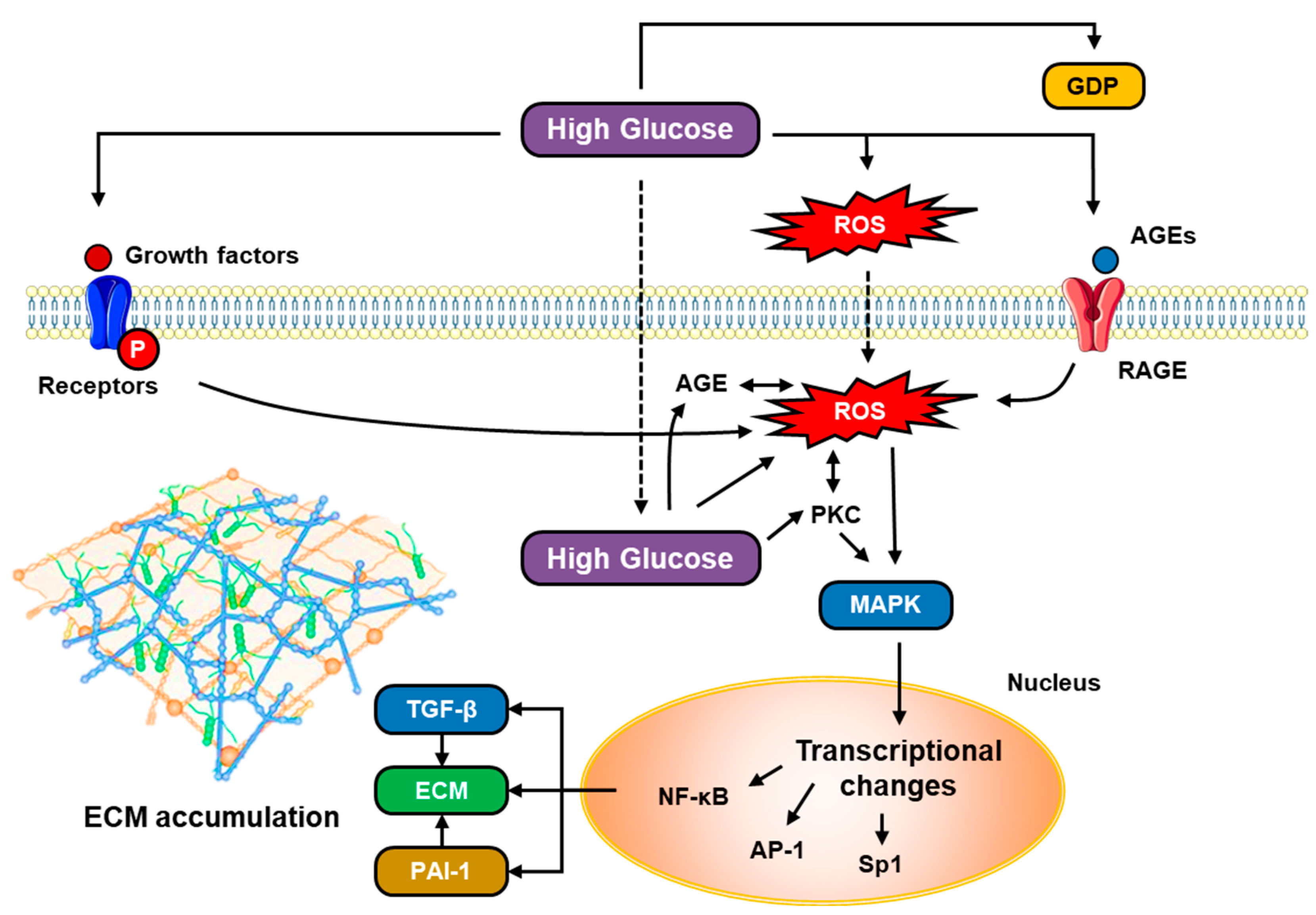
5.4. The Role of Glucose Metabolism
5.5. The Role of Epigenetics and Gut Microbiome
5.6. The Role of Macrophage Infiltration
5.7. Peritoneal Remodelling in Diabetes Mellitus
5.8. Expressions of Glucose Receptors SGLT-2, GLUT, and DPP-4 in Peritoneum and Their Roles in the Formation of Peritoneal Fibrosis
5.9. TGF-β and VEGF in Hyperglycemia
6. Diagnostic Markers of Peritoneal Fibrosis in PD
7. Managements of Peritoneal Fibrosis in PD
8. Peritoneal Rest in PD-Associated Peritoneal Fibrosis
9. Potential Roles of New-Generation Glucose-Lowering Drugs to Limit PD-Associated Peritoneal Fibrosis
10. Peritoneal Function Test as a Non-Invasive Modality to Monitor Peritoneal Membrane Quality
11. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashmi, M.F.; Benjamin, O.; Lappin, S.L. End-Stage Renal Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gupta, R.; Woo, K.; Yi, J.A. Epidemiology of End-Stage Kidney Disease. Semin. Vasc. Surg. 2021, 34, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fleming, G.M. Renal Replacement Therapy Review. Organogenesis 2011, 7, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perl, J.; Bargman, J.M. Peritoneal Dialysis: From Bench to Bedside and Bedside to Bench. Am. J. Physiol.-Ren. Physiol. 2016, 311, F999–F1004. [Google Scholar] [CrossRef] [Green Version]
- Sinnakirouchenan, R.; Holley, J.L. Peritoneal Dialysis versus Hemodialysis: Risks, Benefits, and Access Issues. Adv. Chronic Kidney Dis. 2011, 18, 428–432. [Google Scholar] [CrossRef]
- Krediet, R.T. Aging of the Peritoneal Dialysis Membrane. Front. Physiol. 2022, 13. [Google Scholar] [CrossRef]
- Wang, A.Y.M.; Brimble, K.S.; Brunier, G.; Holt, S.G.; Jha, V.; Johnson, D.W.; Kang, S.-W.; Kooman, J.P.; Lambie, M.; McIntyre, C.; et al. ISPD Cardiovascular and Metabolic Guidelines in Adult Peritoneal Dialysis Patients Part II—Management of Various Cardiovascular Complications. Perit. Dial. Int. 2015, 35, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Li, P.K.-T.; Chow, K.M.; Cho, Y.; Fan, S.; Figueiredo, A.E.; Harris, T.; Kanjanabuch, T.; Kim, Y.-L.; Madero, M.; Malyszko, J.; et al. ISPD Peritonitis Guideline Recommendations: 2022 Update on Prevention and Treatment. Perit. Dial. Int. 2022, 42, 110–153. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, N.; Sacchi, G.; Garosi, G.; Taganelli, P.; Gaggiotti, E. Simple Peritoneal Sclerosis and Sclerosing Peritonitis: Related or Distinct Entities? Int. J. Artif. Organs 2005, 28, 117–128. [Google Scholar] [CrossRef]
- Davies, S.J.; Williams, J.D. CHAPTER 93—Complications of Peritoneal Dialysis. In Comprehensive Clinical Nephrology, 4th ed.; Floege, J., Johnson, R.J., Feehally, J., Eds.; Mosby: Philadelphia, PA, USA, 2010; pp. 1092–1101. ISBN 978-0-323-05876-6. [Google Scholar]
