Safety Profile of Vitamin D in Italy: An Analysis of Spontaneous Reports of Adverse Reactions Related to Drugs and Food Supplements
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic and Clinical Data
3.2. AR Reports from the Italian Phytovigilance System (ISS)
3.3. ADR Reports from the Italian Pharmacovigilance system (AIFA)
3.4. Seriousness and Causality Assessment
3.5. Risk Factors for Serious ARs
3.6. VitDps and Pharmacological Interactions
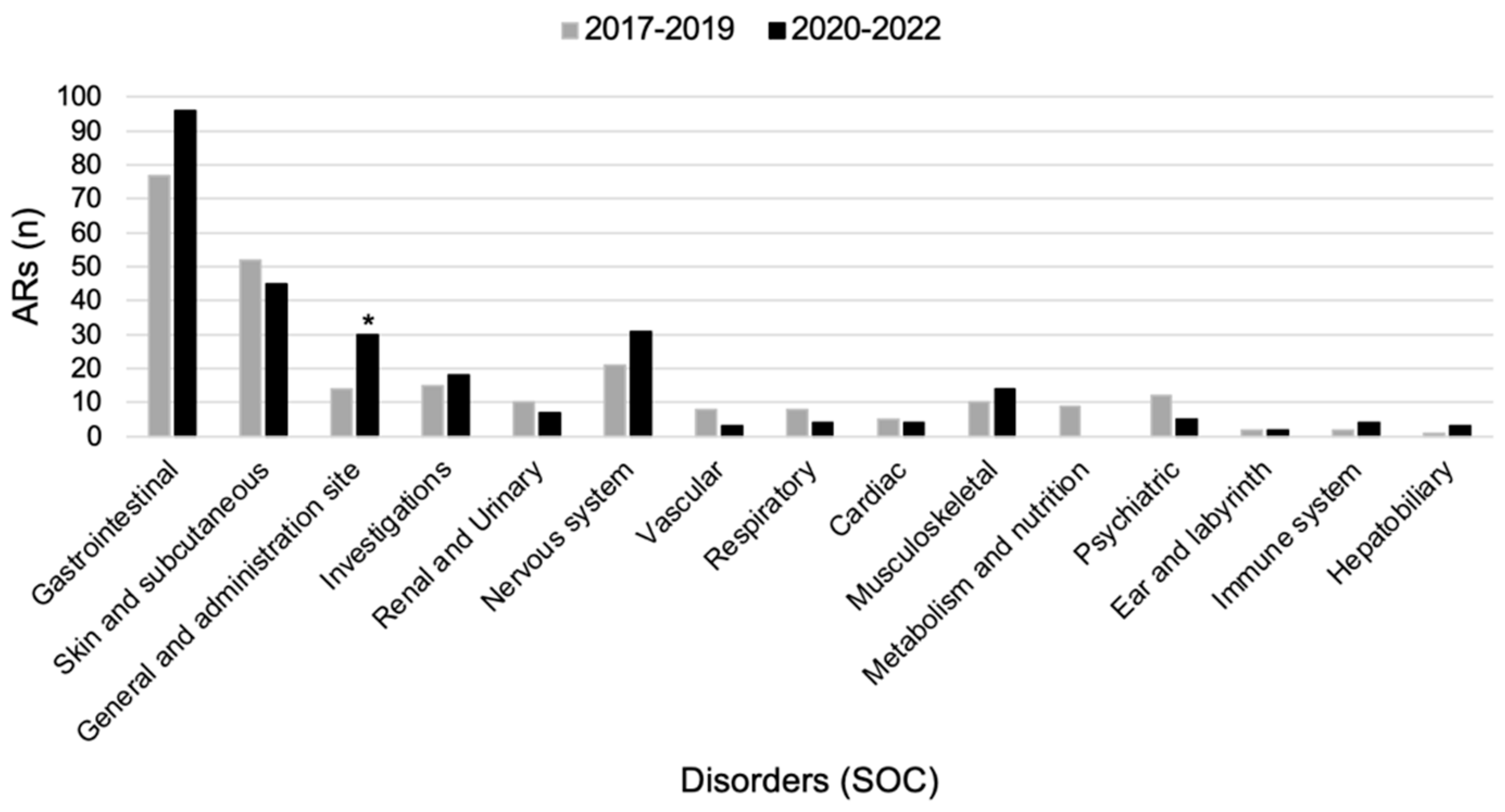
3.7. ARs Comparison between Pre-COVID-19 and during COVID-19 Pandemic
4. Discussion
4.1. Pharmacological Interactions and Dosage
4.2. COVID-19 Pandemic Influence
4.3. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, S.H.; Jang, H.N.; Kim, J.H.; Kim, S.W.; Shin, C.S. Effect of Vitamin D Supplementation on Risk of Fractures and Falls According to Dosage and Interval: A Meta-Analysis. Endocrinol. Metab. 2022, 37, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kuang, X.; Li, K.; Guo, X.; Deng, Q.; Li, D. Effects of Combined Calcium and Vitamin D Supplementation on Osteoporosis in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Food Funct. 2020, 11, 10817–10827. [Google Scholar] [CrossRef] [PubMed]
- Manoj, P.; Derwin, R.; George, S. What Is the Impact of Daily Oral Supplementation of Vitamin D3 (cholecalciferol) plus Calcium on the Incidence of Hip Fracture in Older People? A Systematic Review and Meta-Analysis. Int. J. Older People Nurs. 2023, 18, e12492. [Google Scholar] [CrossRef] [PubMed]
- LeBoff, M.S.; Chou, S.H.; Ratliff, K.A.; Cook, N.R.; Khurana, B.; Kim, E.; Cawthon, P.M.; Bauer, D.C.; Black, D.; Gallagher, J.C.; et al. Supplemental Vitamin D and Incident Fractures in Midlife and Older Adults. N. Engl. J. Med. 2022, 387, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sánchez, L.; Clark, P.; Winzenberg, T.M.; Tugwell, P.; Correa-Burrows, P.; Costello, R. Calcium and Vitamin D for Increasing Bone Mineral Density in Premenopausal Women. Cochrane Database Syst. Rev. 2023, 1, CD012664. [Google Scholar] [CrossRef] [Green Version]
- Bischoff-Ferrari, H.A.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; da Silva, J.A.P.; Blauth, M.; Felson, D.T.; McCloskey, E.V.; Watzl, B.; Hofbauer, L.C.; et al. Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults. JAMA 2020, 324, 1855. [Google Scholar] [CrossRef]
- Nota 96. Available online: https://aifa.gov.it/nota-96 (accessed on 28 February 2023).
