Adrenal Abscesses: A Systematic Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
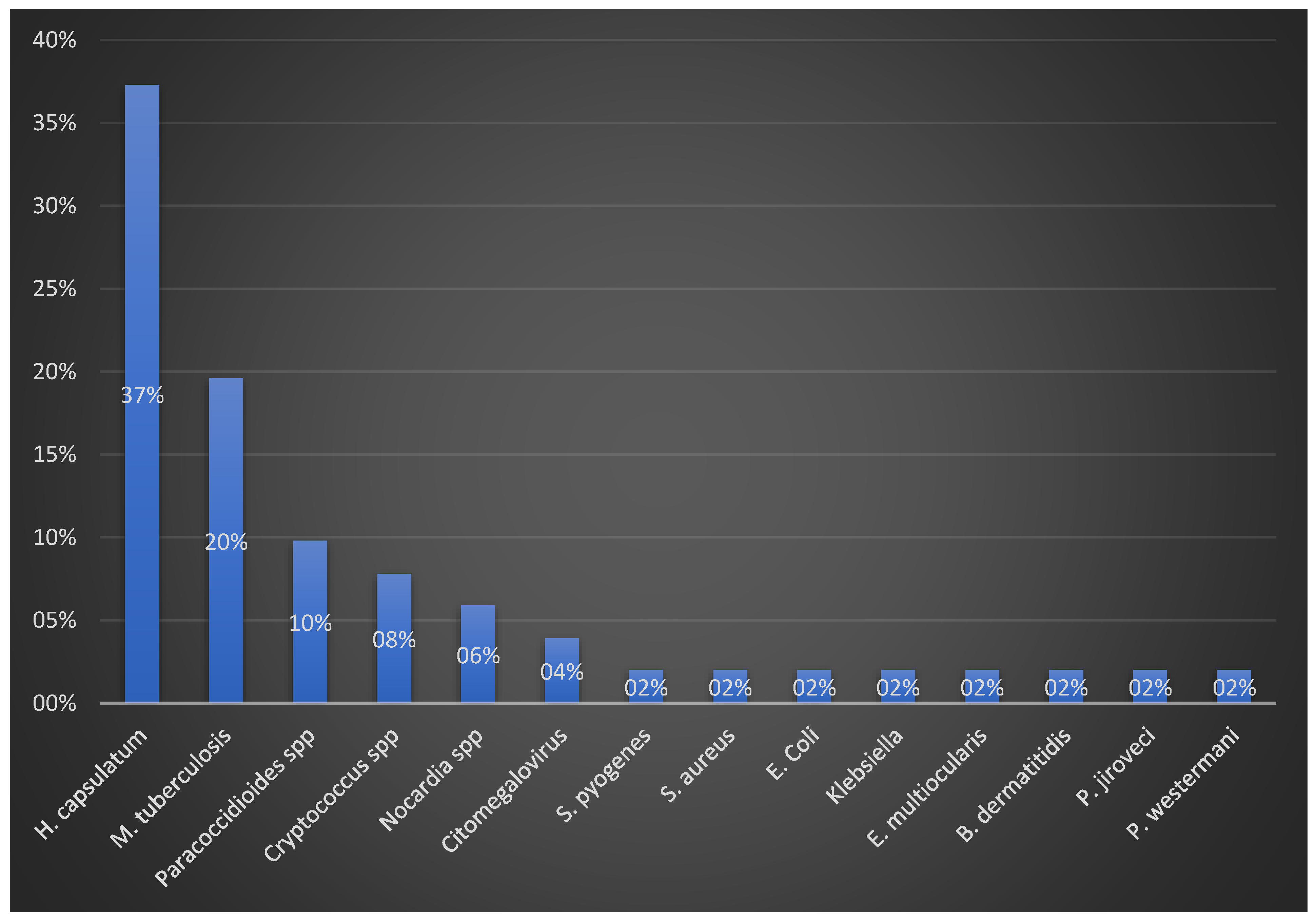
3. Results
4. Discussion
4.1. Demographics
4.2. Symptoms
4.2.1. Weight Loss
4.2.2. Fever
4.2.3. Hyperpigmentation
4.2.4. Abdominal Pain
4.3. Laboratory Markers
4.3.1. Anemia
4.3.2. Thrombocytopenia and Sepsis
4.3.3. Hyponatremia
4.3.4. Adrenal Insufficiency (AI)
4.4. Adrenal Histopathology
4.5. Type of Adrenal Involvement
4.6. Imaging Findings
4.7. Therapy
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pignatti, E.; Leng, S.; Carlone, D.L.; Breault, D.T. Regulation of zonation and homeostasis in the adrenal cortex. Mol. Cell. Endocrinol. 2017, 441, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, S.; Shariff, M.; Bartlett, S.E. The role of the glucocorticoids in developing resilience to stress and addiction. Front. Psychiatry 2013, 4, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, E.; Borges, R.; Eiden, L.E.; García, A.G.; Hernández-Cruz, A. Chromaffin Cells of the Adrenal Medulla: Physiology, Pharmacology, and Disease. Compr. Physiol. 2019, 9, 1443–1502. [Google Scholar] [PubMed]
- Burford, N.G.; Webster, N.A.; Cruz-Topete, D. Hypothalamic-Pituitary-Adrenal Axis Modulation of Glucocorticoids in the Cardiovascular System. Int. J. Mol. Sci. 2017, 18, 2150. [Google Scholar] [CrossRef] [Green Version]
- Turgut, A.O.M.; Akpinar, V.; MacLennan, D.; MacLennan, G. Congenital and Acquired Nonneoplastic Adrenal Diseases. In Genitourinary Radiology: Male Genital Tract, Adrenal and Retroperitoneum; Dogra, V.M.G., Ed.; Springer: London, UK, 2013; pp. 219–238. [Google Scholar]
- Michels, A.W.; Eisenbarth, G.S. Immunologic endocrine disorders. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S226–S237. [Google Scholar] [CrossRef] [Green Version]
- Azeez, T.A.; Irojah, O.A.; Lakoh, S.; Lawal, A.; Ajiboso, O. A systematic review of adrenal insufficiency among patients with pulmonary tuberculosis in Sub-Saharan Africa. Int. J. Mycobacteriol. 2021, 10, 1–7. [Google Scholar] [CrossRef]
- Gupta, S.; Ansari, M.A.M.; Gupta, A.K.; Chaudhary, P.; Bansal, L.K. Current Approach for Diagnosis and Treatment of Adrenal Tuberculosis-Our Experience and Review of Literature. Surg. J. 2022, 8, e92–e97. [Google Scholar] [CrossRef]
- Upadhyay, J.; Sudhindra, P.; Abraham, G.; Trivedi, N. Tuberculosis of the adrenal gland: A case report and review of the literature of infections of the adrenal gland. Int. J. Endocrinol. 2014, 2014, 876037. [Google Scholar] [CrossRef]
- Kathuria, S.; Capoor, M.R.; Yadav, S.; Singh, A.; Ramesh, V. Disseminated histoplasmosis in an apparently immunocompetent individual from north India: A case report and review. Med. Mycol. 2013, 51, 774–778. [Google Scholar] [CrossRef]
- Cabrera, N.L.; Malek, A.E.; Shelburne, S.; Taremi, M.; Awadh, H.; Francisco, D.; Robins, A.; Jabbour, E.; Chemaly, R.F. Disseminated cytomegalovirus infection with bilateral adrenal pseudotumors masquerading as recurrent hematologic malignancy. Infection 2020, 48, 477–481. [Google Scholar] [CrossRef]
- Jin, W.; Miao, Q.; Wang, M.; Zhang, Y.; Ma, Y.; Huang, Y.; Wu, H.; Lin, Y.; Hu, B.; Pan, J. A rare case of adrenal gland abscess due to anaerobes detected by metagenomic next-generation sequencing. Ann. Transl. Med. 2020, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Talan, D.A. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N. Engl. J. Med. 2014, 370, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, M.C.; Coutinho, J.M.; van de Beek, D. Clinical characteristics and outcome of brain abscess: Systematic review and meta-analysis. Neurology 2014, 82, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Losie, J.A.; Lam, J.C.; Gregson, D.B.; Parkins, M.D. Epidemiology and risk factors for pyogenic liver abscess in the Calgary Health Zone revisited: A population-based study. BMC Infect. Dis. 2021, 21, 939. [Google Scholar] [CrossRef] [PubMed]