- Danford, C.J.; Lin, S.C.; Smith, M.P.; Wolf, J.L. Encapsulating Peritoneal Sclerosis. World J. Gastroenterol. 2018, 24, 3101–3111. [Google Scholar] [CrossRef]
- Terri, M.; Trionfetti, F.; Montaldo, C.; Cordani, M.; Tripodi, M.; Lopez-Cabrera, M.; Strippoli, R. Mechanisms of Peritoneal Fibrosis: Focus on Immune Cells–Peritoneal Stroma Interactions. Front. Immunol. 2021, 12, 607204. [Google Scholar] [CrossRef]
- Jones, C.B.; Roumeliotis, A.K.; Bargman, J.M. 33—Noninfectious Complications of Peritoneal Dialysis. In Chronic Kidney Disease, Dialysis, and Transplantation, 4th ed.; Himmelfarb, J., Ikizler, T.A., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 520–537.e6. ISBN 978-0-323-52978-5. [Google Scholar]
- Petrie, M.C.; Traynor, J.P.; Mactier, R.A. Incidence and Outcome of Encapsulating Peritoneal Sclerosis. Clin. Kidney J. 2016, 9, 624–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriles, K.E.; Hashmi, M.F. Encapsulating Peritoneal Sclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yung, S.; Chan, T.M. Pathophysiology of the Peritoneal Membrane during Peritoneal Dialysis: The Role of Hyaluronan. J. Biomed. Biotechnol. 2011, 2011, 180594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.H.; Teitelbaum, I. Physiology of Peritoneal Dialysis. In Applied Peritoneal Dialysis: Improving Patient Outcomes; Rastogi, A., Lerma, E.V., Bargman, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 11–23. ISBN 978-3-030-70897-9. [Google Scholar]
- Goldstein, M.; Carrillo, M.; Ghai, S. Continuous Ambulatory Peritoneal Dialysis—A Guide to Imaging Appearances and Complications. Insights Imaging 2013, 4, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morinelli, T.A.; Luttrell, L.M.; Strungs, E.G.; Ullian, M.E. Angiotensin II Receptors and Peritoneal Dialysis-Induced Peritoneal Fibrosis. Int. J. Biochem. Cell Biol. 2016, 77, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Masola, V.; Bonomini, M.; Borrelli, S.; Di Liberato, L.; Vecchi, L.; Onisto, M.; Gambaro, G.; Palumbo, R.; Arduini, A. Fibrosis of Peritoneal Membrane as Target of New Therapies in Peritoneal Dialysis. Int. J. Mol. Sci. 2022, 23, 4831. [Google Scholar] [CrossRef]
- Zhou, Q.; Bajo, M.-A.; del Peso, G.; Yu, X.; Selgas, R. Preventing Peritoneal Membrane Fibrosis in Peritoneal Dialysis Patients. Kidney Int. 2016, 90, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Zhang, L.; Wang, Y.; Deng, M.; Peng, C.; Liang, W.; Ding, G.; Shen, B.; Wang, H. Loss of JNK-Associated Leucine Zipper Protein Promotes Peritoneal Dialysis-Related Peritoneal Fibrosis. Kidney Dis. 2022, 8, 168–179. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Tao, M.; Zhuang, S.; Liu, N. Peritoneal Fibrosis and Epigenetic Modulation. Perit. Dial. Int. 2021, 41, 168–178. [Google Scholar] [CrossRef]