- Hussain, S.; Yates, C.; Campbell, M.J. Vitamin D and Systems Biology. Nutrients 2022, 14, 5197. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Srifuengfung, M.; Srifuengfung, S.; Pummangura, C.; Pattanaseri, K.; Oon-Arom, A.; Srisurapanont, M. Efficacy and Acceptability of Vitamin D Supplements for Depressed Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrition 2023, 108, 111968. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Agah, S.; Alibakhshi, P.; Heydari, H.; Hoseini, A.S.; Palmowski, A.; Toupchian, O.; Abdollahi, S.; Rezamand, G.; Heshmati, J. Effects of Calcium and Vitamin D Co-Supplementation on the Lipid Profile: A Systematic Review and Meta-Analysis. Clin. Ther. 2021, 43, 274–296. [Google Scholar] [CrossRef]
- Wu, C.; Song, Y.; Wang, X. Vitamin D Supplementation for the Outcomes of Patients with Gestational Diabetes Mellitus and Neonates: A Meta-Analysis and Systematic Review. Int. J. Clin. Pract. 2023, 2023, 1907222. [Google Scholar] [CrossRef]
- Farapti, F.; Fadilla, C.; Yogiswara, N.; Adriani, M. Effects of Vitamin D Supplementation on 25(OH)D Concentrations and Blood Pressure in the Elderly: A Systematic Review and Meta-Analysis. F1000Research 2020, 9, 633. [Google Scholar]
- Zhang, Y.; Xue, Y.; Zhang, D.; Liu, Y.; Xu, Z.; Gao, J.; Li, W.; Li, X. Effect of Vitamin D Supplementation on Glycemic Control in Prediabetes: A Meta-Analysis. Nutrients 2021, 13, 4464. [Google Scholar] [CrossRef]
- Kazemi, A.; Ryul Shim, S.; Jamali, N.; Hassanzadeh-Rostami, Z.; Soltani, S.; Sasani, N.; Mohsenpour, M.A.; Firoozi, D.; Basirat, R.; Hosseini, R.; et al. Comparison of Nutritional Supplements for Glycemic Control in Type 2 Diabetes: A Systematic Review and Network Meta-Analysis of Randomized Trials. Diabetes Res. Clin. Pract. 2022, 191, 110037. [Google Scholar] [CrossRef]
- Fu, J.; Sun, J.; Zhang, C. Vitamin D Supplementation and Risk of Stroke: A Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2022, 13, 970111. [Google Scholar] [CrossRef]
- Kalaitzopoulos, D.R.; Samartzis, N.; Daniilidis, A.; Leeners, B.; Makieva, S.; Nirgianakis, K.; Dedes, I.; Metzler, J.M.; Imesch, P.; Lempesis, I.G. Effects of Vitamin D Supplementation in Endometriosis: A Systematic Review. Reprod. Biol. Endocrinol. 2022, 20, 176. [Google Scholar] [CrossRef]
- Hao, M.; Xu, R.; Luo, N.; Liu, M.; Xie, J.; Zhang, W. The Effect of Vitamin D Supplementation in Children With Asthma: A Meta-Analysis. Front. Pediatr. 2022, 10, 840617. [Google Scholar] [CrossRef]
- Williamson, A.; Martineau, A.R.; Sheikh, A.; Jolliffe, D.; Griffiths, C.J. Vitamin D for the Management of Asthma. Cochrane Database Syst. Rev. 2023, 2, CD011511. [Google Scholar] [CrossRef]
- Nicoll, R.; Henein, M.Y. COVID-19 Prevention: Vitamin D Is Still a Valid Remedy. J. Clin. Med. Res. 2022, 11, 6818. [Google Scholar] [CrossRef]
- Brunvoll, S.H.; Nygaard, A.B.; Ellingjord-Dale, M.; Holland, P.; Istre, M.S.; Kalleberg, K.T.; Søraas, C.L.; Holven, K.B.; Ulven, S.M.; Hjartåker, A.; et al. Prevention of Covid-19 and Other Acute Respiratory Infections with Cod Liver Oil Supplementation, a Low Dose Vitamin D Supplement: Quadruple Blinded, Randomised Placebo Controlled Trial. BMJ 2022, 378, e071245. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Holt, H.; Greenig, M.; Talaei, M.; Perdek, N.; Pfeffer, P.; Vivaldi, G.; Maltby, S.; Symons, J.; Barlow, N.L.; et al. Effect of a Test-and-Treat Approach to Vitamin D Supplementation on Risk of All Cause Acute Respiratory Tract Infection and Covid-19: Phase 3 Randomised Controlled Trial (CORONAVIT). BMJ 2022, 378, e071230. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Gluud, L.L.; Nikolova, D.; Whitfield, K.; Wetterslev, J.; Simonetti, R.G.; Bjelakovic, M.; Gluud, C. Vitamin D Supplementation for Prevention of Mortality in Adults. Cochrane Database Syst. Rev. 2014, 1, CD007470. [Google Scholar] [CrossRef] [PubMed]