- Falhammar, H.; Wallin, G.; Calissendorff, J. Acute suppurative thyroiditis with thyroid abscess in adults: Clinical presentation, treatment and outcomes. BMC Endocr. Disord. 2019, 19, 130. [Google Scholar] [CrossRef] [Green Version]
- Grover, S.; Selhi, P.K.; Sood, N.; Sood, R.; Kaur, H.; Mbbs, R.S. “Polka Dot Macrophages” on cytology of bilateral adrenal masses-Nailing disseminated histoplasmosis. Diagn. Cytopathol. 2017, 45, 943–946. [Google Scholar] [CrossRef]
- Grüter, B.E.; Reuss, A.M.; Rushing, E.J.; Pangalu, A.; Oertel, M.F. An unexpected intracerebral lesion—Case report of a superinfected aspergillosis mimicking a brain metastasis. BMC Infect. Dis. 2021, 21, 537. [Google Scholar] [CrossRef]
- Robinson, L.J.; Lu, M.; Elsayed, S.; Joy, T.R. Bilateral adrenal histoplasmosis manifesting as primary adrenal insufficiency. CMAJ 2019, 191, E1217–E1221. [Google Scholar] [CrossRef]
- Tran, N.Q.; Phan, C.C.; Doan, T.T.P.; Tran, T.V. Bilateral adrenal masses due to tuberculosis: How to diagnose without extra-adrenal tuberculosis. Endocrinol. Diabetes Metab. Case Rep. 2021, 2021, 21-0093. [Google Scholar] [CrossRef]
- Koh, S.A. Addison’s disease due to bilateral adrenal tuberculosis on 18F-fluorodeoxyglucose positron emission tomography computed tomography. Infect. Dis. Rep. 2018, 10, 7773. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Zhou, L.; Mo, X.; Huang, G.; Wu, P. Successful treatment of severe electrolyte imbalance-induced cardiac arrest caused by adrenal tuberculosis with ECMO in the ED. Int. J. Emerg. Med. 2021, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Chee, D.; Moritz, A.W.; Profit, A.P.; Agarwal, A.N.; Anstead, G.M. Fatal coccidioidomycosis involving the lungs, brain, tongue, and adrenals in a cirrhotic patient. An autopsy case. IDCases 2021, 23, e01049. [Google Scholar] [CrossRef] [PubMed]
- Roxas, M.C.A.; Sandoval, M.A.S.; Salamat, M.S.; Matias, P.J.; Cabal, N.P.; Bartolo, S.S. Bilateral adrenal histoplasmosis presenting as adrenal insufficiency in an immunocompetent host in the Philippines. BMJ Case Rep. 2020, 13, e234935. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.A.; Gan, E.H.; Kanaan, M.Z.; Price, D.A.; Hoare, T.; Pearce, S. An unusual cause of adrenal insufficiency and bilateral adrenal masses. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018, 18-0030. [Google Scholar] [CrossRef] [PubMed]
- Lieu, A.; Church, D.; Vaughan, S. Bilateral Adrenal Histoplasmosis in an Immunocompetent Host. Am. J. Trop. Med. Hyg. 2021, 105, 1437–1438. [Google Scholar] [CrossRef]
- Kurian, M.E.; Jebasingh, F.K.; Kodiatte, T.A.; Thomas, N. Adrenal histoplasmosis: An uncommon presentation with an ulcer of the tongue. BMJ Case Rep. 2021, 14, e244296. [Google Scholar] [CrossRef]
- Ramesh, V.; Narreddy, S.; Gowrishankar, S.; Barigala, R.; Nanda, S. A challenging case of pyrexia of unknown origin: Adrenal histoplasmosis mimicking tuberculosis in a patient with chronic hepatitis C. Trop. Dr. 2021, 51, 621–623. [Google Scholar] [CrossRef]
- Zhao, N.; Gao, Y.; Ni, C.; Zhang, D.; Zhao, X.; Li, Y.; Sun, B. An autopsy case of unexpected death due to Addison’s disease caused by adrenal tuberculosis. Eur. J. Med. Res. 2021, 26, 137. [Google Scholar] [CrossRef]
- Ito, M.; Hinata, T.; Tamura, K.; Koga, A.; Ito, T.; Fujii, H.; Hirata, F.; Sakuta, H. Disseminated Cryptococcosis with Adrenal Insufficiency and Meningitis in an Immunocompetent Individual. Intern. Med. 2017, 56, 1259–1264. [Google Scholar] [CrossRef] [Green Version]
