Predicting Penumbra Salvage and Infarct Growth in Acute Ischemic Stroke: A Multifactor Survival Game
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Clinical Variables
2.3. Imaging Protocol
2.4. Statistical Analysis
3. Results
3.1. Predictors of Penumbra Salvage
3.2. Predictors of Infarct Growth
3.3. Impact of Penumbra Salvage and Infarct Growth on Clinical Outcome
4. Discussion
4.1. Factors Associated with Penumbra Salvage Only
4.2. Factors Associated with Infarct Growth Only
4.3. Factors Associated with Both Penumbra Salvage and Infarct Growth
4.4. Outcome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ackerman, R.H.; Correia, J.A.; Alpert, N.M.; Baron, J.C.; Gouliamos, A.; Grotta, J.C.; Brownell, G.L.; Taveras, J.M. Positron imaging in ischemic stroke disease using compounds labeled with oxygen 15. Initial results of clinicophysiologic correlations. Arch. Neurol. 1981, 38, 537–543. [Google Scholar] [CrossRef]
- Baron, J.C.; Bousser, M.G.; Comar, D.; Soussaline, F.; Castaigne, P. Noninvasive tomographic study of cerebral blood flow and oxygen metabolism in vivo. Potentials, limitations, and clinical applications in cerebral ischemic disorders. Eur. Neurol. 1981, 20, 273–284. [Google Scholar] [CrossRef]
- Lenzi, G.L.; Frackowiak, R.S.; Jones, T. Cerebral oxygen metabolism and blood flow in human cerebral ischemic infarction. J. Cereb. Blood Flow Metab. 1982, 2, 321–335. [Google Scholar] [CrossRef]
- Powers, W.J.; Grubb, R.L., Jr.; Darriet, D.; Raichle, M.E. Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. J. Cereb. Blood Flow Metab. 1985, 5, 600–608. [Google Scholar] [CrossRef] [Green Version]
- Rosner, G.; Graf, R.; Kataoka, K.; Heiss, W.D. Selective functional vulnerability of cortical neurons following transient MCA-occlusion in the cat. Stroke 1986, 17, 76–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabuchi, T.; Lucero, J.; Feng, A.; Koziol, J.A.; del Zoppo, G.J. Focal cerebral ischemia preferentially affects neurons distant from their neighboring microvessels. J. Cereb. Blood Flow Metab. 2005, 25, 257–266. [Google Scholar] [CrossRef]
- del Zoppo, G.J.; Sharp, F.R.; Heiss, W.D.; Albers, G.W. Heterogeneity in the penumbra. J. Cereb. Blood Flow Metab. 2011, 31, 1836–1851. [Google Scholar] [CrossRef] [Green Version]
- Olivot, J.M.; Mlynash, M.; Thijs, V.N.; Purushotham, A.; Kemp, S.; Lansberg, M.G.; Wechsler, L.; Gold, G.E.; Bammer, R.; Marks, M.P.; et al. Geography, structure, and evolution of diffusion and perfusion lesions in Diffusion and perfusion imaging Evaluation For Understanding Stroke Evolution (DEFUSE). Stroke 2009, 40, 3245–3251. [Google Scholar] [CrossRef] [Green Version]
- Albers, G.W.; Thijs, V.N.; Wechsler, L.; Kemp, S.; Schlaug, G.; Skalabrin, E.; Bammer, R.; Kakuda, W.; Lansberg, M.G.; Shuaib, A.; et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann. Neurol. 2006, 60, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Lansberg, M.G.; Lee, J.; Christensen, S.; Straka, M.; De Silva, D.A.; Mlynash, M.; Campbell, B.C.; Bammer, R.; Olivot, J.M.; Desmond, P.; et al. RAPID automated patient selection for reperfusion therapy: A pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke 2011, 42, 1608–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansberg, M.G.; Straka, M.; Kemp, S.; Mlynash, M.; Wechsler, L.R.; Jovin, T.G.; Wilder, M.J.; Lutsep, H.L.; Czartoski, T.J.; Bernstein, R.A.; et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): A prospective cohort study. Lancet Neurol. 2012, 11, 860–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, S.; Aoki, J.; Hussain, M.S.; Wisco, D.; Tateishi, Y.; Toth, G.; Hui, F.K.; Uchino, K. Predictors of infarct growth after endovascular therapy for acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef]
- Thomalla, G.; Boutitie, F.; Ma, H.; Koga, M.; Ringleb, P.; Schwamm, L.H.; Wu, O.; Bendszus, M.; Bladin, C.F.; Campbell, B.C.V.; et al. Intravenous alteplase for stroke with unknown time of onset guided by advanced imaging: Systematic review and meta-analysis of individual patient data. Lancet 2020, 396, 1574–1584. [Google Scholar] [CrossRef]