- Brustad, N.; Yousef, S.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.L. Safety of High-Dose Vitamin D Supplementation Among Children Aged 0 to 6 Years: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e227410. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Crescioli, G.; Maggini, V.; Brilli, V.; Menniti-Ippolito, F.; Ippoliti, I.; Potenza, S.; Renda, F.; Mazzanti, G.; Vitalone, A.; et al. Adverse Events Related to Herbal Dietary Supplements and over-the-Counter Medications Containing Laxatives: A 10-Year Update from the Italian Phytovigilance and Pharmacovigilance Systems. Ann. Ist. Super. Sanita 2022, 58, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Crescioli, G.; Bettiol, A.; Menniti-Ippolito, F.; Maggini, V.; Gallo, E.; Mugelli, A.; Vannacci, A.; Firenzuoli, F. Safety of Complementary and Alternative Medicine in Children: A 16-Years Retrospective Analysis of the Italian Phytovigilance System Database. Phytomedicine 2019, 61, 152856. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Crescioli, G.; Maggini, V.; Ippoliti, I.; Menniti-Ippolito, F.; Gallo, E.; Brilli, V.; Lanzi, C.; Mannaioni, G.; Firenzuoli, F.; et al. Acute Liver Injury Following Turmeric Use in Tuscany: An Analysis of the Italian Phytovigilance Database and Systematic Review of Case Reports. Br. J. Clin. Pharmacol. 2021, 87, 741–753. [Google Scholar] [CrossRef]
- Gallo, E.; Pugi, A.; Lucenteforte, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Pharmacovigilance of Herb-Drug Interactions among Preoperative Patients. Altern. Ther. Health Med. 2014, 20, 13–17. [Google Scholar]
- Crescioli, G.; Lombardi, N.; Bettiol, A.; Menniti-Ippolito, F.; Da Cas, R.; Parrilli, M.; Del Lungo, M.; Gallo, E.; Mugelli, A.; Maggini, V.; et al. Adverse Events Following Cannabis for Medical Use in Tuscany: An Analysis of the Italian Phytovigilance Database. Br. J. Clin. Pharmacol. 2020, 86, 106–120. [Google Scholar] [CrossRef]
- Lombardi, N.; Crescioli, G.; Bettiol, A.; Tuccori, M.; Rossi, M.; Bonaiuti, R.; Ravaldi, C.; Levi, M.; Mugelli, A.; Ricci, S.; et al. Vaccines Safety in Children and in General Population: A Pharmacovigilance Study on Adverse Events Following Anti-Infective Vaccination in Italy. Front. Pharmacol. 2019, 10, 948. [Google Scholar] [CrossRef] [Green Version]
- Edwards, I.R.; Aronson, J.K. Adverse Drug Reactions: Definitions, Diagnosis, and Management. Lancet 2000, 356, 1255–1259. [Google Scholar] [CrossRef]
- WHO-UMC. The WHO-UMC System for Standardized Case Causality Assessment. Available online: https://www.who.int/docs/default-source/medicines/pharmacovigilance/whocausality-assessment.pdf (accessed on 3 January 2023).
- MedDRA Hierarchy|MedDRA. Available online: https://www.meddra.org/how-to-use/basics/hierarchy (accessed on 3 January 2023).
- Malihi, Z.; Wu, Z.; Mm Lawes, C.; Scragg, R. Noncalcemic Adverse Effects and Withdrawals in Randomized Controlled Trials of Long-Term Vitamin D2 or D3 Supplementation: A Systematic Review and Meta-Analysis. Nutr. Rev. 2017, 75, 1007–1034. [Google Scholar] [CrossRef] [Green Version]
- Spiller, H.A.; Good, T.F.; Spiller, N.E.; Aleguas, A. Vitamin D Exposures Reported to US Poison Centers 2000-2014: Temporal Trends and Outcomes. Hum. Exp. Toxicol. 2016, 35, 457–461. [Google Scholar] [CrossRef]
- Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Pilz, S. Long-Term Supplementation with 3200 to 4000 IU of Vitamin D Daily and Adverse Events: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Nutr. 2023, 62, 1833–1844. [Google Scholar] [CrossRef]
- Malihi, Z.; Wu, Z.; Stewart, A.W.; Lawes, C.M.; Scragg, R. Hypercalcemia, Hypercalciuria, and Kidney Stones in Long-Term Studies of Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2016, 104, 1039–1051. [Google Scholar] [CrossRef] [Green Version]
- Alshahrani, F.; Aljohani, N. Vitamin D: Deficiency, Sufficiency and Toxicity. Nutrients 2013, 5, 3605–3616. [Google Scholar] [CrossRef] [Green Version]
- Tonon, C.R.; Silva, T.A.A.L.; Pereira, F.W.L.; Queiroz, D.A.R.; Junior, E.L.F.; Martins, D.; Azevedo, P.S.; Okoshi, M.P.; Zornoff, L.A.M.; de Paiva, S.A.R.; et al. A Review of Current Clinical Concepts in the Pathophysiology, Etiology, Diagnosis, and Management of Hypercalcemia. Med. Sci. Monit. 2022, 28, e935821. [Google Scholar] [CrossRef]
- Avenell, A.; Mak, J.C.S.; O’Connell, D. Vitamin D and Vitamin D Analogues for Preventing Fractures in Post-Menopausal Women and Older Men. Cochrane Database Syst. Rev. 2014, 2014, CD000227. [Google Scholar] [CrossRef]
- Selby, P.L.; Davies, M.; Marks, J.S.; Mawer, E.B. Vitamin D Intoxication Causes Hypercalcaemia by Increased Bone Resorption Which Responds to Pamidronate. Clin. Endocrinol. 1995, 43, 531–536. [Google Scholar] [CrossRef]
