Incidence and Predictors of Ventricular Arrhythmias in Transthyretin Amyloid Cardiomyopathy
Abstract
:1. Background
2. Methods
2.1. Study Cohort
2.2. Assessment of Ventricular Arrhythmias
2.3. ECG, Echocardiography and Laboratory Analyses
2.4. Statistical Analysis
3. Results
3.1. Prevalence of Arrhythmias on Holter
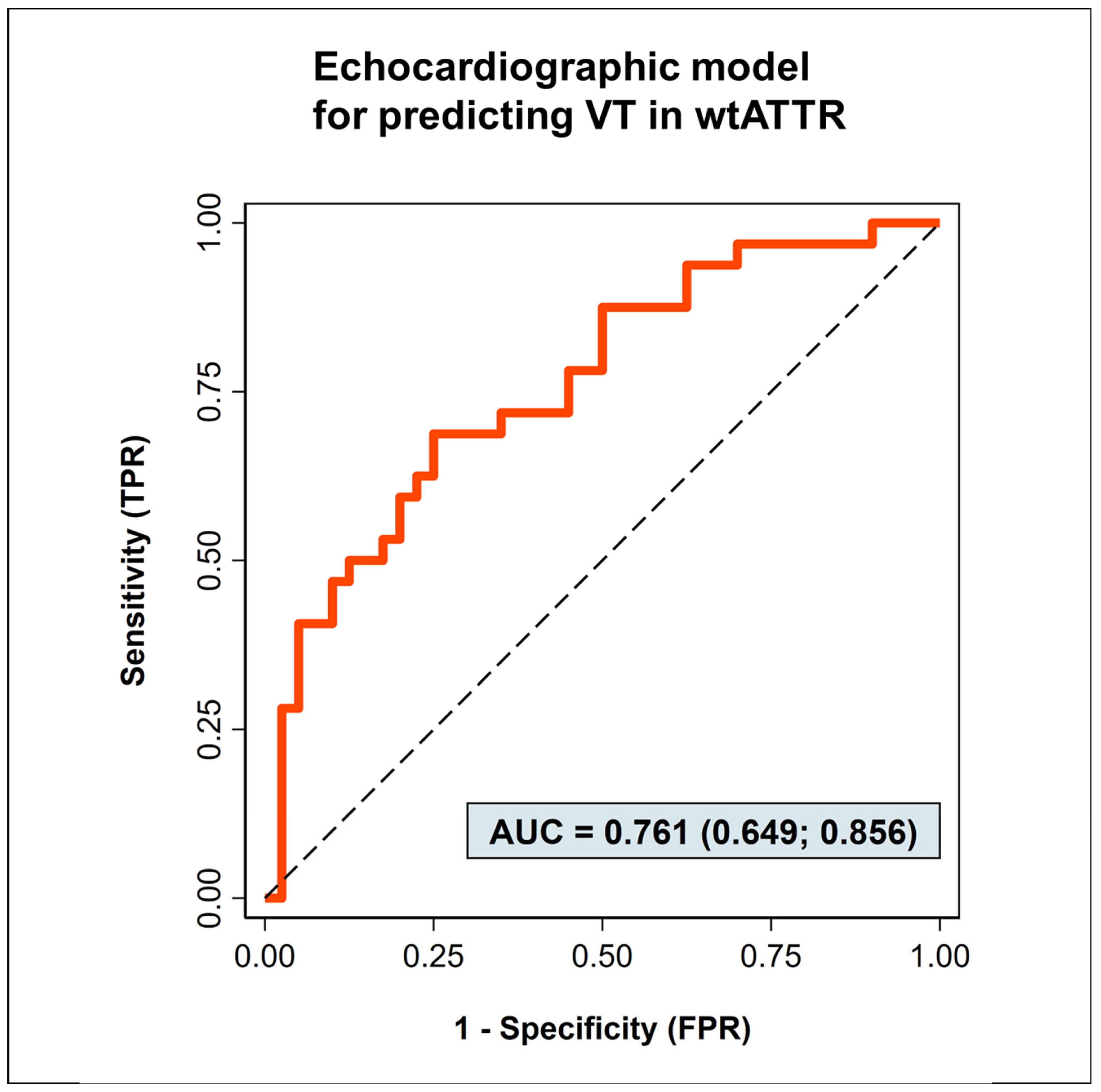
3.2. Predictors of Ventricular Tachycardias
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e7–e22. [Google Scholar] [CrossRef] [PubMed]
- González-López, E.; Gallego-Delgado, M.; Guzzo-Merello, G.; de Haro-Del Moral, F.J.; Cobo-Marcos, M.; Robles, C.; Bornstein, B.; Salas, C.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015, 36, 2585–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappelli, F.; Vignini, E.; Martone, R.; Perlini, S.; Mussinelli, R.; Sabena, A.; Morini, S.; Gabriele, M.; Taborchi, G.; Bartolini, S.; et al. Baseline ECG Features and Arrhythmic Profile in Transthyretin versus Light Chain Cardiac Amyloidosis. Circ. Heart Fail. 2020, 13, e006619. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, Y.B.; Liu, J.; Chou, J.; Hoffman, J.; Comenzo, R.L.; Steingart, R.M. Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am. J. Cardiol. 2009, 104, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Malamani, G.; Cò, F.; Pistorio, A.; Recusani, F.; Anesi, E.; Garini, P.; Merlini, G. Holter monitoring in AL amyloidosis: Prognostic implications. Pacing Clin. Electrophysiol. 2001, 24, 1228–1233. [Google Scholar] [CrossRef]
- Hörnsten, R.; Wiklund, U.; Olofsson, B.O.; Jensen, S.M.; Suhr, O.B. Liver transplantation does not prevent the development of life-threatening arrhythmia in familial amyloidotic polyneuropathy, Portuguese-type (ATTR Val30Met) patients. Transplantation 2004, 78, 112–116. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Hanna, M.; Saliba, W.; Jaber, W.; Kanj, M. Primary prevention implantable cardioverter-defibrillators in transthyretin cardiac amyloidosis. Pacing Clin. Electrophysiol. 2020, 43, 1401–1403. [Google Scholar] [CrossRef]
- Dale, Z.; Al-Rashdan, L.; Elman, M.; Chandrashekar, P.; Heitner, S.B.; Nazer, B.; Masri, A. Mode of death and outcomes of implantable cardioverter defibrillators in transthyretin amyloid cardiomyopathy. Int. J. Cardiol. 2022, 349, 99–102. [Google Scholar] [CrossRef]
- Brown, M.T.; Yalamanchili, S.; Evans, S.T.; Ram, P.; Blank, E.A.; Lyle, M.A.; Merchant, F.M.; Bhatt, K.N. Ventricular arrhythmia burden and implantable cardioverter-defibrillator outcomes in transthyretin cardiac amyloidosis. Pacing Clin. Electrophysiol. 2022, 45, 443–451. [Google Scholar] [CrossRef]
