Does COVID-19 Infection within 1 Week after Total Knee Arthroplasty Affect Patients’ Early Clinical Outcomes? A Matched Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
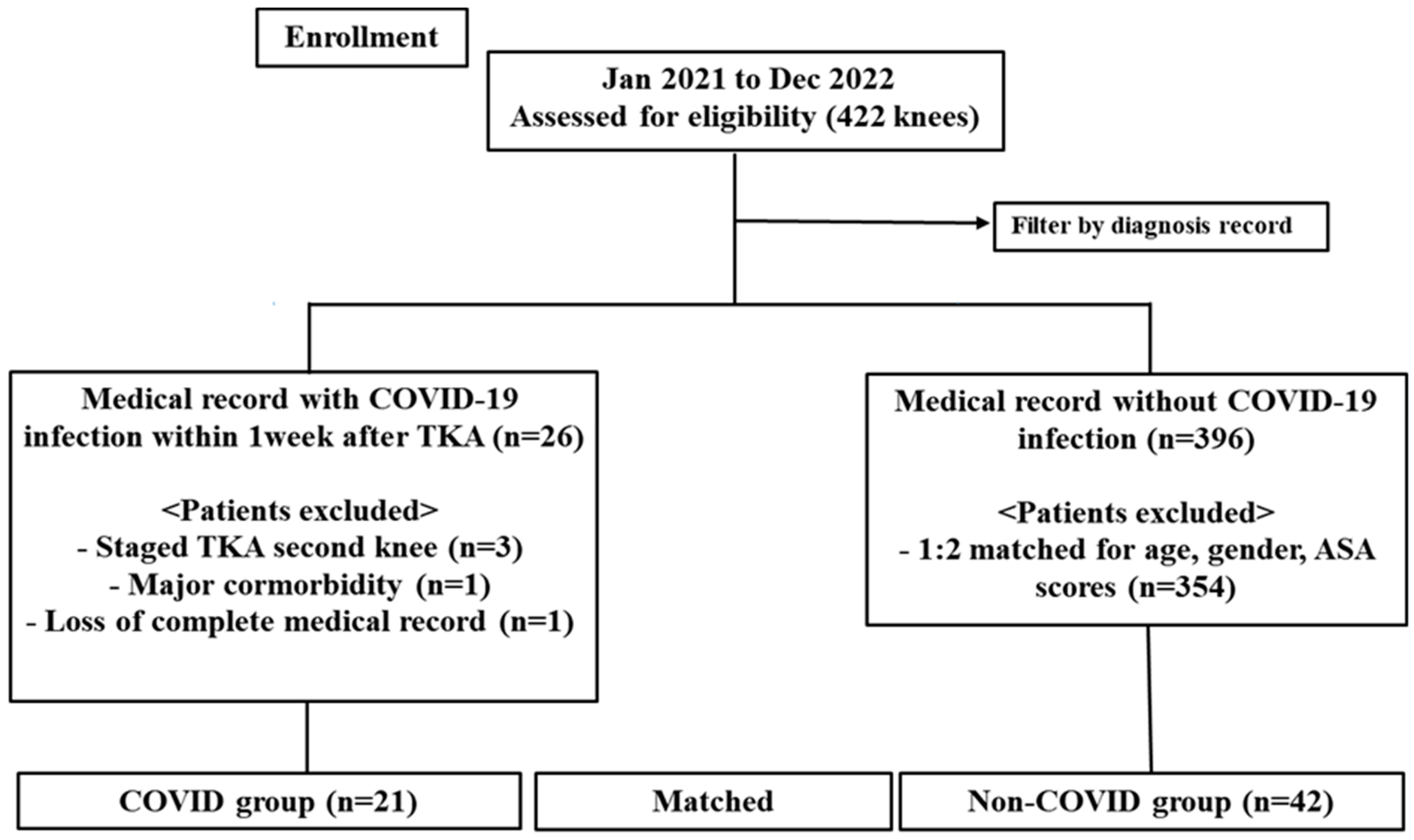
2.1. Study Design and Patients
2.2. Surgical Methods and Postoperative Treatment
2.3. Confirmed Criteria and Treatment of COVID-19 Infection
2.4. Clinical Evaluation and Complications
2.5. Laboratory Evaluation
2.6. Statistical Analysis
3. Results
3.1. Clinical Evaluation
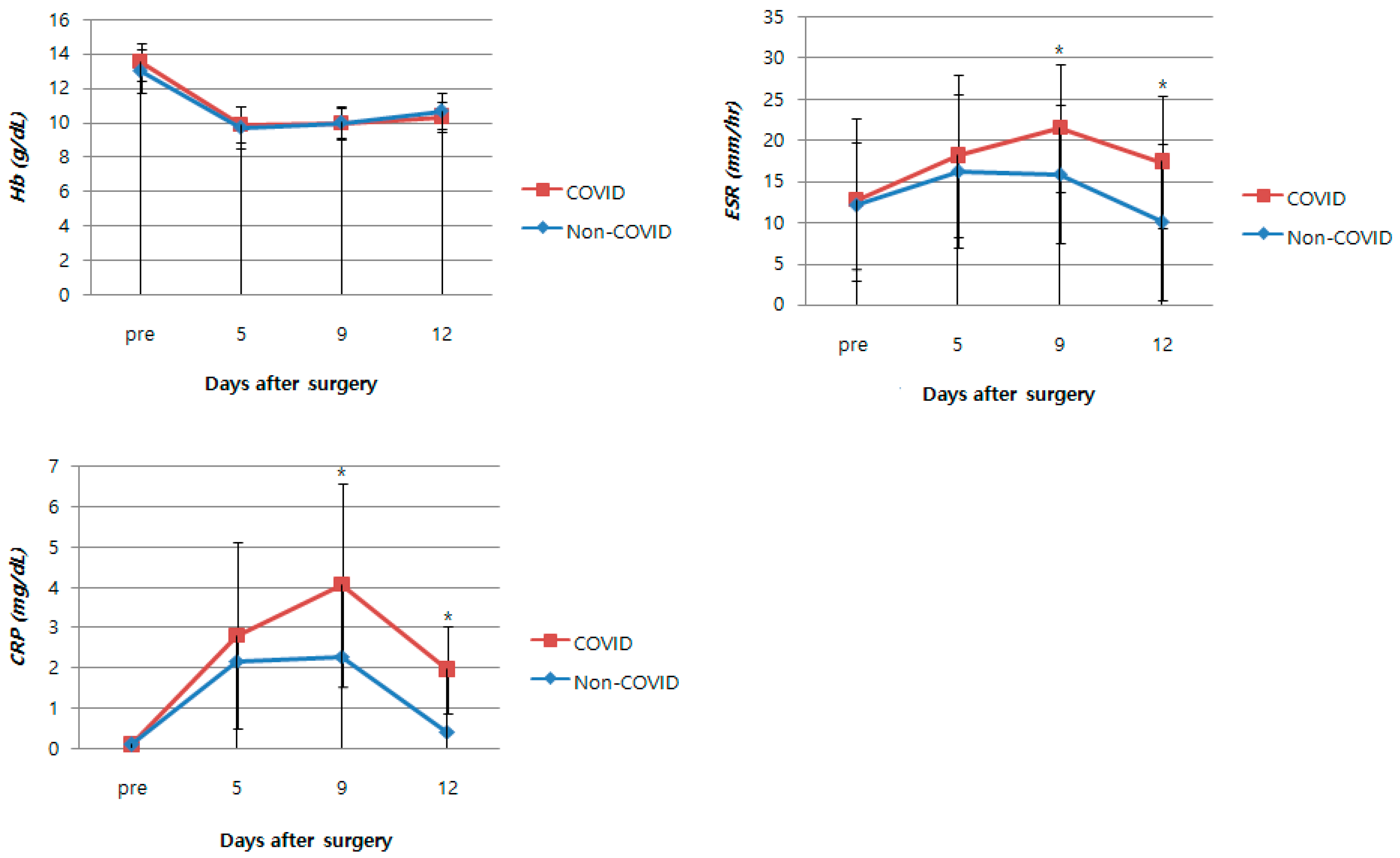
3.2. Perioperative Laboratory Results
3.3. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anoushiravani, A.A.; O’Connor, C.M.; DiCaprio, M.R.; Iorio, R. Economic impacts of the COVID-19 crisis: An orthopaedic perspective. J. Bone Jt. Surg. Am. 2020, 102, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Okeagu, C.N.; Pham, A.D.; Silva, R.A.; Hurley, J.J.; Arron, B.L.; Sarfraz, N.; Lee, H.N.; Ghali, G.E.; Gamble, J.W. Economic impact of COVID-19 pandemic on healthcare facilities and systems: International perspectives. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Soong, J.T.; Wong, A.L.; O’Connor, I.; Marinova, M.; Fisher, D.; Bell, D. Acute medical units during the first wave of the COVID-19 pandemic: A cross-national exploratory study of impact and responses. Clin. Med. 2021, 21, e462. [Google Scholar] [CrossRef]
- Nakai, T.; Iwasaki, H.; Nishikawa, T.; Higuchi, R.; Sakata, K.; Matsuoka, H.; Iwata, H.; Sogo, E.; Nanno, K.; Nakamura, S. Challenges and responses of elective orthopaedic surgery during the second wave of COVID-19. J. Orthop. Sci. 2022, 27, 713–716. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Anoushiravani, A.A.; DiCaprio, M.R.; Healy, W.L.; Iorio, R. Economic recovery after the COVID-19 pandemic: Resuming elective orthopedic surgery and total joint arthroplasty. J. Arthroplast. 2020, 35, S32–S36. [Google Scholar] [CrossRef]
- Nüesch, E.; Dieppe, P.; Reichenbach, S.; Williams, S.; Iff, S.; Jüni, P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: Population based cohort study. BMJ 2011, 342, d1165. [Google Scholar] [CrossRef]
- Reinbacher, P.; Wittig, U.; Hauer, G.; Draschl, A.; Leithner, A.; Sadoghi, P. Impact of the COVID-19 pandemic on early clinical outcome after total knee arthroplasty: A retrospective comparative analysis. Arch. Orthop. Trauma Surg. 2022, 143, 3319–3326. [Google Scholar] [CrossRef]