- Cianferotti, L.; Cricelli, C.; Kanis, J.A.; Nuti, R.; Reginster, J.-Y.; Ringe, J.D.; Rizzoli, R.; Brandi, M.L. The Clinical Use of Vitamin D Metabolites and Their Potential Developments: A Position Statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF). Endocrine 2015, 50, 12–26. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF) European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, N.; Campodonico, J.; Milazzo, V.; De Metrio, M.; Brambilla, M.; Camera, M.; Marenzi, G. Vitamin D and Cardiovascular Disease: Current Evidence and Future Perspectives. Nutrients 2021, 13, 3603. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Pilz, S. Vitamin D and Cardiovascular Disease: An Update. Anticancer Res. 2019, 39, 4627–4635. [Google Scholar] [CrossRef]
- Zhang, Y.; Post, W.S.; Dalal, D.; Bansal, S.; Blasco-Colmenares, E.; Jan De Beur, S.; Alonso, A.; Soliman, E.Z.; Whitsel, E.A.; Brugada, R.; et al. Serum 25-Hydroxyvitamin D, Calcium, Phosphorus, and Electrocardiographic QT Interval Duration: Findings from NHANES III and ARIC. J. Clin. Endocrinol. Metab. 2011, 96, 1873–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikawa, T.; Tsumabuki, S.; Nakagawa, M.; Takakura, T.; Tamura, M.; Maeda, T.; Ito, S.; Ito, M. QT Intervals as an Index of High Serum Calcium in Hypercalcemia. Clin. Cardiol. 1988, 11, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, C.; Bloise, R.; Monteforte, N.; Priori, S.G. Sudden Cardiac Death and Genetic Ion Channelopathies: Long QT, Brugada, Short QT, Catecholaminergic Polymorphic Ventricular Tachycardia, and Idiopathic Ventricular Fibrillation. Circulation 2012, 125, 2027–2034. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Post, W.S.; Dalal, D.; Blasco-Colmenares, E.; Tomaselli, G.F.; Guallar, E. QT-Interval Duration and Mortality Rate: Results from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2011, 171, 1727–1733. [Google Scholar] [CrossRef] [Green Version]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Zittermann, A.; Koerfer, R. Protective and Toxic Effects of Vitamin D on Vascular Calcification: Clinical Implications. Mol. Asp. Med. 2008, 29, 423–432. [Google Scholar] [CrossRef]
- Zittermann, A.; Prokop, S.; Gummert, J.F.; Börgermann, J. Safety Issues of Vitamin D Supplementation. Anticancer Agents Med. Chem. 2013, 13, 4–10. [Google Scholar] [CrossRef]
- Zittermann, A.; Ernst, J.B.; Prokop, S.; Fuchs, U.; Dreier, J.; Kuhn, J.; Knabbe, C.; Birschmann, I.; Schulz, U.; Berthold, H.K.; et al. Effect of Vitamin D on All-Cause Mortality in Heart Failure (EVITA): A 3-Year Randomized Clinical Trial with 4000 IU Vitamin D Daily. Eur. Heart J. 2017, 38, 2279–2286. [Google Scholar] [CrossRef] [Green Version]
- Lucenteforte, E.; Bettiol, A.; Lombardi, N.; Mugelli, A.; Vannacci, A. Risk of Bone Fractures among Users of Oral Anticoagulants: An Administrative Database Cohort Study. Eur. J. Intern. Med. 2017, 44, e30–e31. [Google Scholar] [CrossRef]
- Robien, K.; Oppeneer, S.J.; Kelly, J.A.; Hamilton-Reeves, J.M. Drug-Vitamin D Interactions: A Systematic Review of the Literature. Nutr. Clin. Pract. 2013, 28, 194–208. [Google Scholar] [CrossRef] [Green Version]
- Hejazi, M.E.; Modarresi-Ghazani, F.; Hamishehkar, H.; Mesgari-Abbasi, M.; Dousti, S.; Entezari-Maleki, T. The Effect of Treatment of Vitamin D Deficiency on the Level of P-Selectin and Hs-CRP in Patients With Thromboembolism: A Pilot Randomized Clinical Trial. J. Clin. Pharmacol. 2017, 57, 40–47. [Google Scholar] [CrossRef]
- Wakeman, M. A Literature Review of the Potential Impact of Medication on Vitamin D Status. Risk Manag. Healthc. Policy 2021, 14, 3357–3381. [Google Scholar] [CrossRef]
- Hung, K.-C.; Yang, S.-H.; Chang, C.-Y.; Wang, L.-K.; Lin, Y.-T.; Yu, C.-H.; Chuang, M.-H.; Chen, J.-Y. Is Circulating Vitamin D Status Associated with the Risk of Venous Thromboembolism? A Meta-Analysis of Observational Studies. Nutrients 2023, 15, 1113. [Google Scholar] [CrossRef]
- Beer, T.M.; Venner, P.M.; Ryan, C.W.; Petrylak, D.P.; Chatta, G.; Dean Ruether, J.; Chi, K.N.; Curd, J.G.; DeLoughery, T.G. High Dose Calcitriol May Reduce Thrombosis in Cancer Patients. Br. J. Haematol. 2006, 135, 392–394. [Google Scholar] [CrossRef]
- Lee, K.E.; Chung, J.E.; Yi, B.; Cho, Y.J.; Kim, H.J.; Lee, G.Y.; Kim, J.H.; Chang, B.C.; Gwak, H.S. Influence of NR3C1 and VDR Polymorphisms on Stable Warfarin Dose in Patients with Mechanical Cardiac Valves. Int. J. Cardiol. 2017, 236, 393–397. [Google Scholar] [CrossRef]