- Shang, J.; He, Q.; Chen, Y.; Yu, D.; Sun, L.; Cheng, G.; Liu, D.; Xiao, J.; Zhao, Z. MiR-15a-5p Suppresses Inflammation and Fibrosis of Peritoneal Mesothelial Cells Induced by Peritoneal Dialysis via Targeting VEGFA. J. Cell. Physiol. 2019, 234, 9746–9755. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Gou, R.; Tang, L.; Liu, P. Noncoding RNAs in Peritoneal Fibrosis: Background, Mechanism, and Therapeutic Approach. Biomed. Pharmacother. 2020, 129, 110385. [Google Scholar] [CrossRef]
- Kimura, Y.; Ohzawa, H.; Miyato, H.; Kaneko, Y.; Saito, A.; Takahashi, K.; Tojo, M.; Yamaguchi, H.; Kurashina, K.; Saito, S.; et al. MiR-29b May Suppresses Peritoneal Metastases through Inhibition of the Mesothelial–Mesenchymal Transition (MMT) of Human Peritoneal Mesothelial Cells. Sci. Rep. 2022, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, N. The Gut-Peritoneum Axis in Peritoneal Dialysis and Peritoneal Fibrosis. Kidney Med. 2023, 5, 100645. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zheng, M.; Liu, Y.; Sun, W.; Shi, J.; Ni, J.; Wang, Q. A Pathogenetic Role for M1 Macrophages in Peritoneal Dialysis-Associated Fibrosis. Mol. Immunol. 2018, 94, 131–139. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z.-P.; Su, N.; Fan, J.-J.; Ruan, Y.-P.; Peng, W.-X.; Li, Y.-F.; Yu, X.-Q. The Role of Peritoneal Alternatively Activated Macrophages in the Process of Peritoneal Fibrosis Related to Peritoneal Dialysis. Int. J. Mol. Sci. 2013, 14, 10369–10382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, K.; Hamada, C.; Nakayama, M.; Miyazaki, M.; Sherif, A.M.; Harada, T.; Hirano, H. Impact of Uremia, Diabetes, and Peritoneal Dialysis Itself on the Pathogenesis of Peritoneal Sclerosis: A Quantitative Study of Peritoneal Membrane Morphology. Clin. J. Am. Soc. Nephrol. 2008, 3, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Debray-García, Y.; Sánchez, E.I.; Rodríguez-Muñoz, R.; Venegas, M.A.; Velazquez, J.; Reyes, J.L. Diabetes and Exposure to Peritoneal Dialysis Solutions Alter Tight Junction Proteins and Glucose Transporters of Rat Peritoneal Mesothelial Cells. Life Sci. 2016, 161, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Schricker, S.; Oberacker, T.; Fritz, P.; Ketteler, M.; Alscher, M.D.; Schanz, M. Peritoneal Expression of SGLT-2, GLUT1, and GLUT3 in Peritoneal Dialysis Patients. Kidney Blood Press. Res. 2021, 47, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Sung, P.-H.; Yang, Y.-H.; Chiang, J.Y.; Yip, H.-K.; Yang, C.-C. Dipeptidyl Peptidase 4 Promotes Peritoneal Fibrosis and Its Inhibitions Prevent Failure of Peritoneal Dialysis. Commun. Biol. 2021, 4, 144. [Google Scholar] [CrossRef]
