High Serum VE-Cadherin and Vinculin Concentrations Are Markers of the Disruption of Vascular Integrity during Type B Acute Aortic Dissection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and the Determination of Serum VEC and Vcn Concentrations
2.3. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics of the Participants
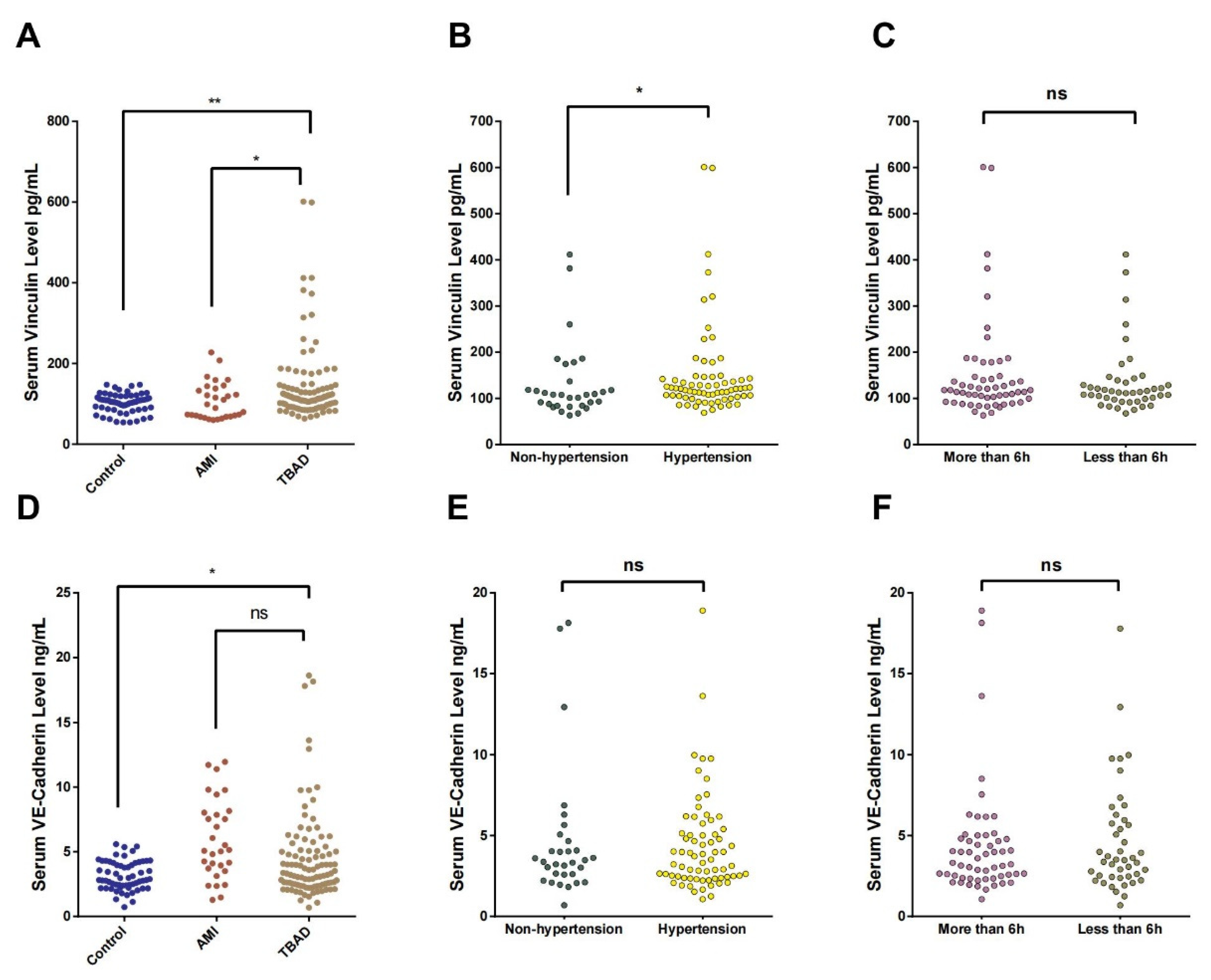
3.2. Serum Vcn and VEC Concentrations in TBAD Patients
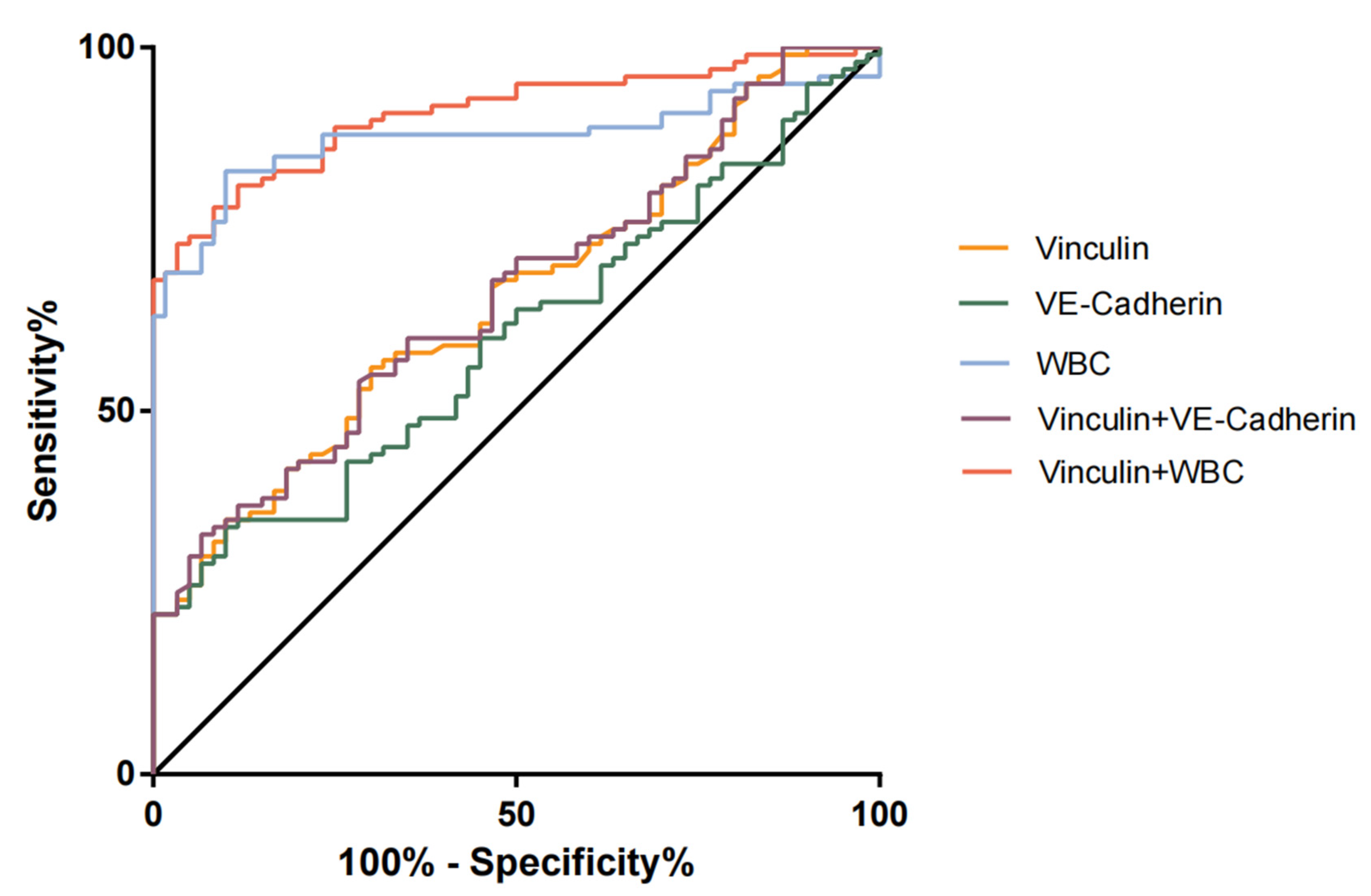
3.3. Performances of Serum Vcn, VEC, and a Combination of the Two for the Diagnosis of TBAD
3.4. Relationships of Serum Vcn and VEC with Blood Parameters
3.5. The Relationships of Serum Vcn and VEC with Acute Preoperative Complications of TBAD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar]
- Erbel, R.; Alfonso, F.; Boileau, C.; Dirsch, O.; Eber, B.; Haverich, A.; Rakowski, H.; Struyven, J.; Radegran, K.; Sechtem, U.; et al. Diagnosis and management of aortic dissection. Eur. Heart J. 2001, 22, 1642–1681. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Zhou, X.L.; Li, J.J.; Hui, R.T. Biomarkers in aortic dissection. Clin. Chim. Acta 2011, 412, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.S.; Nelson, W.J. VE-cadherin: At the front, center, and sides of endothelial cell organization and function. Curr. Opin. Cell Biol. 2010, 22, 651–658. [Google Scholar] [CrossRef]
- Lee, H.T.; Sharek, L.; O’brien, E.T.; Urbina, F.L.; Gupton, S.L.; Superfine, R.; Burridge, K.; Campbell, S.L. Vinculin and metavinculin exhibit distinct effects on focal adhesion properties, cell migration, and mechanotransduction. PLoS ONE 2019, 14, e0221962. [Google Scholar] [CrossRef]
- Maziveyi, M.; Alahari, S.K. Cell matrix adhesions in cancer: The proteins that form the glue. Oncotarget 2017, 8, 48471–48487. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, P.; Xu, F.; He, Y.; Xie, X.; Jiang, X. Vinculin promotes gastric cancer proliferation and migration and predicts poor prognosis in patients with gastric cancer. J. Cell Biochem. 2019, 120, 14107–14115. [Google Scholar] [CrossRef]
- Hazan, R.B.; Kang, L.; Roe, S.; Borgen, P.I.; Rimm, D.L. Vinculin Is Associated with the E-cadherin Adhesion Complex. J. Biol. Chem. 1997, 272, 32448–32453. [Google Scholar] [CrossRef]