- Khan, S.A.; Logan, P.; Asokan, A.; Handford, C.; Rajgor, H.D.; Khadabadi, N.A.; Moores, T.; Targett, J. The incidence of venous thromboembolism in total joint replacement during COVID-19 pandemic: Has lockdown had an influence? Bone Jt. Open 2020, 1, 751–756. [Google Scholar] [CrossRef]
- Rosas, S.; Pollock, D.C.; Roche, M.W.; Najafi, F.; Hollingsworth, N.; Buller, L.T.; Krueger, C.A. Patients with Previous COVID-19 Infection Can Safely Undergo Primary Total Joint Arthroplasty. J. Arthroplast. 2022, 38, 649–654. [Google Scholar] [CrossRef]
- Ong, C.B.; Cororaton, A.D.; Westrich, G.H.; Cushner, F.D.; Haas, S.B.; Della Valle, A.G. COVID-19 disruptions to elective postoperative care did not adversely affect early complications or patient reported outcomes of primary TKA. Arch. Orthop. Trauma Surg. 2022, 143, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Faes, C.; Abrams, S.; Van Beckhoven, D.; Meyfroidt, G.; Vlieghe, E.; Hens, N.; Belgian Collaborative Group on COVID-19 Hospital Surveillance. Time between symptom onset, hospitalisation and recovery or death: Statistical analysis of Belgian COVID-19 patients. Int. J. Environ. Res. Public Health 2020, 17, 7560. [Google Scholar] [CrossRef] [PubMed]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Jian, S.-W.; Liu, D.-P.; Ng, T.-C.; Huang, W.-T.; Lin, H.-H. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern. Med. 2020, 180, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.S.J.; Boone, J.L.; Rosenzweig, S.D.; Steger-May, K.; Barrack, R.L. Patellar resurfacing compared with nonresurfacing in total knee arthroplasty: A concise follow-up of a randomized trial. J. Bone Jt. Surg. Am. 2009, 91, 2562–2567. [Google Scholar] [CrossRef] [PubMed]
- Deroche, E.; Batailler, C.; Swan, J.; Sappey-Marinier, E.; Neyret, P.; Servien, E.; Lustig, S. No difference between resurfaced and non-resurfaced patellae with a modern prosthesis design: A prospective randomized study of 250 total knee arthroplasties. Knee Surg. Sports Traumatol. Arthrosc. 2021, 30, 1025–1038. [Google Scholar] [CrossRef]
- Cho, K.-Y.; Kim, K.-I.; Khurana, S.; Bae, D.-K.; Jin, W. Is routine chemoprophylaxis necessary for prevention of venous thromboembolism following knee arthroplasty in a low incidence population? Arch. Orthop. Trauma Surg. 2013, 133, 551–559. [Google Scholar] [CrossRef]