- Griffin, G.; Hewison, M.; Hopkin, J.; Kenny, R.; Quinton, R.; Rhodes, J.; Subramanian, S.; Thickett, D. Vitamin D and COVID-19: Evidence and Recommendations for Supplementation. R Soc. Open Sci. 2020, 7, 201912. [Google Scholar] [CrossRef]
- Rizzoli, R. Vitamin D Supplementation: Upper Limit for Safety Revisited? Aging Clin. Exp. Res. 2021, 33, 19–24. [Google Scholar] [CrossRef]
- Melamed, M.L.; Michos, E.D.; Post, W.; Astor, B. 25-Hydroxyvitamin D Levels and the Risk of Mortality in the General Population. Arch. Intern. Med. 2008, 168, 1629–1637. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Baron, J.A.; Snellman, G.; Gedeborg, R.; Byberg, L.; Sundström, J.; Berglund, L.; Arnlöv, J.; Hellman, P.; Blomhoff, R.; et al. Plasma Vitamin D and Mortality in Older Men: A Community-Based Prospective Cohort Study. Am. J. Clin. Nutr. 2010, 92, 841–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zittermann, A.; Ernst, J.B.; Gummert, J.F.; Börgermann, J. Vitamin D Supplementation, Body Weight and Human Serum 25-Hydroxyvitamin D Response: A Systematic Review. Eur. J. Nutr. 2014, 53, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wei, W.; Wang, G.; Zhou, H.; Fu, Y.; Liu, N. Circulating Vitamin D Concentration and Risk of Prostate Cancer: A Dose-Response Meta-Analysis of Prospective Studies. Ther. Clin. Risk Manag. 2018, 14, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterhouse, M.; Risch, H.A.; Bosetti, C.; Anderson, K.E.; Petersen, G.M.; Bamlet, W.R.; Cotterchio, M.; Cleary, S.P.; Ibiebele, T.I.; La Vecchia, C.; et al. Vitamin D and Pancreatic Cancer: A Pooled Analysis from the Pancreatic Cancer Case-Control Consortium. Ann. Oncol. 2016, 27, 208. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.E.; Baxter, C.; Romero, B.D.; McLeod, D.S.A.; English, D.R.; Armstrong, B.K.; Ebeling, P.R.; Hartel, G.; Kimlin, M.G.; O’Connell, R.; et al. The D-Health Trial: A Randomised Controlled Trial of the Effect of Vitamin D on Mortality. Lancet Diabetes Endocrinol. 2022, 10, 120–128. [Google Scholar] [CrossRef]
- Freyberg, R.H.; Bauer, J.M. Vitamin D Intoxication with Metastatic Calcification; Fatal Case in an Adult with Autopsy. Proc. Am. Fed. Clin. Res. 1945, 2, 53. [Google Scholar]
- Jelke, H. Vitamin D Intoxication in a Case of Parathyroprival Tetany; Report of a Fatal Case with Autopsy Findings. Acta Med. Scand. 1949, 132, 339–352. [Google Scholar] [CrossRef]
- Dewind, L.T. Hypervitaminosis D with Osteosclerosis. Arch. Dis. Child. 1961, 36, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Sasahara, A.A.; Elfman, N.A. Fatal Vitamin D Intoxication Masked by Multiple Sclerosis. Report of a Case with Post Mortem Findings. Conn. Med. 1962, 26, 428–433. [Google Scholar]
- Davies, J.S.; Poole, C.D.; Feldschreiber, P. The Medico-Legal Aspects of Prescribing Vitamin D. Br. J. Clin. Pharmacol. 2014, 78, 1257–1263. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.N.; Davies, J.S. A Review of the Growing Risk of Vitamin D Toxicity from Inappropriate Practice. Br. J. Clin. Pharmacol. 2018, 84, 1121–1127. [Google Scholar] [CrossRef] [Green Version]
- Benemei, S.; Gallo, E.; Giocaliere, E.; Bartolucci, G.; Menniti-Ippolito, F.; Firenzuoli, F.; Mugelli, A.; Vannacci, A. It’s Time for New Rules on Vitamin D Food Supplements. Br. J. Clin. Pharmacol. 2013, 76, 825–826. [Google Scholar] [CrossRef] [Green Version]
- OSMED—Rapporto-Sull-Uso-Dei-Farmaci-Durante-L-Epidemia-Covid-19-Anno-2020. Available online: https://www.aifa.gov.it/-/rapporto-sull-uso-dei-farmaci-durante-l-epidemia-covid-19-anno-2020 (accessed on 2 March 2023).
- Rossi, C.; Ruggiero, R.; Sportiello, L.; Pentella, C.; Gaio, M.; Pinto, A.; Rafaniello, C. Did the COVID-19 Pandemic Affect Contrast Media-Induced Adverse Drug Reaction’s Reporting? A Pharmacovigilance Study in Southern Italy. J. Clin. Med. Res. 2022, 11, 5104. [Google Scholar] [CrossRef]
- Mallhi, T.H.; Khan, Y.H.; Butt, M.H.; Salman, M. Self-Medication during the Era of COVID-19; Potential Implications for Drug Policy Makers and Pharmacovigilance. Curr. Drug Saf. 2023, 18, 122–124. [Google Scholar] [CrossRef]
- Mazzanti, G.; Vitalone, A.; Da Cas, R.; Menniti-Ippolito, F. Suspected Adverse Reactions Associated with Herbal Products Used for Weight Loss: Spontaneous Reports from the Italian Phytovigilance System. Eur. J. Clin. Pharmacol. 2019, 75, 1599–1615. [Google Scholar] [CrossRef]