- Goldmann, W.H.; Ingber, D.E. Intact vinculin protein is required for control of cell shape, cell mechanics, and rac-dependent lamellipodia formation. Biochem. Biophys. Res. Commun. 2002, 290, 749–755. [Google Scholar] [CrossRef]
- Twiss, F.; Le Duc, Q.; Van Der Horst, S.; Tabdili, H.; Van Der Krogt, G.; Wang, N.; Rehmann, H.; Huveneers, S.; Leckband, D.E.; De Rooij, J. Vinculin-dependent Cadherin mechanosensing regulates efficient epithelial barrier formation. Biol. Open 2012, 1, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Nienaber, C.A.; Eagle, K.A. Aortic dissection: New frontiers in diagnosis and management: Part I: From etiology to diagnostic strategies. Circulation 2003, 108, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Nienaber, C.A.; Clough, R.E.; Sakalihasan, N.; Suzuki, T.; Gibbs, R.; Mussa, F.; Jenkins, M.P.; Thompson, M.M.; Evangelista, A.; Yeh, J.S.M.; et al. Aortic dissection. Nat. Rev. Dis. Primers 2016, 2, 16053. [Google Scholar] [CrossRef]
- He, R.; Guo, D.-C.; Estrera, A.L.; Safi, H.J.; Huynh, T.T.; Yin, Z.; Cao, S.-N.; Lin, J.; Kurian, T.; Buja, L.M.; et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J. Thorac. Cardiovasc. Surg. 2006, 131, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Zhou, X.L.; Li, J.J.; Hui, R.T. Inflammatory response is associated with aortic dissection. Ageing Res. Rev. 2009, 8, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Huveneers, S.; Oldenburg, J.; Spanjaard, E.; van der Krogt, G.; Grigoriev, I.; Akhmanova, A.; Rehmann, H.; de Rooij, J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J. Cell Biol. 2012, 196, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Beppu, M.; Sawai, S.; Satoh, M.; Mori, M.; Kazami, T.; Misawa, S.; Shibuya, K.; Ishibashi, M.; Sogawa, K.; Kado, S.; et al. Autoantibodies against vinculin in patients with chronic inflammatory demyelinating polyneuropathy. J. Neuroimmunol. 2015, 287, 9–15. [Google Scholar] [CrossRef]
- Zemljic-Harpf, A.E.; Miller, J.C.; Henderson, S.A.; Wright, A.T.; Manso, A.M.; Elsherif, L.; Dalton, N.D.; Thor, A.K.; Perkins, G.A.; McCulloch, A.D.; et al. Cardiac-myocyte-specific excision of the vinculin gene disrupts cellular junctions, causing sudden death or dilated cardiomyopathy. Mol. Cell. Biol. 2007, 27, 7522–7537. [Google Scholar] [CrossRef]