- Peçanha-Pietrobom, P.M.; Falqueto, A.; Rodrigues Gandarella, A.D.; Moyzés, J.V.; Rangel, K.A.; Miranda, L.B.; Hemerly, M.C.; Careta, R.S.; Peçanha, P.M. Case Report: Paracoccidioidomycosis in Solid Organ Transplantation: Disseminated Disease in a Liver Recipient and Literature Review. Am. J. Trop. Med. Hyg. 2019, 101, 1100–1106. [Google Scholar] [CrossRef]
- Liu, H.; Tang, T.J.; An, Z.M.; Yu, Y.R. Unilateral adrenal tuberculosis whose computed tomography imaging characteristics mimic a malignant tumor: A case report. World J. Clin. Cases 2022, 10, 5783–5788. [Google Scholar] [CrossRef]
- Soedarso, M.A.; Nugroho, K.H.; Meira Dewi, K.A. A case report: Addison disease caused by adrenal tuberculosis. Urol. Case Rep. 2018, 20, 12–14. [Google Scholar] [CrossRef]
- van Haren Noman, S.; Visser, H.; Muller, A.F.; Limonard, G.J. Addison’s Disease Caused by Tuberculosis: Diagnostic and Therapeutic Difficulties. Eur. J. Case Rep. Intern. Med. 2018, 5, 000911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herreros, B.; Plaza, I.; García, R.; Chichón, M.; Guerrero, C.; Pintor, E. Miliary Tuberculosis Presenting with Hyponatremia and ARDS in an 82-Year-Old Immunocompetent Female. Pathogens 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govind, P.; Subramanian, K.; Kumar, S. Diagnostic and Therapeutic Implications of Organic Delusional Disorder due to Tuberculous Adrenalitis. Case Rep. Psychiatry 2022, 2022, 5056976. [Google Scholar] [CrossRef] [PubMed]
- Maciel, G.V.R.; Tavares, M.C.F.; Pereira, L.S.; Silva, G.L.C.; De Oliveira, N.R.; Paulino, E.; Pascoal-Xavier, M.A. Disseminated mycosis in a patient with yellow fever. Autops. Case Rep. 2018, 8, e2018038. [Google Scholar] [CrossRef]
- Hatakeyama, Y.; Nakakubo, S.; Kusaka, H.; Watanabe, N.; Yoshida, Y.; Shinzaki, H.; Hiroumi, H.; Kishida, N.; Konno, S. Listeria monocytogenes bacteremia mimicking the systemic metastasis of adrenal cancer: A case report. BMC Infect. Dis. 2022, 22, 789. [Google Scholar] [CrossRef]
- Arambewela, M.; Ross, R.; Pirzada, O.; Balasubramanian, S.P. Tuberculosis as a differential for bilateral adrenal masses in the UK. BMJ Case Rep. 2019, 12, e228532. [Google Scholar] [CrossRef]
- de Oliveira, F.M.; Fragoso, M.C.B.V.; Meneses, A.F.; Vilela, L.A.P.; Almeida, M.Q.; Palhares, R.B.; Mattos, T.V.D.A.; Scalissi, N.M.; Lima, J.V. Adrenal insufficiency caused by paracoccidioidomycosis: Three case reports and review. AACE Clin. Case Rep. 2019, 5, e238–e243. [Google Scholar]
- Qu, F.; Qu, Z.; Lv, Y.; Song, B.; Wu, B. Disseminated Cryptococcosis revealed by transverse myelitis in Immunocompetent patient: A case report and review of the literature. BMC Neurol. 2020, 20, 13. [Google Scholar] [CrossRef]
- Muhammed, H.; Nampoothiri, R.V.; Gaspar, B.L.; Jain, S. Infectious causes of Addison’s disease: 1 organ-2 organisms! BMJ Case Rep. 2018, 2018, bcr2017223633. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.S.; Man, C.H.; Wong, K.W.; Ng, K.C. Emergency single-port laparoscopic partial adrenalectomy for adrenal abscess in an adult with disseminated Streptococcus pyogenes bacteraemia: A case report. Hong Kong Med. J. 2021, 27, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, J.; Bansal, N.; Arora, A. Disseminated histoplasmosis in India presenting as addisonian crisis with epiglottis involvement. IDCases 2020, 21, e00844. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Kapoor, V.; Latif, A.; Zangeneh, T. A 30-year delayed presentation of disseminated histoplasmosis in a heart transplant recipient: Diagnostic challenges in a non-endemic area. BMJ Case Rep. 2017, 2017, bcr2017222012. [Google Scholar] [CrossRef]
- Gaspar, G.G.; Cocio, T.A.; Guioti-Puga, F.; Nascimento, E.; Fabro, A.T.; Kress, M.R.V.Z.; Bagagli, E.; Martinez, R. Paracoccidioidomycosis due to Paracoccidioides lutzii complicated with adrenal injury and pulmonary arterial hypertension. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e89. [Google Scholar] [CrossRef]