- Asfour, M.H.; Abd El-Alim, S.H.; Kassem, A.A.; Salama, A.; Gouda, A.S.; Nazim, W.S.; Nashaat, N.H.; Hemimi, M.; Abdel Meguid, N. Vitamin D-Loaded Nanoemulsions as a Potential Drug Delivery System for Autistic Children: Formulation Development, Safety, and Pharmacokinetic Studies. AAPS PharmSciTech 2023, 24, 58. [Google Scholar] [CrossRef]
- Alomar, M.; Tawfiq, A.M.; Hassan, N.; Palaian, S. Post Marketing Surveillance of Suspected Adverse Drug Reactions through Spontaneous Reporting: Current Status, Challenges and the Future. Ther. Adv. Drug Saf. 2020, 11, 2042098620938595. [Google Scholar] [CrossRef]
- Gatti, M.; Ippoliti, I.; Poluzzi, E.; Antonazzo, I.C.; Moro, P.A.; Moretti, U.; Menniti-Ippolito, F.; Mazzanti, G.; De Ponti, F.; Raschi, E. Assessment of Adverse Reactions to α-Lipoic Acid Containing Dietary Supplements through Spontaneous Reporting Systems. Clin. Nutr. 2021, 40, 1176–1185. [Google Scholar] [CrossRef]
- Bettiol, A.; Lombardi, N.; Marconi, E.; Crescioli, G.; Bonaiuti, R.; Maggini, V.; Gallo, E.; Mugelli, A.; Firenzuoli, F.; Ravaldi, C.; et al. The Use of Complementary and Alternative Medicines during Breastfeeding: Results from the Herbal Supplements in Breastfeeding InvesTigation (HaBIT) Study. Br. J. Clin. Pharmacol. 2018, 84, 2040–2047. [Google Scholar] [CrossRef] [Green Version]
- Bischoff-Ferrari, H.A.; Willett, W.C.; Orav, E.J.; Lips, P.; Meunier, P.J.; Lyons, R.A.; Flicker, L.; Wark, J.; Jackson, R.D.; Cauley, J.A.; et al. A Pooled Analysis of Vitamin D Dose Requirements for Fracture Prevention. N. Engl. J. Med. 2012, 367, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Petrović, D.; Runjić, E.; Buljan, I.; Jeličić Kadić, A.; Markić, J. Knowledge and Practice of Pediatricians Regarding Hypovitaminosis D-A Survey across 33 European Countries. Children 2022, 9, 1831. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; März, W.; Pilz, S. Vitamin D and Cardiovascular Disease: An Updated Narrative Review. Int. J. Mol. Sci. 2021, 22, 2896. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Maggini, V.; Berardi, M.; Pugi, A.; Notaro, R.; Talini, G.; Vannozzi, G.; Bagnoli, S.; Forte, P.; Mugelli, A.; et al. Is Green Tea a Potential Trigger for Autoimmune Hepatitis? Phytomedicine 2013, 20, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, G.; Bonaiuti, R.; Corradetti, R.; Mannaioni, G.; Vannacci, A.; Lombardi, N. Pharmacovigilance and Pharmacoepidemiology as a Guarantee of Patient Safety: The Role of the Clinical Pharmacologist. J. Clin. Med. Res. 2022, 11, 3552. [Google Scholar] [CrossRef]

| Italian Phytovigilance System (ISS) | Italian Pharmacovigilance System (AIFA) | |||||
|---|---|---|---|---|---|---|
| Characteristics | Overall | Serious a | Non-Serious a | Overall | Serious b | Non-Serious b |
| n = 127 (%) c | n = 26 (%) c | n = 100 (%) c | n = 643 (%) c | n = 146 (%) c | n = 468 (%) c | |
| Age (years; mean ± SD) | 37.8 ± 26.4 | 43.0 ± 22.8 | 36.0 ± 27.6 | 59.9 ± 21.1 | 59.5 ± 23.1 | 59.7 ± 20.9 |
| Sex | ||||||
| Male | 36 (28.4) | 9 (34.6) | 27 (27.0) | 123 (19.1) | 39 (26.7) | 82 (17.6) |
| Female | 86 (67.7) | 17 (65.4) | 68 (68.0) | 516 (80.3) | 106 (72.6) | 383 (81.8) |
| Not reported | 5 (3.9) | - | 5 (5.0) | 4 (0.6) | 1 (0.7) | 3 (0.6) |
| Comedications | ||||||
| ≥5 drugs | 10 (7.9) | 4 (15.4) | 6 (6.0) | 66 (10.3) | 27 (18.5) | 37 (7.9) |
| 1–4 drugs | 32 (25.2) | 11 (42.3) | 20 (20.0) | 203 (31.6) | 31 (21.3) | 157 (33.5) |
| No drugs | 6 (4.7) | 1 (3.8) | 5 (5.0) | 322 (50.1) | 70 (47.9) | 240 (51.3) |
| Not reported | 79 (62.2) | 10 (38.5) | 69 (69.0) | 52 (8.1) | 18 (12.3) | 34 (7.3) |
| Reason of use | ||||||
| Vitamin integration d | 28 (22.0) | 9 (34.7) | 19 (19.0) | 191 (29.7) | 47 (32.2) | 143 (30.5) |
| Adaptogen/Tonic e | 7 (5.5) | 1 (3.8) | 6 (6.0) | - | - | - |
| Pain | 5 (3.9) | 1 (3.8) | 4 (4.0) | 11 (1.7) | 2 (1.4) | 8 (1.7) |
| Pregnancy | 5 (4.1) | 1 (3.8) | 3 (3.0) | - | - | - |
| Menopause | 3 (2.4) | 1 (3.8) | 2 (2.0) | 1 (0.2) | - | 1 (0.2) |
| Osteoporosis/Osteopenia | - | - | - | 219 (34.1) | 32 (21.9) | 168 (35.9) |
| Thyroid-related | - | - | - | 19 (2.9) | 13 (8.9) | 5 (1.1) |
| Others | 19 (14.9) | 4 (15.4) | 15 (15.0) | 26 (4.1) | 8 (5.5) | 17 (3.6) |
| Not reported | 60 (47.2) | 9 (34.7) | 51 (51.0) | 176 (27.4) | 44 (30.1) | 126 (27.0) |
| Duration of treatment | ||||||
| ≤7 days | 31 (24.4) | 5 (19.2) | 26 (26.0) | 189 (29.4) | 33 (22.6) | 149 (31.8) |
| 8–30 days | 17 (13.4) | 6 (23.1) | 11 (11.0) | 72 (11.2) | 11 (7.5) | 51 (10.9) |