- Lee, W.-S.; Kim, K.-I.; Lee, H.-J.; Kyung, H.-S.; Seo, S.-S. The incidence of pulmonary embolism and deep vein thrombosis after knee arthroplasty in Asians remains low: A meta-analysis. Clin. Orthop. Relat. Res. 2013, 471, 1523–1532. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Alonso, R.; Camon, A.M.; Cardozo, C.; Albiach, L.; Agüero, D.; Marcos, M.A.; Ambrosioni, J.; Bodro, M.; Chumbita, M. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J. Antimicrob. Chemother. 2021, 76, 3296–3302. [Google Scholar] [CrossRef]
- Bae, J.-K.; Kim, K.-I.; Lee, S.-H.; Yoo, M.-C. Mid-to long-term survival of total knee arthroplasty in hemophilic arthropathy. J. Clin. Med. 2020, 9, 3247. [Google Scholar] [CrossRef]
- Risitano, S.; Cacciola, G.; Capella, M.; Bosco, F.; Giustra, F.; Fusini, F.; Indelli, P.F.; Massé, A.; Sabatini, L. Comparison between gaits after a medial pivot and posterior stabilized primary total knee arthroplasty: A systematic review of the literature. Arthroplasty 2023, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 definition of periprosthetic hip and knee infection: An evidence-based and validated criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Cho, K.-Y.; Khurana, S.; Kim, K.-I. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: A prospective randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2611–2617. [Google Scholar] [CrossRef]
- Singh, V.; Berdis, G.; Goel, A.; Shahi, A.; Oliashirazi, A. Stiffness after Primary Total Knee Arthroplasty. In Knee Surgery-Recostruction and Replacement; Intech Open: London, UK, 2019. [Google Scholar]
- Nepogodiev, D.; Bhangu, A.; Glasbey, J.C.; Li, E.; Omar, O.M.; Simoes, J.F.; Abbott, T.E.; Alser, O.; Arnaud, A.P.; Bankhead-Kendall, B.K. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: An international cohort study. Lancet 2020, 396, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Jonker, P.K.; Van der Plas, W.Y.; Steinkamp, P.J.; Poelstra, R.; Emous, M.; Van der Meij, W.; Thunnissen, F.; Bierman, W.F.; Struys, M.M.; de Reuver, P.R. Perioperative SARS-CoV-2 infections increase mortality, pulmonary complications, and thromboembolic events: A Dutch, multicenter, matched-cohort clinical study. Surgery 2021, 169, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Clement, N.D.; Hall, A.J.; Makaram, N.S.; Robinson, P.G.; Patton, R.F.; Moran, M.; Macpherson, G.J.; Duckworth, A.D.; Jenkins, P.J. IMPACT-Restart: The influence of COVID-19 on postoperative mortality and risk factors associated with SARS-CoV-2 infection after orthopaedic and trauma surgery. Bone Jt. J. 2020, 102, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Abate, S.M.; Mantefardo, B.; Basu, B. Postoperative mortality among surgical patients with COVID-19: A systematic review and meta-analysis. Patient Saf. Surg. 2020, 14, 1–14. [Google Scholar] [CrossRef]
- Forlenza, E.M.; Higgins, J.D.; Burnett, R.A.; Serino, J.; Della Valle, C.J. COVID-19 infection after total joint arthroplasty is associated with increased complications. J. Arthroplast. 2022, 37, S457–S464. [Google Scholar] [CrossRef]
- Khan, I.A.; Zaid, M.B.; Gold, P.A.; Austin, M.S.; Parvizi, J.; Bedard, N.A.; Jevsevar, D.S.; Hannon, C.P.; Fillingham, Y.A.; Committee, A.E. Making a joint decision regarding the timing of surgery for elective arthroplasty surgery after being infected with COVID-19: A systematic review. J. Arthroplast. 2022, 37, 2106–2113.e1. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Mulvey, T.; Thornhill, T. Infected total knee arthroplasty. In Surgery of the Knee; Churchill Livingston: New York, NY, USA, 2001; pp. 1875–1895. [Google Scholar]
- Swanson, K.; Windsor, R. Diagnosis of infection after total knee arthroplasty. In The Adult Knee; Callaghan, J.J., Rosenberg, A.G., Rubash, H.E., Simonian, P.T., Wickiewicz, T.L., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2003; pp. 1485–1491. [Google Scholar]
- Park, K.K.; Kim, T.K.; Chang, C.B.; Yoon, S.W.; Park, K.U. Normative temporal values of CRP and ESR in unilateral and staged bilateral TKA. Clin. Orthop. Relat. Res. 2008, 466, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Azboy, I.; Çatal, B.; Başarır, K.; Mutlu, M.; Bilgen, Ö.F.; Parvizi, J. The natural course of serum D-dimer, C-reactive protein, and erythrocyte sedimentation rate levels after uneventful primary total joint arthroplasty. J. Arthroplast. 2021, 36, 3118–3122. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Cai, X.; Wang, H.; He, G.; Lin, Y.; Lu, B.; Chen, C.; Pan, Y.; Hu, X. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin. Chim. Acta 2020, 507, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth-Weinberger, A.; Bendich, I.; Westrich, G.H.; Su, E.P.; Valle, A.G.D.; Boettner, F. Preoperative ferritin and hemoglobin levels are lower in patients with a history of COVID-19 but blood loss and transfusion requirements are not increased. Arch. Orthop. Trauma Surg. 2021, 143, 311–315. [Google Scholar] [CrossRef]