- Schwarz, G.; Agostoni, E.C.; Saliou, G.; Hajdu, S.D.; Salerno, A.; Dunet, V.; Michel, P.; Strambo, D. Perfusion Imaging Mismatch Profiles in the Early Thrombectomy Window: A Single-Center Analysis. Stroke 2023, 54, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W. Late Window Paradox. Stroke 2018, 49, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Michel, P.; Aghaebrahim, A.; Patrie, J.T.; Xin, W.; Eskandari, A.; Zhang, W.; Wintermark, M. Prediction of recanalization trumps prediction of tissue fate: The penumbra: A dual-edged sword. Stroke 2013, 44, 1014–1019. [Google Scholar] [CrossRef] [Green Version]
- Vagal, A.; Aviv, R.; Sucharew, H.; Reddy, M.; Hou, Q.; Michel, P.; Jovin, T.; Tomsick, T.; Wintermark, M.; Khatri, P. Collateral Clock Is More Important Than Time Clock for Tissue Fate. Stroke 2018, 49, 2102–2107. [Google Scholar] [CrossRef]
- Michel, P.; Odier, C.; Rutgers, M.; Reichhart, M.; Maeder, P.; Meuli, R.; Wintermark, M.; Maghraoui, A.; Faouzi, M.; Croquelois, A.; et al. The Acute STroke Registry and Analysis of Lausanne (ASTRAL): Design and baseline analysis of an ischemic stroke registry including acute multimodal imaging. Stroke 2010, 41, 2491–2498. [Google Scholar] [CrossRef] [Green Version]
- Wintermark, M.; Flanders, A.E.; Velthuis, B.; Meuli, R.; van Leeuwen, M.; Goldsher, D.; Pineda, C.; Serena, J.; van der Schaaf, I.; Waaijer, A.; et al. Perfusion-CT assessment of infarct core and penumbra: Receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006, 37, 979–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology 2007, 18, 800–804. [Google Scholar] [CrossRef] [Green Version]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Stroke Organisation Executive, C.; Committee, E.S.O.W. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc. Dis. 2008, 25, 457–507. [Google Scholar] [CrossRef]
- Michel, P.; Engelter, S.; Arnold, M.; Hungerbühler, H.J.; Nedeltchev, K.; Georgiadis, D.; Müller, F.; Bönig, L.; Müller, M.; Barth, A.; et al. Thrombolyse de l’attaque cérébrale ischémique: Recommandations actualisées. Swiss Med. Forum 2009, 9, 892–894. [Google Scholar] [CrossRef]
- Blennow, K.; Wallin, A.; Uhlemann, C.; Gottfries, C.G. White-matter lesions on CT in Alzheimer patients: Relation to clinical symptomatology and vascular factors. Acta Neurol. Scand. 1991, 83, 187–193. [Google Scholar] [CrossRef]
- Puetz, V.; Dzialowski, I.; Hill, M.D.; Demchuk, A.M. The Alberta Stroke Program Early CT Score in clinical practice: What have we learned? Int. J. Stroke 2009, 4, 354–364. [Google Scholar] [CrossRef]
- Puetz, V.; Dzialowski, I.; Hill, M.D.; Subramaniam, S.; Sylaja, P.N.; Krol, A.; O’Reilly, C.; Hudon, M.E.; Hu, W.Y.; Coutts, S.B.; et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: The clot burden score. Int. J. Stroke 2008, 3, 230–236. [Google Scholar] [CrossRef]
- Tan, J.C.; Dillon, W.P.; Liu, S.; Adler, F.; Smith, W.S.; Wintermark, M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann. Neurol. 2007, 61, 533–543. [Google Scholar] [CrossRef]
- Hacke, W.; Kaste, M.; Fieschi, C.; von Kummer, R.; Davalos, A.; Meier, D.; Larrue; Bluhmki, E.; Davis, S.; Donnan, G.; et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998, 352, 1245–1251. [Google Scholar] [CrossRef]
- Vanacker, P.; Lambrou, D.; Eskandari, A.; Maeder, P.; Meuli, R.; Ntaios, G.; Michel, P. Improving prediction of recanalization in acute large-vessel occlusive stroke. J. Thromb. Haemost. 2014, 12, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Allison, P.D. Multiple Regression: A Primer, 1st ed.; Pine Forge Press: Thousand Oaks, CA, USA, 1998. [Google Scholar]
- Van Buuren, S.; Oudshoorn, C.G. Multivariate Imputation by Chained Equations in R. Journal of Statistical Software 2011, 45(3), 1–67. [Google Scholar] [CrossRef] [Green Version]
