The Prediction Score of Acute Kidney Injury in Patients with Severe COVID-19 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Renal Outcomes
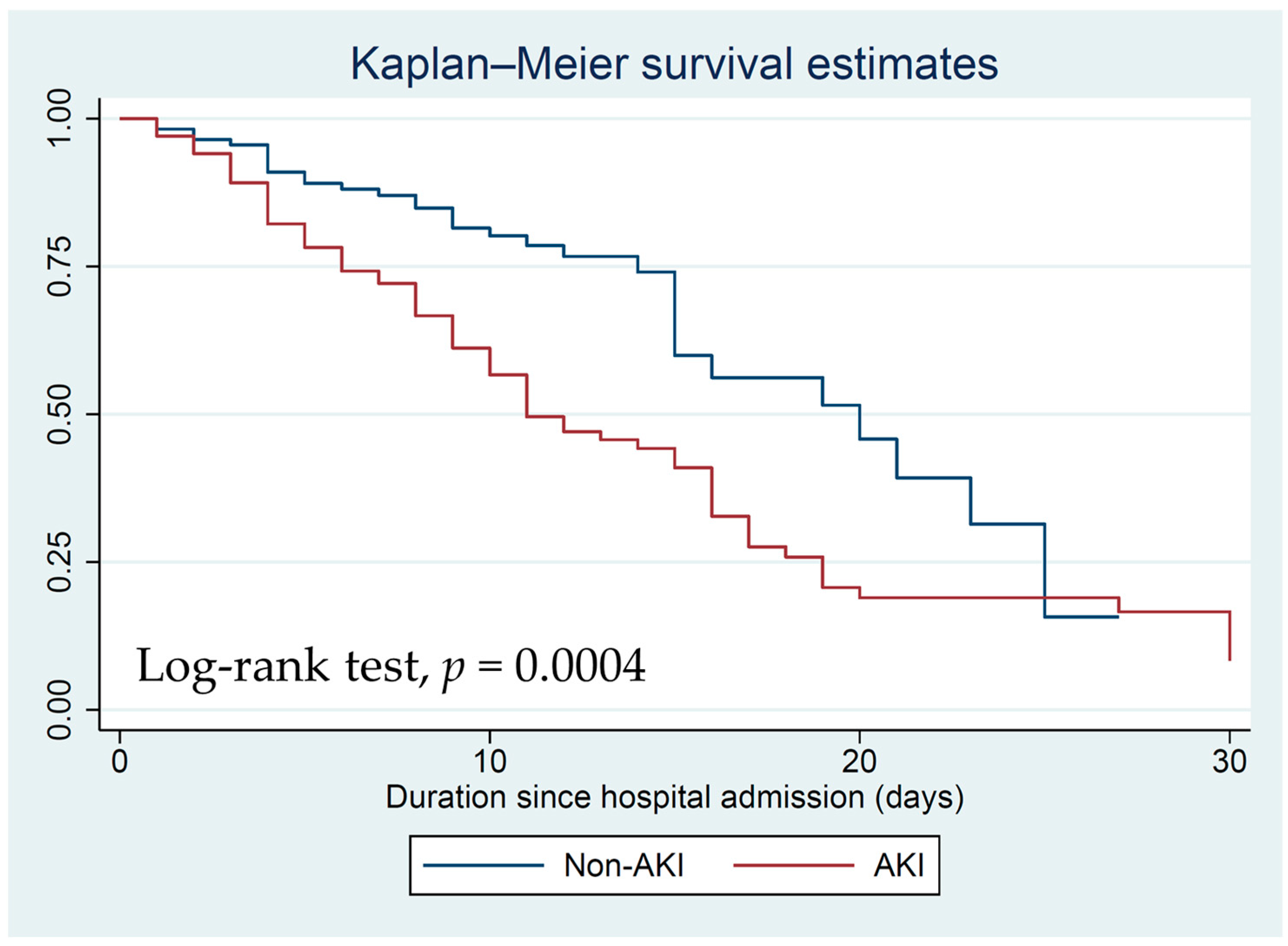
3.3. Mortality
3.4. Risk Factors of AKI in Severe COVID-19 Infection
3.5. Prediction Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jewell, P.D.; Bramham, K.; Galloway, J.; Post, F.; Norton, S.; Teo, J.; Fisher, R.; Saha, R.; Hutchings, S.; Hopkins, P.; et al. COVID-19-related acute kidney injury; incidence, risk factors and outcomes in a large UK cohort. BMC Nephrol. 2021, 22, 359. [Google Scholar]
- Xu, Z.; Tang, Y.; Huang, Q.; Fu, S.; Li, X.; Lin, B.; Xu, A.; Chen, J. Systematic review and subgroup analysis of the incidence of acute kidney injury (AKI) in patients with COVID-19. BMC Nephrol. 2021, 22, 52. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, D.C.; Franco, M.; Dos Santos, D.R.P.; Santos, M.C.; Maltoni, I.S.; Mascotte, F.; de Souza, A.A.; Pietrobom, P.M.; Medeiros, E.A.; Ferreira, P.R.A.; et al. Acute kidney injury: Incidence, risk factors, and outcomes in severe COVID-19 patients. PLoS ONE 2021, 16, e0251048. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Mahajan, Z.A.; Vasistha, P.; Chakraborty, R.; Mukunda, K.; Tibrewal, A.; Neyra, J.A. Incidence and Outcomes of Acute Kidney Injury in COVID-19: A Systematic Review. Blood Purif. 2022, 51, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Prowle, J.R. COVID-19-associated AKI. Curr. Opin. Crit. Care 2022, 28, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Abate, S.M.; Ahmed Ali, S.; Mantfardo, B.; Basu, B. Rate of Intensive Care Unit admission and outcomes among patients with coronavirus: A systematic review and Meta-analysis. PLoS ONE 2020, 15, e0235653. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, X.; Ren, J.; Sun, Y.; Yu, R.; Li, K.; Zheng, L.; Yang, J. Risk factors and prognosis for COVID-19-induced acute kidney injury: A meta-analysis. BMJ Open 2020, 10, e042573. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wu, G.; Zhang, J.; Yang, L. Risk Factors for Acute Kidney Injury in Adult Patients With COVID-19: A Systematic Review and Meta-Analysis. Front. Med. (Lausanne) 2021, 8, 719472. [Google Scholar] [CrossRef]