- Weinberg, J.B.; Pippen, A.M.; Greenberg, C.S. Extravascular fibrin formation and dissolution in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis. Rheum. 1991, 34, 996–1005. [Google Scholar] [CrossRef]
- Zacharski, L.R.; Brown, F.E.; Memoli, V.A.; Kisiel, W.; Kudryk, B.J.; Hunt, J.A.; Dunwiddie, C.; Nutt, E.M. Pathways of coagulation activation in situ in rheumatoid synovial tissue. Clin. Immunol. Immunopathol. 1992, 63, 155–162. [Google Scholar] [CrossRef]
- Stein, P.D.; Hull, R.D.; Patel, K.C.; Olson, R.E.; Ghali, W.A.; Brant, R.; Biel, R.K.; Bharadia, V.; Kalra, N.K. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: A systematic review. Ann. Intern. Med. 2004, 140, 589–602. [Google Scholar] [CrossRef]
- Wells, P.S.; Anderson, D.R.; Rodger, M.; Forgie, M.; Kearon, C.; Dreyer, J.; Kovacs, G.; Mitchell, M.; Lewandowski, B.; Kovacs, M.J. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N. Engl. J. Med. 2003, 349, 1227–1235. [Google Scholar] [CrossRef]
- Shimoyama, Y.; Sawai, T.; Tatsumi, S.; Nakahira, J.; Oka, M.; Nakajima, M.; Jotoku, T.; Minami, T. Perioperative risk factors for deep vein thrombosis after total hip arthroplasty or total knee arthroplasty. J. Clin. Anesth. 2012, 24, 531–536. [Google Scholar] [CrossRef]
- Bytniewski, P.; Machała, W.; Romanowski, L.; Wiśniewski, W.; Kosowski, K. The dynamics of D-dimer level fluctuation in patients after the cemented and cementless total hip and total knee replacement. J. Orthop. Surg. Res. 2014, 9, 1–7. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, Y.-K.; Han, S.B.; Nam, C.H.; Parvizi, J.; Koo, K.-H. Natural progress of D-dimer following total joint arthroplasty: A baseline for the diagnosis of the early postoperative infection. J. Orthop. Surg. Res. 2018, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth-Weinberger, A.; Bendich, I.; Hanreich, C.; Gonzalez Della Valle, A.; Blevins, J.L.; Westrich, G.H.; Boettner, F. History of COVID-19 infection is not associated with increased d-dimer levels and risk of deep-vein thrombosis in total joint arthroplasty. Arch. Orthop. Trauma Surg. 2021, 143, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Kollias, A.; Kyriakoulis, K.G.; Dimakakos, E.; Poulakou, G.; Stergiou, G.S.; Syrigos, K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br. J. Haematol. 2020, 189, 846–847. [Google Scholar] [CrossRef] [PubMed]