- Rubin, D. Multivariate Imputation for Nonresponse in Surveys; J. Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Doehner, W.; Schenkel, J.; Anker, S.D.; Springer, J.; Audebert, H.J. Overweight and obesity are associated with improved survival, functional outcome, and stroke recurrence after acute stroke or transient ischaemic attack: Observations from the TEMPiS trial. Eur. Heart J. 2013, 34, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartolomeo, P.; Thiebaut de Schotten, M.; Doricchi, F. Left unilateral neglect as a disconnection syndrome. Cereb. Cortex 2007, 17, 2479–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Park, J.; Chang, J.Y.; Kim, S.H.; Lee, J.E. Inflammation after Ischemic Stroke: The Role of Leukocytes and Glial Cells. Exp. Neurobiol. 2016, 25, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, T.H.; Nighoghossian, N.; Mikkelsen, I.K.; Derex, L.; Hermier, M.; Pedraza, S.; Fiehler, J.; Ostergaard, L.; Berthezene, Y.; Baron, J.C. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke 2015, 46, 1582–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meseguer, E.; Labreuche, J.; Gonzalez-Valcarcel, J.; Sirimarco, G.; Guidoux, C.; Cabrejo, L.; Lavallee, P.C.; Klein, I.F.; Amarenco, P.; Mazighi, M. The smoking paradox: Impact of smoking on recanalization in the setting of intra-arterial thrombolysis. Cerebrovasc. Dis. Extra 2014, 4, 84–91. [Google Scholar] [CrossRef]
- Kurmann, R.; Engelter, S.T.; Michel, P.; Luft, A.R.; Wegener, S.; Branscheidt, M.; Eskioglou, E.; Sirimarco, G.; Lyrer, P.A.; Gensicke, H.; et al. Impact of Smoking on Clinical Outcome and Recanalization After Intravenous Thrombolysis for Stroke: Multicenter Cohort Study. Stroke 2018, 49, 1170–1175. [Google Scholar] [CrossRef]
- Sifat, A.E.; Vaidya, B.; Villalba, H.; Albekairi, T.H.; Abbruscato, T.J. Neurovascular unit transport responses to ischemia and common coexisting conditions: Smoking and diabetes. Am. J. Physiol. Cell Physiol. 2018, 316, C2–C15. [Google Scholar] [CrossRef] [Green Version]
- Ntaios, G.; Egli, M.; Faouzi, M.; Michel, P. J-shaped association between serum glucose and functional outcome in acute ischemic stroke. Stroke 2010, 41, 2366–2370. [Google Scholar] [CrossRef] [Green Version]
- Bruno, A.; Levine, S.R.; Frankel, M.R.; Brott, T.G.; Lin, Y.; Tilley, B.C.; Lyden, P.D.; Broderick, J.P.; Kwiatkowski, T.G.; Fineberg, S.E.; et al. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology 2002, 59, 669–674. [Google Scholar] [CrossRef]
- Ribo, M.; Tomasello, A.; Lemus, M.; Rubiera, M.; Vert, C.; Flores, A.; Coscojuela, P.; Pagola, J.; Rodriguez-Luna, D.; Bonet, S.; et al. Maximal Admission Core Lesion Compatible with Favorable Outcome in Acute Stroke Patients Undergoing Endovascular Procedures. Stroke 2015, 46, 2849–2852. [Google Scholar] [CrossRef] [Green Version]
- Olivot, J.M.; Mosimann, P.J.; Labreuche, J.; Inoue, M.; Meseguer, E.; Desilles, J.P.; Rouchaud, A.; Klein, I.F.; Straka, M.; Bammer, R.; et al. Impact of diffusion-weighted imaging lesion volume on the success of endovascular reperfusion therapy. Stroke 2013, 44, 2205–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, H.M.; Mlynash, M.; Inoue, M.; Tipirnini, A.; Liggins, J.; Bammer, R.; Lansberg, M.G.; Kemp, S.; Zaharchuk, G.; Straka, M.; et al. The growth rate of early DWI lesions is highly variable and associated with penumbral salvage and clinical outcomes following endovascular reperfusion. Int. J. Stroke 2015, 10, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Kluytmans, M.; van der Grond, J.; van Everdingen, K.J.; Klijn, C.J.; Kappelle, L.J.; Viergever, M.A. Cerebral hemodynamics in relation to patterns of collateral flow. Stroke 1999, 30, 1432–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acampa, M.; Romano, D.G.; Lazzerini, P.E.; Leonini, S.; Guideri, F.; Tassi, R.; Casseri, T.; Bracco, S.; Martini, G. Increased Arterial Stiffness is Associated with Poor Collaterals in Acute Ischemic Stroke from Large Vessel Occlusion. Curr. Neurovasc. Res. 2018, 15, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Vanacker, P.; Heldner, M.R.; Seiffge, D.; Mueller, H.; Eskandari, A.; Traenka, C.; Ntaios, G.; Mosimann, P.J.; Sztajzel, R.; Mendes Pereira, V.; et al. ASTRAL-R score predicts non-recanalisation after intravenous thrombolysis in acute ischaemic stroke. Thromb. Haemost. 