- Higgins, A.Y.; Annapureddy, A.R.; Wang, Y.; Minges, K.E.; Lampert, R.; Rosenfeld, L.E.; Jacoby, D.L.; Curtis, J.P.; Miller, E.J.; Freeman, J.V. Survival Following Implantable Cardioverter-Defibrillator Implantation in Patients With Amyloid Cardiomyopathy. J. Am. Heart Assoc. 2020, 9, e016038. [Google Scholar] [CrossRef]
- Kim, E.J.; Holmes, B.B.; Huang, S.; Lugo, R.; Al Aboud, A.; Goodman, S.; Hung, R.R.; Slosky, D.; Stevenson, W.G.; Michaud, G.F.; et al. Outcomes in patients with cardiac amyloidosis and implantable cardioverter-defibrillator. Europace 2020, 22, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, J.; Jaber, W.; Maurer, M.; Sperry, B.; Hanna, M.; Collier, P.; Patel, D.R.; Wazni, O.M.; Donnellan, E. Electrophysiological Manifestations of Cardiac Amyloidosis: JACC: CardioOncology State-of-the-Art Review. JACC Cardio Oncol. 2021, 3, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Ioannou, A.; Patel, R.K.; Razvi, Y.; Porcari, A.; Sinagra, G.; Venneri, L.; Bandera, F.; Masi, A.; Williams, G.E.; O’Beara, S.; et al. Impact of Earlier Diagnosis in Cardiac ATTR Amyloidosis Over the Course of 20 Years. Circulation 2022, 146, 1657–1670. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, F.; Cipriani, A.; Russo, D.; Tini, G.; Zampieri, M.; Zocchi, C.; Sinigiani, G.; Tassetti, L.; Licchelli, L.; Perfetto, F.; et al. Prevalence and prognostic role of nonsustained ventricular tachycardia in cardiac amyloidosis. Amyloid 2022, 29, 211–212. [Google Scholar] [CrossRef]
- Lin, G.; Dispenzieri, A.; Kyle, R.; Grogan, M.; Brady, P.A. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J. Cardiovasc. Electrophysiol. 2013, 24, 793–798. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Kuo, M.J.; Chung, F.P.; Lin, Y.J.; Chien, K.L.; Hsieh, Y.C.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Chao, T.F.; et al. Risks of Ventricular Tachyarrhythmia and Mortality in Patients with Amyloidosis—A Long-Term Cohort Study. Acta Cardiol. Sin. 2022, 38, 464–474. [Google Scholar] [CrossRef]
- Varr, B.C.; Zarafshar, S.; Coakley, T.; Liedtke, M.; Lafayette, R.A.; Arai, S.; Schrier, S.L.; Witteles, R.M. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm 2014, 11, 158–162. [Google Scholar] [CrossRef]