- Ha, H.; Lee, H.B. Peritoneal Mesothelial Cell Biology in Peritoneal Dialysis. Nephrology 2002, 7, 220–226. [Google Scholar] [CrossRef]
- Geraldes, P.; King, G.L. Activation of Protein Kinase C Isoforms and Its Impact on Diabetic Complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [Green Version]
- Aufricht, C.; Beelen, R.; Eberl, M.; Fischbach, M.; Fraser, D.; Jörres, A.; Kratochwill, K.; LópezCabrera, M.; Rutherford, P.; Schmitt, C.-P.; et al. Biomarker Research to Improve Clinical Outcomes of Peritoneal Dialysis: Consensus of the European Training and Research in Peritoneal Dialysis (EuTRiPD) Network. Kidney Int. 2017, 92, 824–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, D.L.; Struijk, D.G.; Krediet, R.T. Peritoneal Effluent MMP-2 and PAI-1 in Encapsulating Peritoneal Sclerosis. Am. J. Kidney Dis. 2015, 65, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Barreto, D.L.; Coester, A.M.; Struijk, D.G.; Krediet, R.T. Can Effluent Matrix Metalloproteinase 2 and Plasminogen Activator Inhibitor 1 Be Used as Biomarkers of Peritoneal Membrane Alterations in Peritoneal Dialysis Patients? Perit. Dial. Int. 2013, 33, 529–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, N.; Chiou, T.T.-Y.; Wu, C.-H.; Lei, Y.-Y.; Liang, P.-L.; Chao, M.-C.; Yang, H.; Chen, J.-B. Longitudinal Changes of PAI-1, MMP-2, and VEGF in Peritoneal Effluents and Their Associations with Peritoneal Small-Solute Transfer Rate in New Peritoneal Dialysis Patients. Biomed Res. Int. 2019, 2019, 2152584. [Google Scholar] [CrossRef]
- Ditsawanon, P.; Wu, Q.; Adler, S.G.; Wang, Y.; LaPage, J.; Nayak, A.; Andalibi, A.; Aramwit, P.; Dai, T. Inflammatory Biomarker Pairs as Outcome Measures in Peritoneal Dialysis: A Pilot Study. Proc. UCLA Healthc. 2016, 20. [Google Scholar]
- Faria, B.; Gaya da Costa, M.; Lima, C.; Willems, L.; Brandwijk, R.; Berger, S.P.; Daha, M.R.; Pestana, M.; Seelen, M.A.; Poppelaars, F. Soluble CD59 in Peritoneal Dialysis: A Potential Biomarker for Peritoneal Membrane Function. J. Nephrol. 2021, 34, 801–810. [Google Scholar] [CrossRef]
- Yang, J.; Cai, M.; Wan, J.; Wang, L.; Luo, J.; Li, X.; Gong, W.; He, Y.; Chen, J. Effluent Decoy Receptor 2 as a Novel Biomarker of Peritoneal Fibrosis in Peritoneal Dialysis Patients. Perit. Dial. Int. 2022, 42, 631–639. [Google Scholar] [CrossRef]