2015, 113, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.A.; Brekenfeld, C.; Ebinger, M.; Christensen, S.; Barber, P.A.; Butcher, K.S.; Levi, C.R.; Parsons, M.W.; Bladin, C.F.; Donnan, G.A.; et al. The benefits of intravenous thrombolysis relate to the site of baseline arterial occlusion in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET). Stroke 2010, 41, 295–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokin, M.; Kass-Hout, T.; Kass-Hout, O.; Dumont, T.M.; Kan, P.; Snyder, K.V.; Hopkins, L.N.; Siddiqui, A.H.; Levy, E.I. Intravenous thrombolysis and endovascular therapy for acute ischemic stroke with internal carotid artery occlusion: A systematic review of clinical outcomes. Stroke 2012, 43, 2362–2368. [Google Scholar] [CrossRef] [Green Version]
- Kappelhof, M.; Marquering, H.A.; Berkhemer, O.A.; Majoie, C.B. Intra-arterial treatment of patients with acute ischemic stroke and internal carotid artery occlusion: A literature review. J. Neurointerv. Surg. 2015, 7, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.R.; Jain, M.; Kanthala, A.R.; Damania, D.; Stead, L.G.; Wang, H.Z.; Jahromi, B.S. Association of CT perfusion parameters with hemorrhagic transformation in acute ischemic stroke. AJNR. Am. J. Neuroradiol. 2013, 34, 1895–1900. [Google Scholar] [CrossRef] [Green Version]
- Yassi, N.; Parsons, M.W.; Christensen, S.; Sharma, G.; Bivard, A.; Donnan, G.A.; Levi, C.R.; Desmond, P.M.; Davis, S.M.; Campbell, B.C. Prediction of poststroke hemorrhagic transformation using computed tomography perfusion. Stroke 2013, 44, 3039–3043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsetsou, S.; Amiguet, M.; Eskandari, A.; Meuli, R.; Maeder, P.; Jiang, B.; Wintermark, M.; Michel, P. Severe cerebral hypovolemia on perfusion CT and lower body weight are associated with parenchymal haemorrhage after thrombolysis. Neuroradiology 2017, 59, 23–29. [Google Scholar] [CrossRef] [PubMed]
- del Zoppo, G.J.; von Kummer, R.; Hamann, G.F. Ischaemic damage of brain microvessels: Inherent risks for thrombolytic treatment in stroke. J. Neurol. Neurosurg. Psychiatry 1998, 65, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamann, G.F.; del Zoppo, G.J.; von Kummer, R. Hemorrhagic transformation of cerebral infarction--possible mechanisms. Thromb. Haemost. 1999, 82 (Suppl. 1), 92–94. [Google Scholar]
- Lee, S.J.; Lee, K.H.; Na, D.G.; Byun, H.S.; Kim, Y.B.; Shon, Y.M.; Cho, S.J.; Lee, J.; Chung, C.S.; Hong, S.C. Multiphasic helical computed tomography predicts subsequent development of severe brain edema in acute ischemic stroke. Arch. Neurol. 2004, 61, 505–509. [Google Scholar] [CrossRef] [Green Version]
- Aronowski, J.; Zhao, X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke 2011, 42, 1781–1786. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Chen, C.; Zhang, J.; Hu, Z. Mechanism and Therapy of Brain Edema after Intracerebral Hemorrhage. Cerebrovasc. Dis. 2016, 42, 155–169. [Google Scholar] [CrossRef]
- Legrand, L.; Naggara, O.; Turc, G.; Mellerio, C.; Roca, P.; Calvet, D.; Labeyrie, M.A.; Baron, J.C.; Mas, J.L.; Meder, J.F.; et al. Clot burden score on admission T2*-MRI predicts recanalization in acute stroke. Stroke 2013, 44, 1878–1884. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.H.; Kim, K.; Yoo, J.; Kim, Y.D.; Nam, H.S.; Kim, E.Y. Computed Tomography-Based Thrombus Imaging for the Prediction of Recanalization after Reperfusion Therapy in Stroke. J. Stroke 2017, 19, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Kaesmacher, J.; Mosimann, P.J.; Giarrusso, M.; El-Koussy, M.; Zibold, F.; Piechowiak, E.; Dobrocky, T.; Meier, R.; Jung, S.; Bellwald, S.; et al. Multivessel Occlusion in Patients Subjected to Thrombectomy: Prevalence, Associated Factors, and Clinical Implications. Stroke 2018, 49, 1355–1362. [Google Scholar] [CrossRef]