- Dejana, E.; Giampietro, C. Vascular endothelial-cadherin and vascular stability. Curr. Opin. Hematol. 2012, 19, 218–223. [Google Scholar] [CrossRef]
- Sidibé, A.; Mannic, T.; Arboleas, M.; Subileau, M.; Gulino-Debrac, D.; Bouillet, L.; Jan, M.; Vandhuick, T.; Le Loët, X.; Vittecoq, O.; et al. Soluble VE-cadherin in rheumatoid arthritis patients correlates with disease activity: Evidence for tumor necrosis factor alpha-induced VE-cadherin cleavage. Arthritis Rheum. 2012, 64, 77–87. [Google Scholar] [CrossRef]
- Vilgrain, I.; Sidibé, A.; Polena, H.; Cand, F.; Mannic, T.; Arboleas, M.; Boccard, S.; Baudet, A.; Gulino-Debrac, D.; Bouillet, L.; et al. Evidence for post-translational processing of vascular endothelial (VE)-cadherin in brain tumors: Towards a candidate biomarker. PLoS ONE 2013, 8, e80056. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; Hashizume, H.; McDonald, D.M. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005, 15, 102–111. [Google Scholar] [CrossRef]
- Weis, S.M. Vascular Permeability in Cardiovascular Disease and Cancer. Curr. Opin. Hematol. 2008, 15, 7. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, L.; Qiu, Z.; Huang, Q.; Chen, Y.; Chen, L. Mechanical stretch aggravates aortic dissection by regulating MAPK pathway and the expression of MMP-9 and inflammation factors. Biomed. Pharmacother. 2018, 108, 1294–1302. [Google Scholar] [CrossRef]
- Keck, T.; Balcom JHt Fernandez-del Castillo, C.; Antoniu, B.A.; Warshaw, A.L. Matrix metalloproteinase-9 promotes neutrophil migration and alveolar capillary leakage in pancreatitis-associated lung injury in the rat. Gastroenterology 2002, 122, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Del Porto, F.; Proietta, M.; Tritapepe, L.; Miraldi, F.; Koverech, A.; Cardelli, P.; Tabacco, F.; De Santis, V.; Vecchione, A.; Mitterhofer, A.P.; et al. Inflammation and immune response in acute aortic dissection. Ann. Med. 2010, 42, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Ngok, S.P.; Geyer, R.; Liu, M.; Kourtidis, A.; Agrawal, S.; Wu, C.; Seerapu, H.R.; Lewis-Tuffin, L.J.; Moodie, K.L.; Huveldt, D.; et al. VEGF and Angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. J. Cell Biol. 2012, 199, 1103–1115. [Google Scholar] [CrossRef]