- Mahajan, V.K.; Raina, R.K.; Singh, S.; Rashpa, R.S.; Sood, A.; Chauhan, P.S.; Mehta, K.S.; Rawat, R.; Sharma, V. Case Report: Histoplasmosis in Himachal Pradesh (India): An Emerging Endemic Focus. Am. J. Trop. Med. Hyg. 2017, 97, 1749–1756. [Google Scholar] [CrossRef] [Green Version]
- Jayathilake, W.A.P.P.; Kumarihamy, K.W.M.P.P.; Ralapanawa, D.M.P.U.K.; Jayalath, W.A.T.A. A Rare Presentation of Possible Disseminated Histoplasmosis with Adrenal Insufficiency Leading to Adrenal Crisis in an Immunocompetent Adult: A Case Report. Case Rep. Med. 2020, 2020, 8506746. [Google Scholar]
- Jackson, C.; McCullar, B.; Joglekar, K.; Seth, A.; Pokharna, H. Disseminated Nocardia Farcinica Pneumonia with Left Adrenal Gland Abscess. Cureus 2017, 9, e1160. [Google Scholar] [CrossRef] [Green Version]
- Xydakis, A.M.; Chatzellis, E.; Kolomodi, D.; Kaltsas, G.A.; Alexandraki, K.I. Adrenal Failure and Orchitis Secondary to Tuberculosis Mimicking Metastatic Malignancy. Am. J. Med. 2020, 133, e518–e520. [Google Scholar] [CrossRef]
- Bender, K.; Waldie, A.M.; Asogan, M.; Figtree, M.C.; Sywak, M.S. Fungal granuloma: A case report of a rare cause for isolated adrenal incidentaloma. ANZ J. Surg. 2019, 89, E525–E526. [Google Scholar] [CrossRef]
- Yu, J.; Lu, Y.; Han, B. Primary adrenal insufficiency due to adrenal tuberculosis: A case report. J. Int. Med. Res. 2020, 48, 300060520980590. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Du, F.; Wang, J.; Bao, J.; Mi, J.; Sun, X. Primary unilateral and epilepsy adrenal tuberculosis misdiagnosed as adrenal tumor: Report of two cases. Asian J. Surg. 2021, 44, 1461–1463. [Google Scholar] [CrossRef] [PubMed]
- Ranawaka, N.; Welikumbura, N.H. Addison’s disease as a primary manifestation of extrapulmonary tuberculosis; A case report. Indian J. Tuberc. 2021, 68, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Ito, Y.; Osugi, Y.; Hashimoto, M.; Hashimoto, N.; Yano, K. Extrapulmonary pneumocystosis in an antiretroviral therapy-naïve, HIV-positive patient. Int. J. Infect. Dis. 2022, 120, 65–67. [Google Scholar] [CrossRef]
- Kusuki, K.; Watanabe, S.; Mizuno, Y. Tuberculous Addison’s disease with increased hydrocortisone requirements due to administration of rifampicin. BMJ Case Rep. 2019, 12, e228293. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Nehara, H.R.; Bhavi, V.K.; Maan, P.; Saran, S. Adrenal histoplasmosis in immunocompetent individuals a case series from the North-Western part of India, Rajasthan province: An emerging endemic focus. Indian J. Med. Microbiol. 2020, 38, 485–488. [Google Scholar] [CrossRef]
- Herndon, J.; Nadeau, A.M.; Davidge-Pitts, C.J.; Young, W.F.; Bancos, I. Primary adrenal insufficiency due to bilateral infiltrative disease. Endocrine 2018, 62, 721–728. [Google Scholar] [CrossRef]
- Aziz, H.; Adam, N.L.; Karim, N.A. Hypercalcaemia associated with disseminated cryptococcosis. BMJ Case Rep. 2021, 14, e245025. [Google Scholar] [CrossRef]
- Stankard, M.; Gopireddy, D.; Lall, C. Role of MRI in the Diagnosis of Large Right Adrenal Abscess. Cureus 2020, 12, e10986. [Google Scholar] [CrossRef]
- Kalinoski, T. Waterhouse-Friderichsen Syndrome with Bilateral Adrenal Hemorrhage Associated with Methicillin-Resistant Staphylococcus aureus (MRSA) Bacteremia in an Adult Patient with History of Intravenous Drug Use. Am. J. Case Rep. 2022, 23, e936096. [Google Scholar] [CrossRef]
- Motta, J.C.; Barrera, E.C. Acute adrenal insufficiency due to paracoccidiodomycosis. Report of 2 cases. Med. Mycol. Case Rep. 2020, 30, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.C. Reversible cerebellar ataxia and bipolar disorder secondary to tuberculous adrenalitis. Int. J. Mycobacteriol. 2021, 10, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, P.; Nallu, R.; Luthra, P. Histoplasmosis: An Unusual Cause of Adrenal Insufficiency. AACE Clin. Case Rep. 2020, 7, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, H.; Munawar, M.M.; Salih, O.; Deonarine, A. Disseminated coccidioidomycosis in a patient who is immunocompromised in the setting of immune reconstitution inflammatory syndrome. BMJ Case Rep. 2021, 14, e227217. [Google Scholar] [CrossRef]