- Barber, P.A.; Darby, D.G.; Desmond, P.M.; Gerraty, R.P.; Yang, Q.; Li, T.; Jolley, D.; Donnan, G.A.; Tress, B.M.; Davis, S.M. Identification of major ischemic change. Diffusion-weighted imaging versus computed tomography. Stroke 1999, 30, 2059–2065. [Google Scholar] [CrossRef]
- Kucinski, T.; Vaterlein, O.; Glauche, V.; Fiehler, J.; Klotz, E.; Eckert, B.; Koch, C.; Rother, J.; Zeumer, H. Correlation of apparent diffusion coefficient and computed tomography density in acute ischemic stroke. Stroke 2002, 33, 1786–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobesky, J.; von Kummer, R.; Frackowiak, M.; Zaro Weber, O.; Lehnhardt, F.G.; Dohmen, C.; Neveling, M.; Moller-Hartmann, W.; Jacobs, A.H.; Heiss, W.D. Early ischemic edema on cerebral computed tomography: Its relation to diffusion changes and hypoperfusion within 6 h after human ischemic stroke. A comparison of CT, MRI and PET. Cerebrovasc. Dis. 2006, 21, 336–339. [Google Scholar] [CrossRef]
- Barber, P.A.; Demchuk, A.M.; Zhang, J.; Buchan, A.M. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000, 355, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Dzialowski, I.; Hill, M.D.; Coutts, S.B.; Demchuk, A.M.; Kent, D.M.; Wunderlich, O.; von Kummer, R. Extent of early ischemic changes on computed tomography (CT) before thrombolysis: Prognostic value of the Alberta Stroke Program Early CT Score in ECASS II. Stroke 2006, 37, 973–978. [Google Scholar] [CrossRef] [Green Version]
- Ay, H.; Arsava, E.M.; Rosand, J.; Furie, K.L.; Singhal, A.B.; Schaefer, P.W.; Wu, O.; Gonzalez, R.G.; Koroshetz, W.J.; Sorensen, A.G. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke 2008, 39, 1409–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rost, N.S.; Fitzpatrick, K.; Biffi, A.; Kanakis, A.; Devan, W.; Anderson, C.D.; Cortellini, L.; Furie, K.L.; Rosand, J. White matter hyperintensity burden and susceptibility to cerebral ischemia. Stroke 2010, 41, 2807–2811. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Gilgen, M.; Slotboom, J.; El-Koussy, M.; Zubler, C.; Kiefer, C.; Luedi, R.; Mono, M.L.; Heldner, M.R.; Weck, A.; et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain 2013, 136, 3554–3560. [Google Scholar] [CrossRef]
- Seyman, E.; Shaim, H.; Shenhar-Tsarfaty, S.; Jonash-Kimchi, T.; Bornstein, N.M.; Hallevi, H. The collateral circulation determines cortical infarct volume in anterior circulation ischemic stroke. BMC Neurol. 2016, 16, 206. [Google Scholar] [CrossRef] [Green Version]
- Cheng-Ching, E.; Frontera, J.A.; Man, S.; Aoki, J.; Tateishi, Y.; Hui, F.K.; Wisco, D.; Ruggieri, P.; Hussain, M.S.; Uchino, K. Degree of Collaterals and Not Time Is the Determining Factor of Core Infarct Volume within 6 Hours of Stroke Onset. Am. J. Neuroradiol. 2015, 36, 1272–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nannoni, S.; Cereda, C.W.; Sirimarco, G.; Lambrou, D.; Strambo, D.; Eskandari, A.; Dunet, V.; Wintermark, M.; Michel, P. Collaterals are a major determinant of the core but not the penumbra volume in acute ischemic stroke. Neuroradiology 2019, 61, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Berkhemer, O.A.; Jansen, I.G.; Beumer, D.; Fransen, P.S.; van den Berg, L.A.; Yoo, A.J.; Lingsma, H.F.; Sprengers, M.E.; Jenniskens, S.F.; Lycklama, A.N.G.J.; et al. Collateral Status on Baseline Computed Tomographic Angiography and Intra-Arterial Treatment Effect in Patients with Proximal Anterior Circulation Stroke. Stroke 2016, 47, 768–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venema, E.; Roozenbeek, B.; Mulder, M.; Brown, S.; Majoie, C.; Steyerberg, E.W.; Demchuk, A.M.; Muir, K.W.; Davalos, A.; Mitchell, P.J.; et al. Prediction of Outcome and Endovascular Treatment Benefit: Validation and Update of the MR PREDICTS Decision Tool. Stroke 2021, 52, 2764–2772. [Google Scholar] [CrossRef]
- Uniken Venema, S.M.; Dankbaar, J.W.; van der Lugt, A.; Dippel, D.W.J.; van der Worp, H.B. Cerebral Collateral Circulation in the Era of Reperfusion Therapies for Acute Ischemic Stroke. Stroke 2022, 53, 3222–3234. [Google Scholar] [CrossRef]