- Edens, H.A.; Parkos, C.A. Neutrophil transendothelial migration and alteration in vascular permeability Focus on neutrophil-derived azurocidin. Curr. Opin. Hematol. 2003, 10, 25–30. [Google Scholar] [CrossRef]
- Wen, D.; Du, X.; Dong, J.Z.; Zhou, X.L.; Ma, C.S. Value of D-dimer and C reactive protein in predicting inhospital death in acute aortic dissection. Heart 2013, 99, 1192–1197. [Google Scholar] [CrossRef]
- Mori, K.; Tamune, H.; Tanaka, H.; Nakamura, M. Admission Values of D-dimer and C-reactive Protein (CRP) Predict the Long-term Outcomes in Acute Aortic Dissection. Intern. Med. 2016, 55, 1837–1843. [Google Scholar] [CrossRef]
- Ohlmann, P.; Faure, A.; Morel, O.; Petit, H.; Kabbaj, H.; Meyer, N.; Cheneau, E.; Jesel, L.; Epailly, E.; Desprez, D.; et al. Diagnostic and prognostic value of circulating D-Dimers in patients with acute aortic dissection. Crit. Care Med. 2006, 34, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Shimony, A.; Filion, K.B.; Mottillo, S.; Dourian, T.; Eisenberg, M.J. Meta-analysis of usefulness of d-dimer to diagnose acute aortic dissection. Am. J. Cardiol. 2011, 107, 1227–1234. [Google Scholar] [CrossRef]
- Morello, F.; Piler, P.; Novak, M.; Kruzliak, P. Biomarkers for diagnosis and prognostic stratification of aortic dissection challenges and perspectives. Biomark. Med. 2014, 8, 931–941. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Gao, C.; Feng, J.; Wang, A. Risk factors for hospital death in patients with acute aortic dissection. Heart Lung Circ. 2015, 24, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Turak, O.; Afşar, B.; Ozcan, F.; Öksüz, F.; Mendi, M.A.; Yayla, Ç.; Covic, A.; Bertelsen, N.; Kanbay, M. The Role of Plasma Triglyceride/High-Density Lipoprotein Cholesterol Ratio to Predict New Cardiovascular Events in Essential Hypertensive Patients. J. Clin. Hypertens. (Greenwich) 2016, 18, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Girona, J.; Amigó, N.; Ibarretxe, D.; Plana, N.; Rodríguez-Borjabad, C.; Heras, M.; Ferré, R.; Gil, M.; Correig, X.; Masana, L. HDL Triglycerides: A New Marker of Metabolic and Cardiovascular Risk. Int. J. Mol. Sci. 2019, 20, 3151. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, G.; He, H.; Pan, X.; Peng, W.; Chai, X. Triglyceride/High-Density Lipoprotein Cholesterol Ratio Is Associated with In-Hospital Mortality in Acute Type B Aortic Dissection. Biomed. Res. Int. 2020, 2020, 5419846. [Google Scholar] [CrossRef]