- Van Bogaert, C.; Vierasu, I.; Mathey, C.; Theunissen, A.; Goldman, S. Bilateral cytomegalovirus infection of the adrenal glands revealed by 18F-FDG PET/CT in a patient with T-cell lymphoma. Clin. Case Rep. 2022, 10, e05005. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Goyal, A.; Agarwal, S.; Khadgawat, R. Hypercalcaemia, adrenal insufficiency and bilateral adrenal histoplasmosis in a middle-aged man: A diagnostic dilemma. BMJ Case Rep. 2019, 12, e231142. [Google Scholar] [CrossRef]
- Gaur, M.; Sethi, J.; Mitra, S.; Gupta, K. Adrenal histoplasmosis presenting as life-threatening adrenal insufficiency. BMJ Case Rep. 2021, 14, e243181. [Google Scholar] [CrossRef]
- Šimeková, K.; Rosoľanka, R.; Szilágyová, M.; Antolová, D.; Nováková, E.; Novák, M.; Laca, Ľ.; Sadloňová, J.; Šoltys, J. Alveolar Echinococcosis of the Liver with a Rare Infiltration of the Adrenal Gland. Helminthologia 2021, 58, 100–105. [Google Scholar] [CrossRef]
- Thijs, E.; Wierckx, K.; Vandecasteele, S.; Van den Bruel, A. Adrenal insufficiency, be aware of drug interactions! Endocrinol. Diabetes Metab. Case Rep. 2019, 2019, 19–0062. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Ma, Y.; Ban, R.; Shi, Q. Case Report: Diagnosis of Human Alveolar Echinococcosis via Next-Generation Sequencing Analysis. Front. Genet. 2021, 12, 666225. [Google Scholar] [CrossRef]
- Jiang, H.; Li, A.; Liao, S.; Ke, S.; Ji, Z.; Tian, M.; Zhang, H. Simultaneous adrenal tuberculosis and renal oncocytoma mimicking malignant masses incidentally detected by 18F-FDG PET/CT in a patient with lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.; Fan, B.; Wang, Y.; Wen, S.; Wang, H.; Liu, T.; Wang, L. Primary adrenal tuberculosis infection in patients with Behcet’s disease presenting as isolated adrenal metastasis by 18F-FDG PET/CT: A rare case report and literature review. Gland Surg. 2021, 10, 3431–3442. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.L.; Tjarks, B.J.; Berg, A.; Oliver, T. Disseminated Blastomycosis Mimicking Malignancy. S. Dak. Med. 2017, 70, 167–171. [Google Scholar]
- Zhou, J.; Lv, J.; Pan, Y.; Xie, J.; Zhang, Y. Unilateral Adrenal Cryptococcosis on FDG PET/CT. Clin. Nucl. Med. 2017, 42, 565–566. [Google Scholar] [CrossRef]
- Kesim, S.; Oksuzoglu, K.; Ozguven, S. Nocardia Infection with Adrenal Gland Abscess Mimicking Metastatic Lung Cancer on FDG PET/CT. Clin. Nucl. Med. 2023, 48, e24–e25. [Google Scholar] [CrossRef]
- Tejura, N.; Sonyey, A. CMV-associated adrenal insufficiency in a renal transplant recipient. IDCases 2017, 11, 44–45. [Google Scholar] [CrossRef]
- Jain, T.K.; Karunanithi, S.; Bal, C.; Kumar, R. 18F-FDG PET/CT Imaging in Adrenal Cryptococcosis. Clin. Nucl. Med. 2017, 42, e194–e195. [Google Scholar] [CrossRef]
- Cataño, J.; Porras, J. Adrenal Paracoccidioidomycosis. Am. J. Trop. Med. Hyg. 2020, 103, 935–936. [Google Scholar] [CrossRef]
- Sharma, N.; Ahlawat, R.S.; Singh, H.; Sharma, C.; Anuradha, S. Pneumocystis jirovecii infection of bilateral adrenal glands in an immunocompetent adult: A case report. J. R. Coll. Physicians Edinb. 2019, 49, 222–224. [Google Scholar] [CrossRef]