- Uniken Venema, S.M.; Dankbaar, J.W.; Wolff, L.; van Es, A.; Sprengers, M.; van der Lugt, A.; Dippel, D.W.J.; van der Worp, H.B.; MR CLEAN Registry Investigators. Collateral status and recanalization after endovascular treatment for acute ischemic stroke. J. Neurointerv. Surg. 2023, 15, 531–538. [Google Scholar] [CrossRef]
- Lev, M.H.; Segal, A.Z.; Farkas, J.; Hossain, S.T.; Putman, C.; Hunter, G.J.; Budzik, R.; Harris, G.J.; Buonanno, F.S.; Ezzeddine, M.A.; et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: Prediction of final infarct volume and clinical outcome. Stroke 2001, 32, 2021–2028. [Google Scholar] [CrossRef] [Green Version]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Zhu, G.; Patrie, J.; Xin, W.; Michel, P.; Eskandari, A.; Jovin, T.; Wintermark, M. Optimal perfusion computed tomographic thresholds for ischemic core and penumbra are not time dependent in the clinically relevant time window. Stroke 2014, 45, 1355–1362. [Google Scholar] [CrossRef]
- Beaulieu, C.; de Crespigny, A.; Tong, D.C.; Moseley, M.E.; Albers, G.W.; Marks, M.P. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: Evolution of lesion volume and correlation with clinical outcome. Ann. Neurol. 1999, 46, 568–578. [Google Scholar] [CrossRef]
- Kim, S.M.; Kwon, S.U.; Kim, J.S.; Kang, D.W. Early infarct growth predicts long-term clinical outcome in ischemic stroke. J. Neurol. Sci. 2014, 347, 205–209. [Google Scholar] [CrossRef]
- Lin, L.; Bivard, A.; Krishnamurthy, V.; Levi, C.R.; Parsons, M.W. Whole-Brain CT Perfusion to Quantify Acute Ischemic Penumbra and Core. Radiology 2016, 279, 876–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austein, F.; Riedel, C.; Kerby, T.; Meyne, J.; Binder, A.; Lindner, T.; Huhndorf, M.; Wodarg, F.; Jansen, O. Comparison of Perfusion CT Software to Predict the Final Infarct Volume After Thrombectomy. Stroke 2016, 47, 2311–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, P.; Bogousslavsky, J. Penumbra is brain: No excuse not to perfuse. Ann. Neurol. 2005, 58, 661–663. [Google Scholar] [CrossRef] [PubMed]
| N | Values | |
|---|---|---|
| Acute Variables | ||
| Demographics | ||
| Age (years) | 551 | 66.3 (14.7) |
| Males | 551 | 280 (50.8) |
| Medical history | ||
| Hypertension | 551 | 340 (61.7) |
| Dyslipidemia | 549 | 360 (65.6) |
| Diabetes | 550 | 82 (14.9) |
| Current smoking | 547 | 148 (27.1) |
| Previous stroke or TIA | 551 | 103 (18.7) |
| Atrial fibrillation | 550 | 185 (33.6) |
| Treatment before stroke | 550 | 185 (33.6) |
| Lipid-lowering drug | 551 | 122 (22.1) |
| Anti-hypertensive drug | 547 | 282 (51.6) |
| Anti-diabetic drug | 551 | 48 (8.7) |
| Antiplatelet | 551 | 171 (31.0) |
| Anticoagulant | 551 | 52 (9.4) |
| Pre-stroke mRS | 551 | 155 (28.1) |
| Clinical measurements | ||
| Body temperature (°C) | 537 | 36.3 (0.7) |
| Systolic Blood Pressure (mmHg) | 549 | 155 (28) |
| BMI (kg/m2) | 398 | 25.6 (4.6) |
| Biological data | ||
| Blood glucose (mmol/L) | 540 | 6.6 (5.8 to 7.8) |
| Total cholesterol (mmol/L) | 496 | 5.38 (1.75) |
| WBC count (G/L) | 540 | 8.0 (6.5 to 10.2) |
| Hemoglobin (g/L) | 540 | 138 (16) |
| Creatinine (µm/L) | 543 | 89 (24) |
| New neurological deficit | ||
| Admission NIHSS | 551 | 13.3 (7.1) |
| Visual field defects | 546 | 320 (58.6) |
| Eye deviation | 546 | 231 (42.3) |
| Aphasia | 547 | 270 (49.4) |
| Neglect | 543 | 244 (44.9) |
| Vigilance impairment | 539 | 75 (13.9) |
| Dysarthria | 547 | 340 (62.2) |
| Paresis | 548 | 506 (92.3) |
| Sensory loss | 547 | 411 (75.1) |
| Simultaneous PCA and/or ACA involvement | 551 | 44 (8.0) |
| Onset-to-door time (minutes) | 550 | 131 (72 to 301) |
| Onset-to-CT time (minutes) | 546 | 170 (102 to 385) |
| Neuroimaging data | ||
| Baseline penumbra, mm | 551 | 62.6 (23.4 to 108.7) |
| Baseline infarct core, mm | 551 | 28.9 (8.1 to 75.7) |
| Early ischemic changes | 551 | 303 (55.0) |
| ASPECTS | 551 | 8 (6 to 10) |
| Old infarct | 551 | 126 (22.9) |
| Leukoaraiosis | 551 | 98 (17.8) |
| Hyperdense MCA | 550 | 448 (81.5) |
| Clot burden score | 442 | 6 (4 to 9.0) |