- Hidayat, A.A.; Gunawan, V.A.; Iragama, F.R.; Alfiansyah, R.; Hertanto, D.M.; Tjempakasari, A.; Thaha, M. Risk Factors and Clinical Characteristics of Acute Kidney Injury in Patients with COVID-19: A Systematic Review and Meta-Analysis. Pathophysiology 2023, 30, 233–247. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of COVID-19: Living Guideline, 13 January 2023; (WHO/2019-nCoV/clinical/2023.1); World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO); Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012, 2, 1–138. [Google Scholar]
- Chávez-Valencia, V.; Orizaga-de-la-Cruz, C.; Lagunas-Rangel, F.A. Acute Kidney Injury in COVID-19 Patients: Pathogenesis, Clinical Characteristics, Therapy, and Mortality. Diseases 2022, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Gupta, S.; Tighiouart, H.; Goyal, N.; Faugno, A.J.; Tariq, A.; Raichoudhury, R.; Sharma, J.H.; Meyer, L.; Kshirsagar, R.K.; et al. Kidney Recovery and Death in Critically Ill Patients with COVID-19-Associated Acute Kidney Injury Treated with Dialysis: The STOP-COVID Cohort Study. Am. J. Kidney Dis. 2022, 79, 404–416.e401. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 2021, 17, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.H.; Bijol, V.; Sparks, M.A.; Sise, M.E.; Izzedine, H.; Jhaveri, K.D. Pathophysiology and Pathology of Acute Kidney Injury in Patients with COVID-19. Adv. Chronic. Kidney Dis. 2020, 27, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Akilesh, S.; Nast, C.C.; Yamashita, M.; Henriksen, K.; Charu, V.; Troxell, M.L.; Kambham, N.; Bracamonte, E.; Houghton, D.; Ahmed, N.I.; et al. Multicenter Clinicopathologic Correlation of Kidney Biopsies Performed in COVID-19 Patients Presenting with Acute Kidney Injury or Proteinuria. Am. J. Kidney Dis. 2021, 77, 82–93.e81. [Google Scholar] [CrossRef] [PubMed]
- Lumlertgul, N.; Pirondini, L.; Cooney, E.; Kok, W.; Gregson, J.; Camporota, L.; Lane, K.; Leach, R.; Ostermann, M. Acute kidney injury prevalence, progression and long-term outcomes in critically ill patients with COVID-19: A cohort study. Ann. Intensive Care 2021, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Palomba, H.; Cubos, D.; Bozza, F.; Zampieri, F.G.; Romano, T.G. Development of a Risk Score for AKI onset in COVID-19 Patients: COV-AKI Score. BMC Nephrol. 2023, 24, 46. [Google Scholar] [CrossRef] [PubMed]