| >30 days | 12 (9.4) | 4 (15.4) | 8 (8.0) | 184 (28.6) | 54 (38.0) | 129 (27.6) |
| Not reported | 67 (52.8) | 11 (42.3) | 55 (55.0) | 198 (30.8) | 48 (32.9) | 139 (29.7) |
| Time to onset f | ||||||
| ≤7 days | 48 (39.3) | 5 (20) | 43 (44.8) | 236 (36.5) | 36 (24.7) | 194 (41.4) |
| 8–30 days | 14 (11.4) | 5 (20) | 9(9.4) | 64 (11.2) | 13 (8.9) | 43 (9.2) |
| >30 days | 14 (11.4) | 5 (20) | 9 (9.4) | 163 (25.3) | 53 (36.3) | 106 (22.7) |
| Not reported | 46 (37.7) | 10 (40) | 35 (36.4) | 180 (28.0) | 44 (30.1) | 125 (26.7) |
| Reporter qualification | ||||||
| Physician | 49 (38.6) | 20 (76.9) | 29 (29.0) | 333 (51.8) | 85 (58.2) | 225 (48.1) |
| Pharmacist | 34 (26.8) | 2 (7.7) | 32 (32.0) | 107 (16.6) | 21 (14.4) | 86 (18.4) |
| Patient | 34 (26.8) | 3 (11.6) | 30 (30.0) | 141 (22.0) | 11 (7.5) | 128 (27.3) |
| Other | 9 (7.0) | 1 (3.8) | 8 (8.0) | 62 (9.6) | 29 (19.9) | 29 (6.2) |
| Not reported | 1 (0.8) | - | 1 (1.0) | - | - | - |
| Seriousness | ||||||
| Hospitalisation/prolongation of existing hospitalisation | - | 19 (73.1%) | - | - | 84 (57.5%) | - |
| Other important medical events | - | 2 (7.7%) | - | - | 40 (27.4%) | - |
| Life-threatening | - | 3 (11.5%) | - | - | 11 (7.5%) | - |
| Persistent or significant disability or incapacity | - | 2 (7.7%) | - | - | 8 (5.5%) | - |
| Fatal | - | - | - | - | 2 (1.4%) | - |
| Birth defect | - | - | - | - | 1 (0.7%) | - |
| Outcomes | ||||||
| Recovered/resolved | 56 (44.1) | 8 (30.8) | 48 (48.0) | 260 (40.4) | 48 (32.9) | 196 (41.9) |
| Recovering/resolving | 12 (9.4) | 8 (30.8) | 4 (4.0) | 160 (24.9) | 47 (32.2) | 112 (23.9) |
| Recovered with sequelae | 2 (1.6) | - | 2 (2.0) | 10 (1.6) | 3 (2.1) | 6 (1.3) |
| Not recovered | 9 (7.1) | 3 (11.5) | 6 (6.0) | 66 (10.3) | 16 (11.0) | 50 (10.7) |
| Fatal | - | - | - | 2 (0.3) | 2 (1.3) | - |
| Unknown | 48 (37.8) | 7 (26.9) | 40 (40.0) | 145 (22.5) | 30 (20.5) | 104 (22.2) |
| Dechallenge | ||||||
| Positive | 43 (33.8) | 9 (34.6) | 34 (34.0) | 349 (54.3) | 74 (50.7) | 262 (56.0) |
| Negative | 2 (1.6) | - | 2 (2.0) | 27 (4.2) | 8 (5.5) | 19 (4.0) |
| Not reported | 82 (64.6) | 17 (65.4) | 64 (64.0) | 267 (41.5) | 64 (43.8) | 187 (40.0) |
| Rechallenge | ||||||
| Positive | 15 (11.8) | - | 15 (15.0) | 38 (5.9) | 5 (3.3) | 31 (6.6) |
| Negative | 1 (0.8) | - | 1 (1.0) | 4 (0.6) | 1 (0.7) | 3 (0.6) |
| Not reported | 111 (87.4) | 26 (100) | 84 (84.0) | 601 (93.5) | 140 (95.9) | 434 (92.8) |
| Causality assessment | ||||||
| Definite | 8 (6.3) | 2 (7.7) | 6 (6.0) | - | - | - |
| Probable/likely | 58 (45.7) | 8 (30.8) | 50 (50.0) | 240 (37.3) | 43 (29.5) | 191 (40.8) |
| Possible | 35 (27.5) | 13 (50.0) | 22 (22.0) | 388 (60.3) | 97 (66.4) | 269 (57.5) |
| Unlikely | 1 (0.8) | - | - | 8 (1.3) | 4 (2.7) | 3 (0.6) |
| Unassessable/unclassifiable | 25 (19.7) | 3 (11.5) | 22 (22.0) | 7 (1.1) | 2 (1.4) | 5 (1.1) |
| System Organ Class (SOC) | ISS ARs n (%) a | AIFA ADRs n (%) a | ARs or ADRs n (%) a | Total % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serious n = 49 | Non-Serious n = 166 | NR n = 1 | Serious n = 283 | Non-Serious n = 825 | NR n = 51 | ISS n = 216 | AIFA n = 1159 | p Value b | on Ars c | on Subjects d | |
| Gastrointestinal disorders | 6 (12.2) | 88 (53.0) | - | 37 (13.1) | 258 (31.3) | 15 (29.4) | 94 (43.5) | 310 (26.7) | <0.001 | 29.4 | 52.5 |
| Skin and subcutaneous tissue disorders | 10 (20.4) | 26 (15.7) | - | 38 (13.4) | 204 (24.7) | 13 (25.5) | 36 (16.7) | 255 (22.0) | 0.08 | 21.2 | 37.8 |
| Nervous system disorders | 2 (4.1) | 7 (4.2) | - | 38 (3.3) | 85 (10.3) | 7 (13.7) | 9 (4.2) | 130 (11.2) | 0.002 | 10.1 | 18.1 |
| Investigations | 9 (18.4) | 6 (3.6) | - | 53 (18.7) | 39 (4.7) | 2 (3.9) | 15 (6.9) | 94 (8.1) | 0.56 | 7.9 | 14.2 |
| General disorders and administration site conditions | 1 (2.0) | 14 (8.4) | - | 18 (6.4) | 59 (7.2) | 2 (3.9) | 15 (6.9) | 79 (6.8) | 0.94 | 6.8 | 12.2 |
| Psychiatric disorders | - | 2 (1.2) | - | 15 (5.3) | 35 (4.2) | 2 (3.9) | 2 (0.9) | 52 (4.5) | 0.01 | 3.9 | 7.0 |
| Musculoskeletal and connective tissue disorders | 1 (2.0) | 3 (1.8) | - | 18 (6.4) | 28 (3.4) | 1 (2.0) | 4 (1.9) | 47 (4.1) | 0.12 | 3.7 | 6.6 |
| Renal and urinary disorders | 6 (12.2) | 5 (3.0) | - | 16 (5.7) | 19 (2.3) | 1 (2.0) | 11 (5.1) | 36 (3.1) | 0.14 | 3.4 | 6.1 |
| Respiratory, thoracic and mediastinal disorders | 4 (8.2) | 1 (0.6) | - | 8 (2.8) | 22 (2.7) | - | 5 (2.3) | 30 (2.6) | 0.81 | 2.5 | 4.5 |