- Artifoni, M.; Danic, G.; Gautier, G.; Gicquel, P.; Boutoille, D.; Raffi, F.; Néel, A.; Lecomte, R. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: Incidence and role of D-dimer as predictive factors. J. Thromb. Thrombolysis 2020, 50, 211–216. [Google Scholar] [CrossRef]
- Schairer, W.W.; Vail, T.P.; Bozic, K.J. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin. Orthop. Relat. Res. 2014, 472, 181–187. [Google Scholar] [CrossRef]
| Variables | COVID-19 Group (n = 21) | Non-COVID-19 Group (n = 42) | p-Value a |
|---|---|---|---|
| Sex (male/female) b | 3/18 | 5/37 | 0.789 |
| Mean age (years) c | 72.8 ± 7.9 | 74.9 ± 6.5 | 0.252 |
| Mean BMI (kg/m2) c | 27.9 ± 5.8 | 26.6 ± 4.6 | 0.312 |
| Mean ASA score c | 2.1 ± 0.4 | 2.0 ± 0.3 | 0.618 |
| Preoperative HKA angle (°) c | −8.3 ± 4.2 | −7.7 ± 6.0 | 0.661 |
| Preoperative K–L grade (3/4) b | 2/19 | 5/37 | 0.776 |
| Length of hospital stay (days) c | 17.1 ± 1.4 | 13.0 ± 0.6 | <0.001 |
| Follow-up period (months) c | 10.6 ± 7.5 | 10.7 ± 8.4 | 0.968 |
| COVID-19 Group (n = 21) | Non-COVID-19 Group (n = 42) | p-Value a | |
|---|---|---|---|
| Preoperative | |||
| AKS knee score b | 30.8 ± 10.6 | 31.4 ± 10.4 | 0.846 |
| AKS function score b | 32.9 ± 14.9 | 30.4 ± 12.9 | 0.485 |
| WOMAC score b | 79.5 ± 3.2 | 80.9 ± 4.1 | 0.156 |
| FC (°) b | 9.3 ± 7.8 | 8.5 ± 7.0 | 0.670 |
| FF (°) b | 123.1 ± 11.8 | 125.8 ± 6.8 | 0.246 |
| Postoperative | |||
| AKS knee score b | 93.9 ± 3.3 | 92.7 ± 4.6 | 0.325 |
| AKS function score b | 89.3 ± 6.0 | 91.6 ± 5.1 | 0.117 |
| WOMAC score b | 20.0 ± 4.2 | 20.9 ± 4.2 | 0.413 |
| FC (°) b | 2.1 ± 3.0 | 1.7 ± 3.1 | 0.559 |
| FF (°) b | 128.3 ± 4.0 | 129.3 ± 1.8 | 0.192 |
| COVID-19 Group (n = 21) | Non-COVID-19 Group (n = 42) | p-Value a | |
|---|---|---|---|
| Hb (g/dL) | |||
| Preoperative b | 13.5 ± 1.1 | 13.0 ± 1.3 | 0.126 |
| PO fifth day b | 9.9 ± 1.1 | 9.7 ± 1.2 | 0.585 |
| PO ninth day b | 9.9 ± 0.9 | 10.0 ± 0.9 | 0.875 |
| PO twelfth day b | 10.4 ± 0.9 | 10.7 ± 1.1 | 0.242 |
| ESR (mm/hr)b | |||