| Cerebral stenosis ≥ 50% or occlusion | 550 | 448 (81.5) |
| Extracranial stenosis ≥ 50% or occlusion | 550 | 170 (30.9) |
| Intracranial stenosis ≥ 50% or occlusion | 550 | 414 (75.3) |
| Good collaterals | 551 | 352 (63.9) |
| Reperfusion therapy | 551 | 273 (49.6) |
| IV thrombolysis | 551 | 256 (46.5) |
| Endovascular treatment | 551 | 17 (3.1) |
| Subacute variables | ||
| Clinical and biological measurements | ||
| Systolic blood pressure (mmHg) | 525 | 137 (21) |
| Body temperature (°C) | 508 | 37 (0.7) |
| Blood glucose (mmol/L) | 432 | 5.6 (4.9 to 6.5) |
| Neuroimaging data | ||
| Final infarct | 551 | 24.7 (5.8 to 72.9) |
| Penumbra salvage | 551 | 59.3 (15.9 to 117.5) |
| Infarct growth | 551 | −0.0 (−15.1 to 18.4) |
| Recanalization | 463 | |
| No occlusion | 131 (28.3) | |
| Recanalized | 218 (47.1) | |
| Non-recanalized | 114 (24.6) | |
| Parenchymal hemorrhage | 460 | 45 (9.8) |
| TOAST Mechanism | 548 | |
| Large artery atherosclerosis | 83 (15.2) | |
| Cardioembolism | 230 (42.0) | |
| SVD and other determined etiology 1 | 123 (22.4) | |
| Undetermined etiology 2 | ||
| 3-month outcome | ||
| mRS | 547 | 2 (1 to 4) |
| c-mRS 0–1 | 547 | 180 (32.9) |
| c-mRS 0–2 | 547 | 289 (52.8) |
| All-cause mortality | 547 | 76 (13.9) |
| 12-month outcome | ||
| mRS | 541 | 2 (1 to 4) |
| c-mRS 0–1 | 541 | 206 (38.1) |
| c-mRS 0–2 | 541 | 297 (54.9) |
| All-cause mortality | 541 | 100 (18.5) |
| Predictors | Acute Model | Acute and Subacute Models | ||
|---|---|---|---|---|
| OR (95%CI) 1 | p Value | OR (95%CI) 1 | p Value | |
| Acute characteristics | ||||
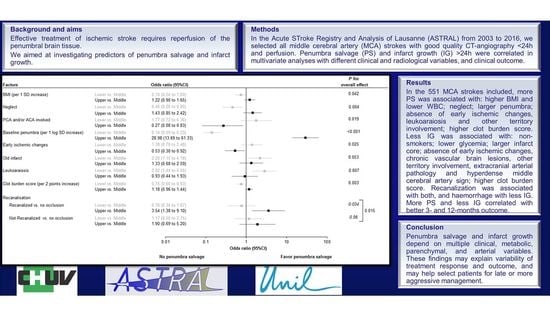
| BMI (per 1 SD increase) | 1.33 (1.06 to 1.67) | 0.011 | 1.31 (1.04 to 1.65) | 0.020 |
| WBC (per 1 log SD increase) | 0.80 (0.65 to 0.99) | 0.032 | 0.82 (0.66 to 1.01) | 0.056 |
| Neglect | 1.81 (1.22 to 2.68) | 0.003 | 1.66 (1.11 to 2.49) | 0.013 |
| Baseline penumbra (per 1 log SD increase) | 18.75 (12.48 to 28.17) | <0.001 | 18.05 (11.90 to 27.36) | <0.001 |
| PCA and/or ACA involved | 0.32 (0.15 to 0.68) | 0.003 | 0.36 (0.17 to 0.77) | 0.008 |
| Early ischemic changes | 0.55 (0.36 to 0.84) | 0.005 | 0.56 (0.36 to 0.85) | 0.006 |
| Clot burden score (per 1 point increase) | 1.09 (1.01 to 1.17) | 0.016 | 1.13 (1.04 to 1.22) | 0.003 |
| Leukoaraiosis | 0.44 (0.26 to 0.74) | 0.001 | 0.46 (0.27 to 0.77) | 0.003 |
| Subacute characteristics | ||||
| Recanalization | Not entered | - | 0.006 2 | |
| No occlusion | 1.00 (reference) | - | ||
| Recanalized | 2.34 (1.27 to 4.29) | 0.006 | ||
| Non-recanalized | 1.28 (0.64 to 2.56) | 0.48 | ||
| Parenchymal hemorrhage at 24 h | Not entered | - | 0.56 (0.29 to 1.07) | 0.078 |
| Predictors | Acute Model | Acute and Subacute Models | ||
|---|---|---|---|---|
| OR (95%CI) 1 | p Value | OR (95%CI) 1 | p Value | |
| Acute characteristics | ||||
| Current smoking | 1.56 (1.06 to 2.27) | 0.022 | 1.54 (1.05 to 2.25) | 0.027 |
| Blood glucose (per 1 log SD increase) | 1.35 (1.13 to 1.61) | <0.001 | 1.33 (1.11 to 1.59) | 0.002 |
| Baseline infarct core (per 1 log SD increase) | 0.41 (0.32 to 0.51) | <0.001 | 0.44 (0.34 to 0.55) | <0.001 |
| PCA and/or ACA involved | 6.32 (3.10 to 12.88) | <0.001 | 5.76 (2.75 to 12.05) | <0.001 |
| Early ischemic changes | 2.26 (1.56 to 3.27) | <0.001 | 2.27 (1.55 to 3.31) | <0.001 |
| Hyperdense MCA | 1.80 (1.15 to 2.78) | 0.009 | 1.91 (1.22 to 2.98) | 0.004 |
| Clot burden score (per 1 point increase) | 0.91 (0.83 to 0.98) | 0.015 | 0.87 (0.80 to 0.95) | 0.001 |
| Leukoaraiosis | 2.04 (1.31 to 3.19) | 0.002 | 2.05 (1.30 to 3.23) | 0.002 |
| Old infarct | 1.55 (1.04 to 2.32) | 0.031 | 1.66 (1.11 to 2.51) | 0.015 |
| Extracranial stenosis ≥ 50% or occlusion | 1.64 (1.11 to 2.41) | 0.012 | 1.55 (1.03 to 2.30) | 0.032 |
| Good collaterals | 0.68 (0.45 to 1.03) | 0.067 | Not selected | - |
| Subacute characteristics | ||||
| Recanalization | Not entered | - | <0.001 2 | |
| No occlusion | 1.00 (reference) | - | ||
| Recanalized | 0.50 (0.31 to 0.83) | 0.006 | ||
| Non-recanalized | 1.11 (0.64 to 1.90) | 0.72 | ||