- Porntharukchareon, T.; Khahakaew, S.; Sriprasart, T.; Paitoonpong, L.; Snabboon, T. Bilateral Adrenal Histoplasmosis. Balk. Med. J. 2019, 36, 359–360. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Lee, H.W.; Kim, H.J. Paragonimus westermani infection manifesting as a pulmonary cavity and adrenal gland mass: A case report. J. Infect. Chemother. 2019, 25, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Tripathi, M. Addison’s disease due to histoplasmosis of bilateral adrenal glands in a previously treated extrapulmonary tuberculosis case. Indian J. Med. Res. 2020, 152 (Suppl. 1), S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Langmaid, T.; Jassal, K.; Meher-Homji, Z.; Lee, J.C.; Serpell, J.; Yeung, M.; McMahon, J.; Grodski, S. Disseminated nocardiosis with adrenal abscess masquerading as metastatic adrenal cancer in an immunocompetent adult. ANZ J. Surg. 2021, 91, E396–E398. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.E.; Shorman, M. A Case of Bilateral Nocardia francinia Adrenal Abscesses in an Intravenous Drug-Using Splenectomized Patient with Tricuspid Endocarditis. Open Forum Infect. Dis. 2018, 5, ofy141. [Google Scholar] [CrossRef]
- Pender, M.; Mehta, N.; Hamilton, B.D.; Swaminathan, S. Nocardia beijingensis Isolated from an Adrenal Abscess in a Diabetic Host. Open Forum Infect. Dis. 2022, 9, ofac328. [Google Scholar] [CrossRef]
- Yip, S.W.Y.; Li, Y.L.; Chu, Y.L.E.; Mak, J.Y.H.; Li, J.Y.Y.; Lee, K.-H.; Lee, R. Adrenal and renal abscesses following glue embolization of gastric varices: A case description. Quant. Imaging Med. Surg. 2020, 10, 1566–1569. [Google Scholar] [CrossRef]
- Rog, C.J.; Rosen, D.G.; Gannon, F.H. Bilateral adrenal histoplasmosis in an immunocompetent man from Texas. Med. Mycol. Case Rep. 2016, 14, 4–7. [Google Scholar] [CrossRef]
- Schrimashaw, N. Effect of infection on nutritional status. Proc. Natl. Sci. Counc. B Life Sci. 1992, 16, 46–64. [Google Scholar]
- Du Bois, E.F. The mechanism of Heat Loss and Temperature regulation. Ann. Intern. Med. 1938, 12, 338–395. [Google Scholar]
- Azzam, I.; Gilad, S.; Limor, R. Ghrelin stimulation by hypothalamic-pituitary-adrenal axis activation depends on increasing cortisol levels. Endocr. Connect. 2017, 6, 847–855. [Google Scholar] [CrossRef] [Green Version]
- Elshimy, G.; Chippa, V.; Jeong, J.M. Adrenal Crisis. 2023 Feb 3. In Stat Pearls (Internet); Stat Pearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sominsky, L.; Spencer, S.J. Eating behavior and stress: A pathway to obesity. Front. Psychol. 2014, 5, 434. [Google Scholar] [CrossRef] [PubMed]
- Erkut, Z.A.; Pool, C.; Swaab, D.F. Glucocorticoids suppress corticotropin-releasing hormone in human hypothalamic neurons. J. Clin. Endocrinol. Metab. 1998, 83, 2066–2073. [Google Scholar] [PubMed] [Green Version]
- El-Radhi, A.S. Pathogenesis of fever. In Clinical Manual of Fever in Children; Springer: Cham, Switzerland, 2019; pp. 53–68. [Google Scholar]
- Jang, W.; Sohn, Y.; Park, J.H.; Pai, H.; Kim, D.S.; Kim, B. Clinical Characteristics of Patients with Adrenal Insufficiency and Fever. J. Korean Med. Sci. 2021, 36, e152. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Kushner, I.; Samols, D. C-reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balli, S.; Shumway, K.R.; Sharan, S. Physiology, Fever. 2022 Sep 11. In StatPearls [Internet]; Stat Pearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Smith, S. The Role of hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Younes, N.; Bourdeau, I.; Lacroix, A. Latent Adrenal Insufficiency: From Concept to Diagnosis. Front. Endocrinol. 2021, 12, 720769. [Google Scholar] [CrossRef]
- Rana, M.V.; Candido, K.D.; Raja, O.; Knezevic, N.N. Celiac plexus block in the management of chronic abdominal pain. Curr. Pain Headache Rep. 2014, 18, 394. [Google Scholar] [CrossRef]
- Megha, R.; Wehrle, C.J.; Kashyap, S.; Leslie, S.W. Anatomy, Abdomen and Pelvis: Adrenal Glands (Suprarenal Glands) [Updated 2022 Oct 17]. In StatPearls [Internet]; Stat Pearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482264/ (accessed on 20 June 2023).