| Preoperative b | 12.8 ± 9.8 | 12.1 ± 7.7 | 0.769 |
| PO fifth day b | 18.2 ± 9.9 | 16.3 ± 9.4 | 0.457 |
| PO ninth day b | 21.5 ± 7.8 | 15.9 ± 8.5 | 0.013 |
| PO twelfth day b | 17.4 ± 8.0 | 10.1 ± 9.5 | 0.003 |
| CRP (mg/dL) | |||
| Preoperative b | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.832 |
| PO fifth day b | 2.8 ± 2.3 | 2.2 ± 2.1 | 0.280 |
| PO ninth day b | 4.1 ± 2.5 | 2.3 ± 1.9 | 0.003 |
| PO twelfth day b | 2.0 ± 1.1 | 0.4 ± 0.4 | <0.001 |
| COVID-19 Group | Non-COVID-19 Group | p-Value a | |
|---|---|---|---|
| Preoperative (ng/mL) b | 496 ± 159 | 482 ± 206 | 0.795 |
| Postoperative 12 days (ng/mL) b | 1906 ± 259 | 1866 ± 288 | 0.595 |
| Variables | COVID-19 Group (n = 21 Knees) | Non-COVID-19 Group (n = 42 Knees) | p-Value a |
|---|---|---|---|
| Hemarthrosis b | 0(0) | 0(0) | 1 |
| Stiffness b | 0(0) | 0(0) | 1 |
| Wound dehiscence b | 0(0) | 1(2.4) | 1 |
| Pneumonia b | 0(0) | 0(0) | 1 |
| PJI b | 0(0) | 0(0) | 1 |
| Total DVT b | 2(9.5) | 3(7.1) | 0.741 |
| Proximal DVT b | 0(0) | 0(0) | 1 |
| Symptomatic PE b | 0(0) | 0(0) | 1 |
| 90-days readmission b | 1(4.8) | 1(2.4) | 0.611 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.-K.; Seo, J.-S.; Shin, S.-K.; Kim, S.-J.; Kim, J.-H. Does COVID-19 Infection within 1 Week after Total Knee Arthroplasty Affect Patients’ Early Clinical Outcomes? A Matched Case–Control Study. J. Clin. Med. 2023, 12, 4496. https://doi.org/10.3390/jcm12134496
Bae J-K, Seo J-S, Shin S-K, Kim S-J, Kim J-H. Does COVID-19 Infection within 1 Week after Total Knee Arthroplasty Affect Patients’ Early Clinical Outcomes? A Matched Case–Control Study. Journal of Clinical Medicine. 2023; 12(13):4496. https://doi.org/10.3390/jcm12134496
Chicago/Turabian StyleBae, Jung-Kwon, Jae-Sung Seo, Seong-Kee Shin, Seo-Jin Kim, and Jun-Ho Kim. 2023. "Does COVID-19 Infection within 1 Week after Total Knee Arthroplasty Affect Patients’ Early Clinical Outcomes? A Matched Case–Control Study" Journal of Clinical Medicine 12, no. 13: 4496. https://doi.org/10.3390/jcm12134496
APA StyleBae, J.-K., Seo, J.-S., Shin, S.-K., Kim, S.-J., & Kim, J.-H. (2023). Does COVID-19 Infection within 1 Week after Total Knee Arthroplasty Affect Patients’ Early Clinical Outcomes? A Matched Case–Control Study. Journal of Clinical Medicine, 12(13), 4496. https://doi.org/10.3390/jcm12134496