- Formosa, A.; Santos, D.M.; Marcuzzi, D.; Common, A.A.; Prabhudesai, V. Low Contrast Dose Catheter-Directed CT Angiography (CCTA). Cardiovasc. Interv. Radiol. 2016, 39, 606–610. [Google Scholar] [CrossRef]
| Variables | Control (n = 60) | AMI (n = 30) | TBAD (n = 100) | p Value |
|---|---|---|---|---|
| AAD vs. Control | ||||
| AAD vs. AMI | ||||
| Age, years | 53.75 ± 1.49 | 57.33 ± 1.75 | 56.09 ± 1.35 | 0.417 |
| 0.898 | ||||
| BMI, kg/m2 | 22.00 ± 0.44 | 22.88 ± 0.63 | 22.68 ± 0.47 | 0.432 |
| 0.659 | ||||
| Male, n (%) | 48 (80) | 24 (80) | 83 (83) | 0.466 |
| Hypertension, n (%) | 25 (41.67) | 12 (40) | 69 (69) | 0.001 |
| Diabetes mellitus, n (%) | 8 (13.33) | 8 (26.67) | 14 (14) | 0.204 |
| Smoking, n (%) | 19 (31.67) | 16 (53.33) | 40 (40) | 0.138 |
| Heart rate, bmp | 82.67 ± 1.29 | 82.87 ± 2.00 | 88.17 ± 1.29 | 0.001 |
| 0.496 | ||||
| WBC, 109/L | 5.85 ± 0.10 | 10.52 ± 0.68 | 10.05 ± 0.38 | <0.001 |
| 0.752 | ||||
| NE, 109/L | 3.70 ± 0.09 | 9.02 ± 0.64 | 7.99 ± 0.39 | <0.001 |
| 0.249 | ||||
| Hb, g/L | 151.73 ± 2.27 | 137.97 ± 3.03 | 142.13 ± 1.75 | 0.002 |
| 0.483 | ||||
| Vinculin, pg/mL | 102.3 ± 3.09 | 107.32 ± 8.34 | 145.4 ± 9.62 | 0.001 |
| 0.036 | ||||
| VE-Cadherin, ng/uL | 3.15 ± 0.14 | 5.95 ± 0.56 | 4.49 ± 0.34 | 0.003 |
| 0.763 | ||||
| CRP, mg/L | - | - | 55.23 ± 5.18 | - |
| D-Dimer, ug/mL | - | - | 6.26 ± 0.70 | - |
| Management in hospital | ||||
| Medical Therapy | - | - | 100 (100) | - |
| TEVAR | - | - | 77 (77) | - |
| Acute phase complications | 44 (44) | |||
| Refractory pain | - | - | 34 (34) | - |
| Uncontrollable hypertension | - | - | 22 (22) | - |
| Rapid growing | - | - | 15 (15) | - |
| Limb malperfusion | - | - | 12 (12) | - |
| Visceral malperfusion | - | - | 26 (26) | - |
| Variables | Cut-off Value | AUC (95% CI) | Sensitivity | Specificity | p Value |
|---|---|---|---|---|---|
| TBAD vs. Ctrl | |||||
| Vinculin, pg/mL | 128.1 | 0.655 (0.571–0.739) | 35.00% | 90.00% | 0.001 |
| VE-Cadherin, ng/uL | 3.975 | 0.599 (0.512–0.686) | 43.00% | 73.30% | 0.036 |