- Newhall, D.A.; Oliver, R.; Lugthart, S. Anaemia: A disease or symptom? Neth. J. Med. 2020, 78, 104–110. [Google Scholar]
- Sharma, S.; Nemeth, E.; Chen, Y.H.; Goodnough, J.; Huston, A.; Roodman, G.; Ganz, T.; Lichtenstein, A. Involvement of hepcidin in the anemia of multiple myeloma. Clin. Cancer Res. 2008, 14, 3262–3267. [Google Scholar] [CrossRef] [Green Version]
- De Mast, Q.; van Dongen-Leses, E.C.; Swinkles, D.W.; Nieman, A.E.; Roestenberg, M.; Druilhe, P.; Arens, T.A.; Luty, A.J.; Hermsen, C.C.; Sauerwein, R.W.; et al. Mild increases in serum hepcidin and interleukin -6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection. Br. J. Haematol. 2009, 145, 657–664. [Google Scholar] [CrossRef]
- Miller, C.B.; Jones, R.J.; Piantados, S.; Abeloff, M.D.; Spivak, J.L. Decreased erythropoietin response in patients with the anemia of cancer. N. Engl. J. Med. 1990, 322, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Mityling, B.L.; Singh, J.A.; Furne, J.K.; Ruddy, J.; Levitt, M.D. Use of breath carbon monoxide measurements to access erythrocyte survival in subjects with chronic diseases. Am. J. Hematol. 2006, 81, 432–438. [Google Scholar] [CrossRef]
- Gauer, R.; Forbes, D.; Boyer, N. Sepsis: Diagnosis and Management. Am. Fam. Physician 2020, 101, 409–418. [Google Scholar] [PubMed]
- Larkin, C.M.; Santos-Martinez, M.J.; Ryan, T.; Radomski, M.W. Sepsis-associated thrombocytopenia. Thromb. Res. 2016, 141, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Veneri, D.; Lippi, G. Thrombocytopenia and infections. Expert Rev. Hematol. 2017, 10, 99–106. [Google Scholar] [CrossRef]
- Bedet, A.; Razazi, K.; Boissier, F.; Surenaud, M.; Hue, S.; Giraudier, S.; Brun-Buisson, C.; Mekontso Dessap, A. Mechanisms of Thrombocytopenia During Septic Shock: A Multiplex Cluster Analysis of Endogenous Sepsis Mediators. Shock 2018, 49, 641–648. [Google Scholar] [CrossRef]
- Giustozzi, M.; Ehrlinder, H.; Bongiovanni, D.; Borovac, J.A.; Guerreiro, R.A.; Gąsecka, A.; Papakonstantinou, P.E.; Parker, W.A. Coagulopathy and sepsis: Pathophysiology, clinical manifestations and treatment. Blood Rev. 2021, 50, 100864. [Google Scholar] [CrossRef]
- Tsirigotis, P.; Chondropoulos, S.; Frantzeskaki, F.; Stamouli, M.; Gkirkas, K.; Bartzeliotou, A.; Papanikolaou, N.; Atta, M.; Papassotiriou, I.; Dimitriadis, G.; et al. Thrombocytopenia in critically ill patients with severe sepsis/septic shock: Prognostic value and association with a distinct serum cytokine profile. J. Crit. Care 2016, 32, 9–15. [Google Scholar] [CrossRef]
- Johansson, D.; Rasmussen, M.; Inghammar, M. Thrombocytopenia in bacteraemia and association with bacterial species. Epidemiol. Infect. 2018, 146, 1312–1317. [Google Scholar] [CrossRef] [Green Version]
- Oelkers, W. Adrenal insufficiency. N. Engl. J. Med. 1996, 335, 1206–1212. [Google Scholar] [CrossRef]
- Adrogué, H.J.; Tucker, B.M.; Madias, N.E. Diagnosis and Management of Hyponatremia: A Review. JAMA 2022, 328, 280–291. [Google Scholar] [CrossRef]
- Liamis, G.; Milionis, H.J.; Elisaf, M. Endocrine disorders: Causes of hyponatremia not to neglect. Ann. Med. 2011, 43, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Masuyama, H.; Yamagata, H.; Miyabayashi, M.; Onishi, S.; Inaba, Y.; Takemoto, M. The Incidence and Risk Factors of Hyponatremia in Pulmonary Tuberculosis. J. Endocr. Soc. 2022, 6, bvac130. [Google Scholar] [CrossRef] [PubMed]
- Orth, D.N. Adrenal insufficiency. Curr. Ther. Endocrinol. Metab. 1994, 5, 124–130. [Google Scholar] [PubMed]
- Napier, C.; Pearce, S.H. Autoimmune Addison’s disease. Presse Med. 2012, 41 Pt 2, e626–e635. [Google Scholar] [CrossRef] [PubMed]
- Betterle, C.; Morlin, L. Autoimmune Addison’s disease. Endocr. Dev. 2011, 20, 161–172. [Google Scholar]
- Grabarczyk, M.; Gorczyca, M.; Cieślik, P.; Hrycek, A.; Holecki, M. Addison’s Disease in the Course of Recurrent Microangiopathic Antiphospholipid Syndrome—A Clinical Presentation and Review of the Literature. Medicina 2023, 59, 4. [Google Scholar] [CrossRef]
- Espinosa, G.; Cervera, R.; Font, J.; Asherson, R.A. Adrenal involvement in the antiphospholipid syndrome. Lupus 2003, 12, 569–572. [Google Scholar] [CrossRef]
- Tallis, P.H.; Rushworth, R.L.; Torpy, D.J.; Falhammar, H. Adrenal insufficiency due to bilateral adrenal metastases—A systematic review and meta-analysis. Heliyon 2019, 5, e01783. [Google Scholar] [CrossRef] [Green Version]
- Alevritis, E.M.; Sarubbi, F.A.; Jordan, R.M.; Peiris, A.N. Infectious causes of adrenal insufficiency. South Med. J. 2003, 96, 888–890. [Google Scholar] [CrossRef]
- Paolo, W.F., Jr.; Nosanchuk, J.D. Adrenal infections. Int. J. Infect. Dis. 2006, 10, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arlt, W.; Allolio, B. Adrenal insufficiency. Lancet 2003, 361, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Araúz, A.B.; Papineni, P. Histoplasmosis. Infect. Dis. Clin. N. Am. 2021, 35, 471–491. [Google Scholar] [CrossRef]
- Maiga, A.W.; Deppen, S.; Scaffidi, B.K.; Baddley, J.; Aldrich, M.C.; Dittus, R.S.; Grogan, E.L. Mapping Histoplasma capsulatum Exposure, United States. Emerg. Infect. Dis. 2018, 24, 1835–1839. [Google Scholar] [CrossRef] [Green Version]