- Thakkar, S.; Patel, H.P.; Chowdhury, M.; Patel, K.; Kumar, A.; Arora, S.; Zahid, S.; Goel, M.; Barssoum, K.; Jain, V.; et al. Impact of Arrhythmias on Hospitalizations in Patients With Cardiac Amyloidosis. Am. J. Cardiol. 2021, 143, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, H.; Zhu, C.; Gu, K.; Yang, G.; Chen, H.; Ju, W.; Li, M.; Zhang, F.; Yang, B.; et al. Clinical characteristics and therapeutic strategy of frequent accelerated idioventricular rhythm. BMC Cardiovasc. Disord. 2021, 21, 425. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devereux, R.B.; Reichek, N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977, 55, 613–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Van Smeden, M.; Moons, K.G.; de Groot, J.A.; Collins, G.S.; Altman, D.G.; Eijkemans, M.J.; Reitsma, J.B. Sample size for binary logistic prediction models: Beyond events per variable criteria. Stat. Methods Med. Res. 2019, 28, 2455–2474. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Pavia, P.; Bengel, F.; Brito, D.; Damy, T.; Duca, F.; Dorbala, S.; Nativi-Nicolau, J.; Obici, L.; Rapezzi, C.; Sekijima, Y.; et al. Expert consensus on the monitoring of transthyretin amyloid cardiomyopathy. Eur. J. Heart Fail. 2021, 23, 895–905. [Google Scholar] [CrossRef]
- Kocher, F.; Kaser, A.; Escher, F.; Doerler, J.; Zaruba, M.M.; Messner, M.; Mussner-Seeber, C.; Mayr, A.; Ulmer, H.; Schneiderbauer-Porod, S.; et al. Heart failure from ATTRwt amyloid cardiomyopathy is associated with poor prognosis. ESC Heart Fail. 2020, 7, 3919–3928. [Google Scholar] [CrossRef]
- Chacko, L.; Karia, N.; Venneri, L.; Bandera, F.; Passo, B.D.; Buonamici, L.; Lazari, J.; Ioannou, A.; Porcari, A.; Patel, R.; et al. Progression of echocardiographic parameters and prognosis in transthyretin cardiac amyloidosis. Eur. J. Heart Fail. 2022, 24, 1700–1712. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Damy, T.; Fontana, M.; Hutchinson, M.; Lachmann, H.J.; Martinez-Naharro, A.; Quarta, C.C.; Rezk, T.; Whelan, C.J.; Gonzalez-Lopez, E.; et al. A new staging system for cardiac transthyretin amyloidosis. Eur. Heart J. 2018, 39, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.; Scott, C.G.; Kyle, R.A.; Zeldenrust, S.R.; Gertz, M.A.; Lin, G.; Klarich, K.W.; Miller, W.L.; Maleszewski, J.J.; Dispenzieri, A. Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J. Am. Coll. Cardiol. 2016, 68, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
| ATTR-CM without Ventricular Tachycardia | ATTR-CM with Ventricular Tachycardia | p-Value | |
|---|---|---|---|
| n = 40 | n = 32 | ||
| Clinical characteristics | |||
| Age (years; median, IQR) | 81 (76–84) | 80 (75–82) | 0.385 |
| Gender (male; n, %) | 38 (95) | 31 (97) | 1.000 |
| Body mass index (kg/m2; median, IQR) | 24.8 (23.3–27.0) | 24.6 (23.1–28.1) | 0.882 |