| Parenchymal hemorrhage at 24 h | Not entered | - | 2.61 (1.39 to 4.87) | 0.003 |
| 24 h Salvage of the Penumbra Volume (Median [Range], mL) | ||||
|---|---|---|---|---|
| <33th Percentile | 33–65th Percentiles | ≥66th Percentiles | ||
| 2.6 [−50.0 to 26.7] | 59.3 [26.8 to 97.6] | 139.8 [97.7 to 250.0] | ||
| (N = 183) | (N = 184) | (N = 184) | p-Value 1 | |
| mRS (shift analysis) | ||||
| Median (IQR) | 2 (1–4) | 3 (1–4) | 2 (1–3) | |
| Baseline adjusted OR (95%CI) 2 | 1.00 (reference) | 2.20 (1.43 to 3.36) | 4.94 (2.96 to 8.25) | <0.0001 |
| Fully adjusted OR (95%CI) 3 | 1.00 (reference) | 1.48 (0.93 to 2.34) | 2.89 (1.65 to 5.06) | 0.0001 |
| Corrected mRS 0–1 | ||||
| Baseline adjusted rates, % | 18.4 | 35.0 | 45.9 | |
| Baseline adjusted OR (95%CI) | 1.00 (reference) | 2.39 (1.36 to 4.19) | 3.76 (1.89 to 7.43) | <0.0001 |
| Fully adjusted OR (95%CI) 3 | 1.00 (reference) | 1.97 (1.00 to 3.87) | 3.44 (1.46 to 8.11) | 0.005 |
| Corrected mRS 0–2 | ||||
| Baseline adjusted rates, % | 35.4 | 52.4 | 71.1 | |
| Baseline adjusted OR (95%CI) | 1.00 (reference) | 2.01 (1.20 to 3.33) | 4.48 (2.41 to 8.31) | <0.0001 |
| Fully adjusted OR (95%CI) 3 | 1.00 (reference) | 1.32 (0.71 to 2.46) | 2.78 (1.30 to 5.93) | 0.004 |
| All-cause mortality | ||||
| Baseline adjusted rates, % | 31.3 | 13.5 | 2.5 | |
| Baseline adjusted OR (95%CI) | 1.00 (reference) | 0.34 (0.18 to 0.63) | 0.06 (0.02 to 0.15) | <0.0001 |
| Fully adjusted OR (95%CI) 3 | 1.00 (reference) | 0.47 (0.22 to 0.98) | 0.10 (0.03 to 0.29) | <0.0001 |
| 24 h Increase of the Infarct Core Median [Range], mL) | ||||
|---|---|---|---|---|
| <33th Percentile | 33–65th Percentiles | ≥66th Percentiles | ||
| −25.7 [−50.0 to −7.3] | −0.0 [−7.2 to 8.4] | 35.0 [18.4 to 250.0] | ||
| (N = 183) | (N = 184) | (N = 184) | p-Value 1 | |
| mRS (shift analysis) | ||||
| median (IQR) | 2 (1–3) | 2 (1–3) | 4 (2–6) | |
| Baseline adjusted OR (95%CI) 2 | 1.00 (reference) | 0.45 (0.29 to 0.69) | 0.14 (0.09 to 0.21) | <0.0001 |
| Fully adjusted OR (95%CI) 3 | 1.00 (reference) | 0.61 (0.38 to 0.96) | 0.27 (0.17 to 0.42) | <0.0001 |
| Corrected mRS 0–1 | ||||
| Baseline adjusted rates, % | 51.6 | 31.7 | 10.3 | |
| Baseline adjusted OR (95%CI) | 1.00 (reference) | 0.44 (0.25 to 0.75) | 0.11 (0.05 to 0.20) | <0.0001 |
| Fully adjusted OR (95%CI) 3 | 1.00 (reference) | 0.66 (0.35 to 1.23) | 0.22 (0.11 to 0.44) | <0.0001 |
| Corrected mRS 0–2 | ||||
| Baseline adjusted rates, % | 72.7 | 56.6 | 28.7 | |
| Baseline adjusted OR (95%CI) | 1.00 (reference) | 0.49 (0.29 to 0.82) | 0.15 (0.09 to 0.25) | <0.0001 |
| Fully adjusted OR (95%CI) 3 | 1.00 (reference) | 0.69 (0.38 to 1.24) | 0.29 (0.16 to 0.53) | <0.0001 |
| All-cause mortality | ||||
| Baseline adjusted rates, % | 3.9 | 9.0 | 21.9 | |
| Baseline adjusted OR (95%CI) | 1.00 (reference) | 2.43 (1.03 to 5.70) | 6.87 (3.47 to 13.61) | <0.0001 |
| Fully adjusted OR (95%CI) 3 | 1.00 (reference) | 2.37 (0.92 to 6.10) | 4.29 (1.90 to 9.63) | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirimarco, G.; Strambo, D.; Nannoni, S.; Labreuche, J.; Cereda, C.; Dunet, V.; Puccinelli, F.; Saliou, G.; Meuli, R.; Eskandari, A.; et al. Predicting Penumbra Salvage and Infarct Growth in Acute Ischemic Stroke: A Multifactor Survival Game. J. Clin. Med. 2023, 12, 4561. https://doi.org/10.3390/jcm12144561
Sirimarco G, Strambo D, Nannoni S, Labreuche J, Cereda C, Dunet V, Puccinelli F, Saliou G, Meuli R, Eskandari A, et al. Predicting Penumbra Salvage and Infarct Growth in Acute Ischemic Stroke: A Multifactor Survival Game. Journal of Clinical Medicine. 2023; 12(14):4561. https://doi.org/10.3390/jcm12144561
Chicago/Turabian StyleSirimarco, Gaia, Davide Strambo, Stefania Nannoni, Julien Labreuche, Carlo Cereda, Vincent Dunet, Francesco Puccinelli, Guillaume Saliou, Reto Meuli, Ashraf Eskandari, and et al. 2023. "Predicting Penumbra Salvage and Infarct Growth in Acute Ischemic Stroke: A Multifactor Survival Game" Journal of Clinical Medicine 12, no. 14: 4561. https://doi.org/10.3390/jcm12144561