| Variables | All (N = 215) | Non-AKI (N = 113) | AKI (N = 102) | p-Value |
|---|---|---|---|---|
| Systolic BP, mmHg (SD) | 131.01 (22.86) | 133.70 (2.093) | 128.04 (24.59) | 0.07 |
| Diastolic BP, mmHg (SD) | 79.19 (15.42) | 76.97 (12.96) | 75.32 (17.79) | 0.43 |
| Pulse, bpm (SD) | 90.96 (18.99) | 89.34 (17.36) | 92.76 (20.60) | 0.18 |
| APACHE II, score (SD) | 11.92 (5.64) | 9.49 (4.72) | 14.60 (5.36) | <0.001 |
| ARDS (%) | 31 (14.42) | 5 (4.42) | 26 (25.49) | <0.001 |
| Laboratory admission | ||||
| Hematocrit, % (SD) | 36.71 (8.00) | 37.56 (7.84) | 35.75 (8.09) | 0.10 |
| WBC, cells/mm3 (SD) | 8770.12 (5540.64) | 7697.88 (4381.90) | 9957 (6406.35) | 0.002 |
| Lymphocyte count, cells/mm3 (SD) | 1096.14 (720.73) | 1135.84 (553.66) | 1052.18 (870.05) | 0.40 |
| Albumin, g/dL (SD) | 3.37 (0.57) | 3.50 (0.52) | 3.22 (0.60) | 0.0004 |
| Baseline Cr, mg/dL (SD) | 0.92 (0.42) | 0.87 (0.42) | 0.98 (0.42) | 0.07 |
| Sodium, mmol/L (SD) | 133.86 (10.12) | 132.87 (12.76) | 134.96 (5.86) | 0.13 |
| Potassium, mmol/L (SD) | 4.02 (0.80) | 3.88 (0.60) | 4.17 (0.95) | 0.007 |
| Bicarbonate mmol/L (SD) | 22.46 (4.23) | 23.31 (3.70) | 21.53 (4.59) | 0.002 |
| Oxygen therapy (%) | <0.001 | |||
| Cannula | 57 (26.51) | 44 (38.94) | 13 (12.75) | |
| Mask with bag | 1 (0.47) | 1 (0.88) | 0 | |
| High flow | 79 (36.74) | 46 (40.71) | 33 (32.35) | |
| BiPAP/CPAP | 5 (2.33) | 2 (1.77) | 3 (2.94) | |
| Mechanical ventilation | 73 (33.95) | 20 (17.70) | 53 (51.96) | |
| Inotrope/Vasopressor (%) | 69 (32.09) | 19 (16.81) | 50 (49.02) | <0.001 |
| Variables | Univariate OR (95% CI) | p-Value | Multivariate OR (95% CI) | p-Value |
|---|---|---|---|---|
| Female | 0.93 (0.53–01.61) | 0.78 | ||
| Age > 60 years | 2.94 (1.47–5.85) | 0.002 | 2.28 (0.90–5.75) | 0.08 |
| BMI > 30 Kg/m2 | 0.70 (0.33–1.45) | 0.34 | ||
| Comorbidities | ||||
| HT | 1.50 (0.82–2.73) | 0.18 | ||
| DM | 1.23 (0.71–2.13) | 0.47 | ||
| CKD | 2.03 (1.03–4.00) | 0.04 | 1.26 (0.53–2.95) | 0.60 |
| AF | 2.64 (0.79–8.84) | 0.12 | ||
| CAD | 1.13 (0.52–2.44) | 0.76 | ||