| WBC, 109/L | 6.685 | 0.876 (0.820–0.933) | 83.00% | 86.67% | <0.001 |
| Vinculin + VE-Cadherin | - | 0.661 (0.577–0.744) | 33.00% | 93.33% | <0.001 |
| Vinculin + WBC | - | 0.909 (0.865–0.954) | 73.00% | 96.67% | <0.001 |
| TBAD vs. AMI | |||||
| Vinculin, pg/ml | 80.57 | 0.650 (0.522–0.779) | 94.00% | 46.67% | 0.013 |
| Blood Parameters | Vinculin (pg/mL) | VE-Cadherin (ng/μL) | ||
|---|---|---|---|---|
| R | p Value | R | p Value | |
| Blood routine | ||||
| WBC, ×109/L | 0.194 | 0.054 | 0.084 | 0.409 |
| RBC, ×1012/L | −0.047 | 0.649 | −0.023 | 0.826 |
| HGB, g/L | −0.056 | 0.58 | −0.075 | 0.459 |
| BA, ×109/L | −0.005 | 0.964 | −0.047 | 0.646 |
| EO, ×109/L | −0.093 | 0.359 | −0.046 | 0.652 |
| LY, ×109/L | −0.154 | 0.125 | −0.136 | 0.179 |
| MO, ×109/L | 0.090 | 0.372 | −0.059 | 0.560 |
| NE, ×109/L | 0.202 | 0.043 * | 0.111 | 0.270 |
| MCH, pg | 0.058 | 0.578 | −0.048 | 0.644 |
| MCHC, g/L | −0.156 | 0.13 | −0.008 | 0.942 |
| MCV, fL | 0.121 | 0.242 | −0.055 | 0.597 |
| HCT, L/L | −0.018 | 0.861 | −0.072 | 0.489 |
| Blood coagulation function | ||||
| APTT, s | −0.176 | 0.09 | −0.046 | 0.633 |
| PT, s | −0.041 | 0.693 | −0.092 | 0.376 |
| PTA, % | −0.019 | 0.855 | 0.086 | 0.405 |
| INR | 0.018 | 0.864 | −0.065 | 0.53 |
| D-Dimer, ug/ml | 0.048 | 0.636 | 0.217 | 0.030 * |
| Cardiovascular injury-related parameters | ||||
| CK, U/L | −0.007 | 0.952 | 0.17 | 0.162 |
| Inflammatory response | ||||
| CRP, mg/L | 0.013 | 0.902 | 0.065 | 0.524 |
| Liver function | ||||
| ALB, g/L | −0.023 | 0.828 | 0.034 | 0.741 |
| ALP, U/L | −0.008 | 0.944 | −0.106 | 0.319 |
| ALT, U/L | −0.014 | 0.895 | −0.103 | 0.321 |
| AST, U/L | 0.144 | 0.19 | −0.12 | 0.277 |
| PA, mg/dL | −0.184 | 0.128 | 0.178 | 0.140 |
| LDH, U/L | 0.055 | 0.646 | −0.139 | 0.242 |
| Serum lipid profile | ||||
| HDL-C, mmol/L | 0.235 | 0.033 * | 0.033 | 0.766 |
| TC, mmol/L | −0.083 | 0.468 | 0.134 | 0.244 |
| TG, mmol/L | −0.32 | 0.003 ** | 0.069 | 0.535 |
| LDL-C, mmol/L | −0.137 | 0.219 | 0.108 | 0.336 |
| Vinculin | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | OR (95% CI) | p Value | β | OR (95% CI) | p Value | β | OR (95% CI) | p Value | |
| Refractory pain | 0.003 | 1.003 (0.999:1.007) | 0.175 | 0.003 | 1.003 (0.998:1.007) | 0.247 | 0.003 | 1.003 (0.998:1.007) | 0.262 |