- Azar, M.M.; Loyd, J.L.; Relich, R.F.; Wheat, L.J.; Hage, C.A. Current Concepts in the Epidemiology, Diagnosis, and Management of Histoplasmosis Syndromes. Semin. Respir. Crit. Care Med. 2020, 41, 13–30. [Google Scholar] [CrossRef]
- Benedict, K.; Derado, G.; Mody, R.K. Histoplasmosis-Associated Hospitalizations in the United States, 2001–2012. Open Forum Infect. Dis. 2016, 3, ofv219. [Google Scholar] [CrossRef] [Green Version]
- Mallereau, C.H.; Todeschi, J.; Ganau, M.; Cebula, H.; Bozzi, M.T.; Romano, A.; Le Van, T.; Ollivier, I.; Zaed, I.; Spatola, G.; et al. Pituitary Abscess: A Challenging Preoperative Diagnosis—A Multicenter Study. Medicina 2023, 59, 565. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Farwell, A.P.; Braverman, L.E. Thyroiditis. N. Engl. J. Med. 2003, 348, 2646–2655. [Google Scholar] [CrossRef]
- Lesh, R.; Hellums, R.; Pichardo, P.; Wong, J.; Pellitteri, P.; Purdy, N.; Stavrides, K.; Haugen, T.W. Thyroid Abscess: A Case Series and Literature Review. Ear Nose Throat J. 2023, 5, 1455613221150128. [Google Scholar] [CrossRef]
- Yin, D.; Ji, C.; Zhang, S.; Wang, J.; Lu, Z.; Song, X.; Jiang, H.; Lau, W.Y.; Liu, L. Clinical characteristics and management of 1572 patients with pyogenic liver abscess: A 12-year retrospective study. Liver Int. 2021, 41, 810–818. [Google Scholar] [CrossRef]
- Singh, A.K.; Karmani, S.; Samanta, J.; Gupta, P.; Gupta, V.; Yadav, T.D.; Kumari, S.; Dutta, U.; Sinha, S.K.; Kochhar, R. Splenic abscess in a tertiary care center in India: Clinical characteristics and prognostic factors. ANZ J. Surg. 2021, 91, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.N.; Roug, S.; Hansen, E.F.; Knudsen, J.D.; Novovic, S. Spectrum of microorganisms in infected walled-off pancreatic necrosis—Impact on organ failure and mortality. Pancreatology 2014, 14, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Mowbray, N.G.; Ben-Ismaeil, B.; Hammoda, M.; Shingler, G.; Al-Sarireh, B. The microbiology of infected pancreatic necrosis. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Rubilotta, E.; Balzarro, M.; Lacola, V.; Sarti, A.; Porcaro, A.; Artibani, W. Current clinical management of renal and perinephric abscesses: A literature review. Urologia 2014, 81, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Beishuizen, A.; Thijs, L.G. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J. Endotoxin. Res. 2003, 9, 3–24. [Google Scholar] [PubMed]
- Nacher, M.; Alsibai, K.D. HIV associated Disseminated Histoplasmosis and Rare Adrenal Involvement: Evidence of Absence of Evidence. Front. Cell. Infect. Microbiol. 2021, 11, 619459. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Kubiha, S.; Tyagi, P. Fungi and Endocrine Dysfunction. (Updated 2021 Jun 25). In Endotext (Internet); Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572246/ (accessed on 28 March 2023).
- Frenkel, J.K. Role of corticosteroids as predisposing factors in fungal diseases. Lab Invest. J. Tech. Methods Pathol. 1962, 11, 1192–1208. [Google Scholar]
- Vinnard, C.; Blumberg, E.A. Endocrine and Metabolic Aspects of Tuberculosis. Microbiol. Spectr. 2017, 5, 436–447. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.T.; Horton, K.M.; Fishman, E.K. Adrenal mass imaging with multidetector CT: Pathologic conditions, pearls, and pitfalls. Radiographics 2009, 29, 1333–1351. [Google Scholar] [CrossRef]
- Papanicolas, I.; Woskie, L.R.; Jha, A.K. Health Care Spending in the United States and Other High-Income Countries. JAMA 2018, 319, 1024–1039. [Google Scholar] [CrossRef]
- Osa, S.R.; Peterson, R.E.; Roberts, R.B. Recovery of adrenal reserve following treatment of disseminated South American blastomycosis. Am. J. Med. 1981, 71, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Rana, C.; Kumari, N.; Krishnani, N. Adrenal histoplasmosis: A diagnosis on fine needle aspiration cytology. Diagn. Cytopathol. 2011, 39, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, C.S.; Mescher, T.; Bubak, A.N.; Medina, E.M.; Hassell, J.E., Jr.; Nagel, M.A. VZV Infection of Primary Human Adrenal Cortical Cells Produces a Proinflammatory Environment without Cell Death. Viruses 2022, 14, 674. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gligorijevic, N.; Kaljevic, M.; Radovanovic, N.; Jovanovic, F.; Joksimovic, B.; Singh, S.; Dumic, I. Adrenal Abscesses: A Systematic Review of the Literature. J. Clin. Med. 2023, 12, 4601. https://doi.org/10.3390/jcm12144601
Gligorijevic N, Kaljevic M, Radovanovic N, Jovanovic F, Joksimovic B, Singh S, Dumic I. Adrenal Abscesses: A Systematic Review of the Literature. Journal of Clinical Medicine. 2023; 12(14):4601. https://doi.org/10.3390/jcm12144601
Chicago/Turabian StyleGligorijevic, Nikola, Marija Kaljevic, Natasa Radovanovic, Filip Jovanovic, Bojan Joksimovic, Sandra Singh, and Igor Dumic. 2023. "Adrenal Abscesses: A Systematic Review of the Literature" Journal of Clinical Medicine 12, no. 14: 4601. https://doi.org/10.3390/jcm12144601