- Branco, P.; Calça, R.; Martins, A.R.; Mateus, C.; Jervis, M.J.; Gomes, D.P.; Azeredo-Lopes, S.; De Melo Junior, A.F.; Sousa, C.; Civantos, E.; et al. Fibrosis of Peritoneal Membrane, Molecular Indicators of Aging and Frailty Unveil Vulnerable Patients in Long-Term Peritoneal Dialysis. Int. J. Mol. Sci. 2023, 24, 5020. [Google Scholar] [CrossRef]
- Htay, H.; Johnson, D.W.; Wiggins, K.J.; Badve, S.V.; Craig, J.C.; Strippoli, G.F.; Cho, Y. Biocompatible Dialysis Fluids for Peritoneal Dialysis. Cochrane Database Syst. Rev. 2018, 10, CD007554. [Google Scholar] [CrossRef] [PubMed]
- Raby, A.-C.; Labéta, M.O. Preventing Peritoneal Dialysis-Associated Fibrosis by Therapeutic Blunting of Peritoneal Toll-Like Receptor Activity. Front. Physiol. 2018, 9, 1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taheri, S.; Thiagaraj, S.S.; Shukla, T.S.; Gutlapalli, S.D.; Farhat, H.; Muthiah, K.; Pallipamu, N.; Hamid, P.; Taheri, S.; Thiagaraj, S.S.; et al. A Review on Major Pathways Leading to Peritoneal Fibrosis in Patients Receiving Continuous Peritoneal Dialysis. Cureus 2022, 14, e31799. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yu, C.; Qin, H.; Zhang, S.; Fang, L.; Wang, Y.; Wang, J.; Cui, B.; Hu, S.; Liu, N.; et al. Nintedanib Attenuates Peritoneal Fibrosis by Inhibiting Mesothelial-to-Mesenchymal Transition, Inflammation and Angiogenesis. J. Cell. Mol. Med. 2021, 25, 6103–6114. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shan, Y.; Yu, M.; Shi, J.; Tang, L.; Cao, H.; Sheng, M. Tetramethylpyrazine Ameliorates Peritoneal Angiogenesis by Regulating VEGF/Hippo/YAP Signaling. Front. Pharmacol. 2021, 12, 649581. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Chang, P.-Y.; Chen, J.-Y.; Wu, B.-S.; Yang, A.-H.; Lee, O.K.-S. Adipose-Derived Mesenchymal Stem Cells Attenuate Dialysis-Induced Peritoneal Fibrosis by Modulating Macrophage Polarization via Interleukin-6. Stem Cell Res. Ther. 2021, 12, 193. [Google Scholar] [CrossRef]
- Nagasaki, K.; Nakashima, A.; Tamura, R.; Ishiuchi, N.; Honda, K.; Ueno, T.; Doi, S.; Kato, Y.; Masaki, T. Mesenchymal Stem Cells Cultured in Serum-Free Medium Ameliorate Experimental Peritoneal Fibrosis. Stem Cell Res. Ther. 2021, 12, 203. [Google Scholar] [CrossRef]
- Xu, L.; Liu, N.; Gu, H.; Wang, H.; Shi, Y.; Ma, X.; Ma, S.; Ni, J.; Tao, M.; Qiu, A.; et al. Histone Deacetylase 6 Inhibition Counteracts the Epithelial–Mesenchymal Transition of Peritoneal Mesothelial Cells and Prevents Peritoneal Fibrosis. Oncotarget 2017, 8, 88730–88750. [Google Scholar] [CrossRef] [Green Version]