| Angina pectoris (n, %) | 6 (15) | 6 (19) | 0.756 |
| Dyspnea (n, %) | 32 (80) | 30 (94) | 0.169 |
| Syncope (n, %) | 4 (10) | 3 (9) | 1.000 |
| Systolic blood pressure (mmHg; median, IQR) | 135 (122–148) | 140 (125–149) | 0.489 |
| Medication at baseline | |||
| Beta blocker (n, %) | 23 (58) | 24 (75) | 0.142 |
| ARNI (n, %) | 2 (5.0) | 2 (6.3) | 1.000 |
| ACE-inhibitor or AT-receptor antagonist (n, %) | 26 (65) | 22 (69) | 0.805 |
| Mineralocorticoid receptor-antagonist (n, %) | 16 (40) | 15 (47) | 0.635 |
| SGLT2-antagonist (n, %) | 7 (18) | 2 (6.3) | 0.282 |
| Diuretic (n, %) | 28 (70) | 26 (81) | 0.412 |
| Amiodarone (n, %) | 1 (2.5) | 0 (0) | 1.000 |
| Tafamidis during Holter (n, %) | 2 (5) | 10 (31) | 0.004 |
| Tafamidis at follow-up (n, %) | 25 (63) | 24 (75) | 0.402 |
| Comorbidities | |||
| Coronary artery disease (n, %) | 23 (58) | 19 (59) | 0.611 |
| Atrial fibrillation (n, %) | 25 (63) | 20 (63) | 1.000 |
| Pacemaker (n, %) | 6 (15) | 2 (6.3) | 0.287 |
| Implantable cardioverter defibrillator (ICD, n, %) | 2 (5.0) | 1 (3.1) | 1.000 |
| Cardiac resynchronization therapy (CRT, n, %) | 1 (2.5) | 1 (3.1) | 1.000 |
| Laboratory values | |||
| NTproBNP (pg/mL; median, IQR) | 2945 (1615–5900) | 3100 (1705–4875) | 0.825 |
| Troponin T (ng/L; median, IQR) | 46 (32–63) | 53 (42–70) | 0.141 |
| Creatinine (mg/dL; median, IQR) | 1.3 (1.03–1.62) | 1.1 (1.07–1.32) | 0.109 |
| GFR (ml/min; median, IQR) | 56 (43–75) | 63 (55–74) | 0.245 |
| Potassium (K+, mmol/L; median, IQR) | 4.37 (4.05–4.62) | 4.18 (3.96–4.43) | 0.124 |
| Sodium (Na2+, mmol/L; median, IQR) | 139 (137–141) | 140 (138–142) | 0.082 |
| ATTR-CM without Ventricular Tachycardia | ATTR-CM with Ventricular Tachycardia | p-Value | |
|---|---|---|---|
| n = 40 | n = 32 | ||
| Echocardiography (all: median, IQR) | |||
| Interventricular septal diameter (mm) | 19 (17–20) | 21 (19–22) | 0.006 |
| Left ventricular end-diastolic diameter (mm) | 43 (39–48) | 44 (39–49) | 0.972 |
| Left ventricular end-systolic diameter (mm) | 31 (27–36) | 33 (30–40) | 0.063 |
| Left ventricular posterior wall diameter (mm) | 15 (13–17) | 17 (15–19) | 0.026 |
| LV mass indexed for body surface area (g/m2) | 170 (132–199) | 198 (175–221) | 0.063 |
| Ejection fraction (Simpson, %) | 55 (48–57) | 47 (41–52) | 0.014 |
| TAPSE (mm) | 13.5 (12–18) | 14.0 (10–15) | 0.470 |
| Left atrial area (mm2) | 28 (24–32) | 31 (27–36) | 0.020 |
| Right atrial area (mm2) | 24 (21–29) | 24 (22–39) | 0.430 |
| E/e’ | 18.3 (13.8–21.5) | 17.7 (13.6–23.1) | 0.773 |