| Cardiac disorders | 1 (2.0) | 3 (1.8) | - | 12 (4.2) | 13 (1.6) | 4 (7.8) | 4 (1.9) | 29 (2.5) | 0.57 | 2.4 | 4.3 |
| Vascular disorders | 3 (6.1) | 3 (1.8) | - | 8 (2.8) | 18 (2.2) | 1 (2.0) | 6 (2.8) | 27 (2.3) | 0.69 | 2.4 | 4.3 |
| Metabolism and nutrition disorders | - | 3 (1.8) | - | 5 (1.8) | 11 (1.3) | - | 3 (1.4) | 16 (1.4) | 0.99 | 1.4 | 2.5 |
| Eye disorders | - | - | - | 1 (0.4) | 12 (1.5) | 2 (3.9) | - | 15 (1.3) | 0.09 | 1.1 | 1.9 |
| Ear and labyrinth disorders | 1 (2.0) | - | - | 3 (1.1) | 9 (1.1) | 1 (2.0) | 1 (0.5) | 13 (1.1) | 0.38 | 1.0 | 1.8 |
| Immune system disorders | - | 1 (0.6) | - | 4 (1.4) | 5 (0.6) | - | 1 (0.5) | 9 (0.8) | 0.62 | 0.7 | 1.3 |
| Hepatobiliary disorders | 4 (8.2) | 1 (0.6) | - | 2 (0.7) | 1 (0.1) | - | 5 (2.3) | 3 (0.3) | < 0.001 | 0.6 | 1.0 |
| Reproductive system and breast disorders | - | 1 (0.6) | - | 1 (0.4) | 5 (0.6) | - | 1 (0.5) | 6 (0.5) | 0.92 | 0.5 | 0.9 |
| Infections and infestations | - | - | - | 2 (0.7) | 2 (0.2) | - | - | 4 (0.3) | 0.39 | 0.3 | 0.5 |
| Pregnancy. puerperium and perinatal conditions | - | 1 (0.6) | 1 (100.0) | 1 (0.4) | - | - | 2 (0.9) | 1 (0.1) | 0.01 | 0.2 | 0.4 |
| Blood and lymphatic system disorders | 1 (2.0) | - | - | 2 (0.7) | - | - | 1 (0.5) | 2 (0.2) | 0.40 | 0.2 | 0.4 |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | - | - | - | 1 (0.4) | - | - | - | 1 (0.1) | 0.67 | 0.1 | 0.1 |
| Not applied e | - | 1 (0.6) | - | - | - | - | 1 (0.5) | - | - | 0.1 | 0.1 |
| SOCs and PTs | n = 332 (%) |
|---|---|
| Investigations | 62 (18.7) |
| Hypercalcaemia | 43 (12.9) |
| Hypervitaminosis D | 4 (1.2) |
| Hypocalcemia | 3 (0.9) |
| Renal and urinary disease | 22 (6.6) |
| Renal failure | 11 (3.3) |
| Nephrocalcinosis or kidney stones | 4 (1.2) |
| Hydronephrosis | 2 (0.6) |
| Muscoloskeletal and connective disorders | |
| Musculoskeletal pain | 7 (2.1%) |
| Jaw osteonecrosis a | 6 (1.8%) |
| Others | 5 (1.5%) |
| Cardiac disorders | 13 (3.9) |
| Tachycardia (including cardiac arrhythmia and palpitations) | 8 (2.4) |
| Chest pain | 4 (1.2) |
| Myocardial infarction | 1 (0.3) |
| Crude OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Age (years) | ||
| <30 | 1 | 1 |
| 30–65 | 1.48 (0.85–2.57) | 0.76 (0.34–1.71) |
| ≥65 | 1.46 (0.84–2.55) | 0.75 (0.33–1.72) |
| Sex | ||
| Male | 1 | 1 |
| Female | 1.34 (0.91–1.97) | 0.95 (0.54–1.70) |
| Number of concomitant products | ||
| 0 | 1 | 1 |
| 1–4 | 0.93 (0.63–1.38) | 0.85 (0.51–1.42) |
| ≥5 | 2.35 (1.41–3.94) | 2.44 (1.30–4.60) |
| Length of exposure (days) | ||
| ≤7 | 1 | 1 |
| 8–30 | 1.70 (0.97–2.98) | 2.08 (1.12–3.86) |
| >30 | 1.62 (1.03–2.53) | 1.91 (1.16–3.15) |
| VitDps reported daily dose (mg) | ||
| ≤1000 | 1 | - |
| >1000 | 2.70 (1.30–5.64) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggini, V.; Crescioli, G.; Ippoliti, I.; Gallo, E.; Menniti-Ippolito, F.; Chiaravalloti, A.; Mascherini, V.; Da Cas, R.; Potenza, S.; Gritti, G.; et al. Safety Profile of Vitamin D in Italy: An Analysis of Spontaneous Reports of Adverse Reactions Related to Drugs and Food Supplements. J. Clin. Med. 2023, 12, 4726. https://doi.org/10.3390/jcm12144726
Maggini V, Crescioli G, Ippoliti I, Gallo E, Menniti-Ippolito F, Chiaravalloti A, Mascherini V, Da Cas R, Potenza S, Gritti G, et al. Safety Profile of Vitamin D in Italy: An Analysis of Spontaneous Reports of Adverse Reactions Related to Drugs and Food Supplements. Journal of Clinical Medicine. 2023; 12(14):4726. https://doi.org/10.3390/jcm12144726
Chicago/Turabian StyleMaggini, Valentina, Giada Crescioli, Ilaria Ippoliti, Eugenia Gallo, Francesca Menniti-Ippolito, Adelaide Chiaravalloti, Vittorio Mascherini, Roberto Da Cas, Simona Potenza, Giulia Gritti, and et al. 2023. "Safety Profile of Vitamin D in Italy: An Analysis of Spontaneous Reports of Adverse Reactions Related to Drugs and Food Supplements" Journal of Clinical Medicine 12, no. 14: 4726. https://doi.org/10.3390/jcm12144726
APA StyleMaggini, V., Crescioli, G., Ippoliti, I., Gallo, E., Menniti-Ippolito, F., Chiaravalloti, A., Mascherini, V., Da Cas, R., Potenza, S., Gritti, G., Galiulo, M. T., Sottosanti, L., Vannacci, A., Lombardi, N., & Firenzuoli, F. (2023). Safety Profile of Vitamin D in Italy: An Analysis of Spontaneous Reports of Adverse Reactions Related to Drugs and Food Supplements. Journal of Clinical Medicine, 12(14), 4726. https://doi.org/10.3390/jcm12144726