- Tamura, R.; Doi, S.; Nakashima, A.; Sasaki, K.; Maeda, K.; Ueno, T.; Masaki, T. Inhibition of the H3K4 Methyltransferase SET7/9 Ameliorates Peritoneal Fibrosis. PLoS ONE 2018, 13, e0196844. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Tao, M.; Wang, Y.; Zang, X.; Ma, X.; Qiu, A.; Zhuang, S.; Liu, N. Genetic or Pharmacologic Blockade of Enhancer of Zeste Homolog 2 Inhibits the Progression of Peritoneal Fibrosis. J. Pathol. 2020, 250, 79–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Yu, C.; Zhuang, S. Histone Methyltransferase EZH2: A Potential Therapeutic Target for Kidney Diseases. Front. Physiol. 2021, 12, 640700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, J.; Wang, R.; Sun, J. Inhibition of EZH2 Mitigates Peritoneal Fibrosis and Lipid Precipitation in Peritoneal Mesothelial Cells Mediated by Klotho. Ren. Fail. 2023, 45, 2149411. [Google Scholar] [CrossRef]
- Ueda, A.; Nagai, K.; Yamagata, K. Preserved Peritoneal Function by Short-Term Two-Day Peritoneal Rest in Hemodialysis Combination Therapy Patients. J. Artif. Organs 2021, 24, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Zareie, M.; Keuning, E.D.; ter Wee, P.M.; Beelen, R.H.J.; van den Born, J. Peritoneal Dialysis Fluid-Induced Changes of the Peritoneal Membrane Are Reversible after Peritoneal Rest in Rats. Nephrol. Dial. Transplant. 2005, 20, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhe, X.; Tian, X.; Cheng, L.; Wang, T. Effects of Peritoneal Resting on Peritoneal Fluid Transport Kinetics. Perit. Dial. Int. 2007, 27, 575–579. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, E.; Del Peso, G.; Alvarez, L.; Ros, S.; Mateus, A.; Aguilar, A.; Selgas, R.; Bajo, M.-A. Peritoneal Resting with Heparinized Lavage Reverses Peritoneal Type I Membrane Failure. A Comparative Study of the Resting Effects on Normal Membranes. Perit. Dial. Int. 2014, 34, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Fan, J.; Zheng, C.; Yin, P.; Wu, H.; Li, X.; Luo, N.; Yu, X.; Chen, C. SGLT-2 Inhibitors Reduce Glucose Absorption from Peritoneal Dialysis Solution by Suppressing the Activity of SGLT-2. Biomed. Pharmacother. 2019, 109, 1327–1338. [Google Scholar] [CrossRef]
- Balzer, M.S.; Rong, S.; Nordlohne, J.; Zemtsovski, J.D.; Schmidt, S.; Stapel, B.; Bartosova, M.; von Vietinghoff, S.; Haller, H.; Schmitt, C.P.; et al. SGLT2 Inhibition by Intraperitoneal Dapagliflozin Mitigates Peritoneal Fibrosis and Ultrafiltration Failure in a Mouse Model of Chronic Peritoneal Exposure to High-Glucose Dialysate. Biomolecules 2020, 10, 1573. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, X.; Liang, X.; Wu, X.; Yao, C. SGLT2 Inhibitors Attenuate Nephrin Loss and Enhance TGF-Β1 Secretion in Type 2 Diabetes Patients with Albuminuria: A Randomized Clinical Trial. Sci. Rep. 2022, 12, 15695. [Google Scholar] [CrossRef]
- Martus, G.; Bergling, K.; de Arteaga, J.; Öberg, C.M. SGLT2 Inhibition Does Not Reduce Glucose Absorption during Experimental Peritoneal Dialysis. Perit. Dial. Int. 2021, 41, 373–380. [Google Scholar] [CrossRef]