| COPD/asthma | 0.84 (0.95–1.99) | 0.69 | ||
| Cancer | 1.04 (0.59–1.81) | 0.89 | ||
| Previous Medication | ||||
| ACEi/ARB | 0.85 (0.47–1.52) | 0.58 | ||
| Diuretic | 6.82 (1.92–24.17) | 0.003 | 8.21 (2.03–33.28) | 0.003 |
| NSAIDs | 1.10 (0.15–7.96) | 0.92 | ||
| APACHE II score ≥ 12 | 5.65 (3.14–10.18) | <0.001 | 2.96 (1.42–6.19) | 0.004 |
| WBC (103 cell/mm3) | 1.07 (1.02–1.13) | 0.01 | 1.05 (0.98–1.12) | 0.17 |
| Lymphocyte count (103 cell/mm3) | 0.85 (0.58–1.24) | 0.40 | ||
| Albumin < 3.5 g/dL | 2.53 (1.44–4.45) | 0.001 | 1.81 (0.90–3.64) | 0.10 |
| Inotrope/Vasopressor | 4.75 (2.54–8.90) | <0.001 | 0.96 (0.33–2.82) | 0.95 |
| Mechanical ventilator | 5.03 (2.70–9.34) | <0.001 | 5.09 (1.73–14.96) | 0.003 |
| Variables | Multivariate Coeff. (95% CI) a | p-Value | Multivariate Coeff. (95% CI) b | p-Value |
|---|---|---|---|---|
| Age > 60 years | 0.72 (−0.15 to 1.60) | 0.10 | 0.72 (−0.11 to 1.55) | 0.10 |
| Prior diuretic use | 2.07 (0.68 to 3.48) | 0.004 | 1.93 (0.62 to 3.24) | 0.004 |
| APACHE II score ≥ 12 | 1.22 (0.53 to 1.91) | 0.001 | 1.16 (0.51 to 1.81) | <0.001 |
| Albumin < 3.5 g/dL | 0.70 (0.02 to 1.38) | 0.04 | 0.70 (0.05 to 1.34) | 0.03 |
| Mechanical ventilator | 1.63 (0.89 to 2.37) | <0.001 | 1.61 (0.90 to 2.31) | <0.001 |
| Variables | Point |
|---|---|
| Age > 60 years | 1 |
| Prior Diuretic use | 3 |
| APACHE II score ≥ 12 | 2 |
| Albumin < 3.5 g/dL | 1 |
| Mechanical ventilator | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anumas, S.; Chueachinda, S.; Tantiyavarong, P.; Pattharanitima, P. The Prediction Score of Acute Kidney Injury in Patients with Severe COVID-19 Infection. J. Clin. Med. 2023, 12, 4412. https://doi.org/10.3390/jcm12134412
Anumas S, Chueachinda S, Tantiyavarong P, Pattharanitima P. The Prediction Score of Acute Kidney Injury in Patients with Severe COVID-19 Infection. Journal of Clinical Medicine. 2023; 12(13):4412. https://doi.org/10.3390/jcm12134412
Chicago/Turabian StyleAnumas, Suthiya, Supoj Chueachinda, Pichaya Tantiyavarong, and Pattharawin Pattharanitima. 2023. "The Prediction Score of Acute Kidney Injury in Patients with Severe COVID-19 Infection" Journal of Clinical Medicine 12, no. 13: 4412. https://doi.org/10.3390/jcm12134412