| GLS | −9.3 (−11.4–−7.9) | −8.4 (−11.5–−6.1) | 0.533 |
| Electrocardiogram | |||
| QRS-complex duration (ms; median, IQR) | 118 (103–140) | 116 (103–140) | 0.830 |
| QTc-interval duration (ms; median, IQR) | 481 (462–507) | 489 (468–509) | 0.325 |
| T wave inversion (n, %) | 9 (23) | 5 (15) | 0.553 |
| LBBB (n, %) | 2 (5) | 1 (3) | 1.000 |
| RBBB (n, %) | 5 (13) | 5 (16) | 0.746 |
| LAHB (n, %) | 12 (31) | 17 (53) | 0.089 |
| LPHB (n, %) | 6 (15) | 0 (0) | 0.029 |
| Low-voltage pattern (n, %) | 13 (33) | 7 (21) | 0.375 |
| Variables Included in the Multivariable Model | p-Value | Odds Ratio [95% Confidence Interval] |
|---|---|---|
| Clinical parameters | ||
| Age | 0.858 | - |
| BMI | 0.499 | - |
| Coronary artery disease | 0.814 | - |
| Dyspnoea (NYHA) | 0.732 | - |
| Syncope | 0.651 | - |
| NTproBNP (pg/mL) | 0.778 | - |
| Troponin T (ng/L) | 0.058 | 1.02 [0.999–1.05] |
| GFR (ml/min) | 0.073 | 1.03 [0.997–1.06] |
| Systolic blood pressure | 0.765 | - |
| Echocardiographic parameters | ||
| Interventricular septal diameter (mm) | 0.763 | - |
| Left ventricular end-diastolic diameter (mm) | 0.021 | 0.85 [0.74–0.98] |
| Left ventricular end-systolic diameter (mm) | 0.022 | 1.19 [1.03–1.39] |
| Left ventricular posterior wall diameter (mm) | 0.591 | - |
| LV mass indexed for body surface area (g/m2) | 0.031 | 1.02 [1.001–1.03] |
| Ejection fraction (Simpson, %) | 0.422 | - |
| TAPSE (mm) | 0.381 | - |
| Left atrial area (mm2) | 0.257 | - |
| Right atrial area (mm2) | 0.553 | - |
| E/e’ | 0.306 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoll, K.; Fuchs, P.; Weidmann, I.; Altunkas, F.; Voss, S.; Lennerz, C.; Kolb, C.; Kessler, T.; Schunkert, H.; Reinhard, W.; et al. Incidence and Predictors of Ventricular Arrhythmias in Transthyretin Amyloid Cardiomyopathy. J. Clin. Med. 2023, 12, 4624. https://doi.org/10.3390/jcm12144624
Knoll K, Fuchs P, Weidmann I, Altunkas F, Voss S, Lennerz C, Kolb C, Kessler T, Schunkert H, Reinhard W, et al. Incidence and Predictors of Ventricular Arrhythmias in Transthyretin Amyloid Cardiomyopathy. Journal of Clinical Medicine. 2023; 12(14):4624. https://doi.org/10.3390/jcm12144624
Chicago/Turabian StyleKnoll, Katharina, Patrick Fuchs, Isabel Weidmann, Fatih Altunkas, Stephanie Voss, Carsten Lennerz, Christof Kolb, Thorsten Kessler, Heribert Schunkert, Wibke Reinhard, and et al. 2023. "Incidence and Predictors of Ventricular Arrhythmias in Transthyretin Amyloid Cardiomyopathy" Journal of Clinical Medicine 12, no. 14: 4624. https://doi.org/10.3390/jcm12144624