- Bergling, K.; Martus, G.; Öberg, C.M. Phloretin Improves Ultrafiltration and Reduces Glucose Absorption during Peritoneal Dialysis in Rats. J. Am. Soc. Nephrol. 2022, 33, 1857–1863. [Google Scholar] [CrossRef]
- Sridhar, V.S.; Fraser, D.J. SGLT2 Inhibition, Glucose Transport and Peritoneal Dialysis: Finding the Sweet Spot. Perit. Dial. Int. 2023, 43, 115–118. [Google Scholar] [CrossRef]
- Mehrotra, R.; Ravel, V.; Streja, E.; Kuttykrishnan, S.; Adams, S.V.; Katz, R.; Molnar, M.Z.; Kalantar-Zadeh, K. Peritoneal Equilibration Test and Patient Outcomes. Clin. J. Am. Soc. Nephrol. 2015, 10, 1990–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, Madhukar; Khanna, Ramesh Peritoneal Equilibration Test—UpToDate. Available online: https://www.uptodate.com/contents/peritoneal-equilibration-test#H1 (accessed on 16 June 2023).
- Twardowski, Z.J. The Fast Peritoneal Equilibration Test. Semin. Dial. 1990, 3, 141–142. [Google Scholar] [CrossRef]
- Kawanishi, K.; Honda, K.; Tsukada, M.; Oda, H.; Nitta, K. Neutral Solution Low in Glucose Degradation Products Is Associated with Less Peritoneal Fibrosis and Vascular Sclerosis in Patients Receiving Peritoneal Dialysis. Perit. Dial. Int. 2013, 33, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plum, J.; Hermann, S.; Fussholler, A.; Schoenicke, G.; Donner, A.; Röhrborn, A.; Grabensee, B. Peritoneal Sclerosis in Peritoneal Dialysis Patients Related to Dialysis Settings and Peritoneal Transport Properties. Kidney Int. 2001, 59, S42–S47. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suryantoro, S.D.; Thaha, M.; Sutanto, H.; Firdausa, S. Current Insights into Cellular Determinants of Peritoneal Fibrosis in Peritoneal Dialysis: A Narrative Review. J. Clin. Med. 2023, 12, 4401. https://doi.org/10.3390/jcm12134401
Suryantoro SD, Thaha M, Sutanto H, Firdausa S. Current Insights into Cellular Determinants of Peritoneal Fibrosis in Peritoneal Dialysis: A Narrative Review. Journal of Clinical Medicine. 2023; 12(13):4401. https://doi.org/10.3390/jcm12134401
Chicago/Turabian StyleSuryantoro, Satriyo Dwi, Mochammad Thaha, Henry Sutanto, and Sarah Firdausa. 2023. "Current Insights into Cellular Determinants of Peritoneal Fibrosis in Peritoneal Dialysis: A Narrative Review" Journal of Clinical Medicine 12, no. 13: 4401. https://doi.org/10.3390/jcm12134401
APA StyleSuryantoro, S. D., Thaha, M., Sutanto, H., & Firdausa, S. (2023). Current Insights into Cellular Determinants of Peritoneal Fibrosis in Peritoneal Dialysis: A Narrative Review. Journal of Clinical Medicine, 12(13), 4401. https://doi.org/10.3390/jcm12134401