| Uncontrollable hypertension | 0.005 | 1.005 (1.001:1.010) | 0.024 | 0.004 | 1.004 (0.999:1.009) | 0.086 | 0.004 | 1.004 (0.999:1.010) | 0.150 |
| Limb malperfusion | 0.004 | 1.004 (1.000:1.009) | 0.075 | 0.003 | 1.003 (0.999:1.008) | 0.170 | 0.004 | 1.004 (0.999:1.010) | 0.109 |
| Visceral malperfusion | 0.007 | 1.008 (1.002:1.013) | 0.006 | 0.007 | 1.007 (1.001:1.013) | 0.013 | 0.007 | 1.007 (1.001:1.013) | 0.014 |
| VE-Cadherin | Model 1 | Model 2 | Model 3 | ||||||
| β | OR (95% CI) | p Value | β | OR (95% CI) | p Value | β | OR (95% CI) | p Value | |
| Refractory pain | 0.16 | 1.173 (1.024:1.344) | 0.021 | 0.158 | 1.172 (1.011:1.358) | 0.036 | 0.159 | 1.172 (1.010:1.361) | 0.036 |
| Uncontrollable hypertension | 0.069 | 1.072 (0.945:1.215) | 0.283 | 0.028 | 1.029 (0.899:1.177) | 0.680 | 0.027 | 1.028 (0.892:1.184) | 0.706 |
| Limb malperfusion | 0.075 | 1.077 (0.929:1.249) | 0.323 | 0.039 | 1.040 (0.888:1.218) | 0.627 | 0.044 | 1.045 (0.889:1.227) | 0.595 |
| Visceral malperfusion | 0.057 | 1.059 (0.936:1.197) | 0.364 | 0.026 | 1.027 (0.902:1.168) | 0.690 | 0.037 | 1.037 (0.905:1.189) | 0.599 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Li, X.; Jiang, H.; Zhang, J. High Serum VE-Cadherin and Vinculin Concentrations Are Markers of the Disruption of Vascular Integrity during Type B Acute Aortic Dissection. J. Clin. Med. 2023, 12, 4730. https://doi.org/10.3390/jcm12144730
Wang S, Li X, Jiang H, Zhang J. High Serum VE-Cadherin and Vinculin Concentrations Are Markers of the Disruption of Vascular Integrity during Type B Acute Aortic Dissection. Journal of Clinical Medicine. 2023; 12(14):4730. https://doi.org/10.3390/jcm12144730
Chicago/Turabian StyleWang, Shiyue, Xin Li, Han Jiang, and Jian Zhang. 2023. "High Serum VE-Cadherin and Vinculin Concentrations Are Markers of the Disruption of Vascular Integrity during Type B Acute Aortic Dissection" Journal of Clinical Medicine 12, no. 14: 4730. https://doi.org/10.3390/jcm12144730
APA StyleWang, S., Li, X., Jiang, H., & Zhang, J. (2023). High Serum VE-Cadherin and Vinculin Concentrations Are Markers of the Disruption of Vascular Integrity during Type B Acute Aortic Dissection. Journal of Clinical Medicine, 12(14), 4730. https://doi.org/10.3390/jcm12144730







