Impact of a VA–ECMO in Combination with an Extracorporeal Cytokine Hemadsorption System in Critically Ill Patients with Cardiogenic Shock–Design and Rationale of the ECMOsorb Trial
Abstract
:1. Introduction
2. Methods and Analysis
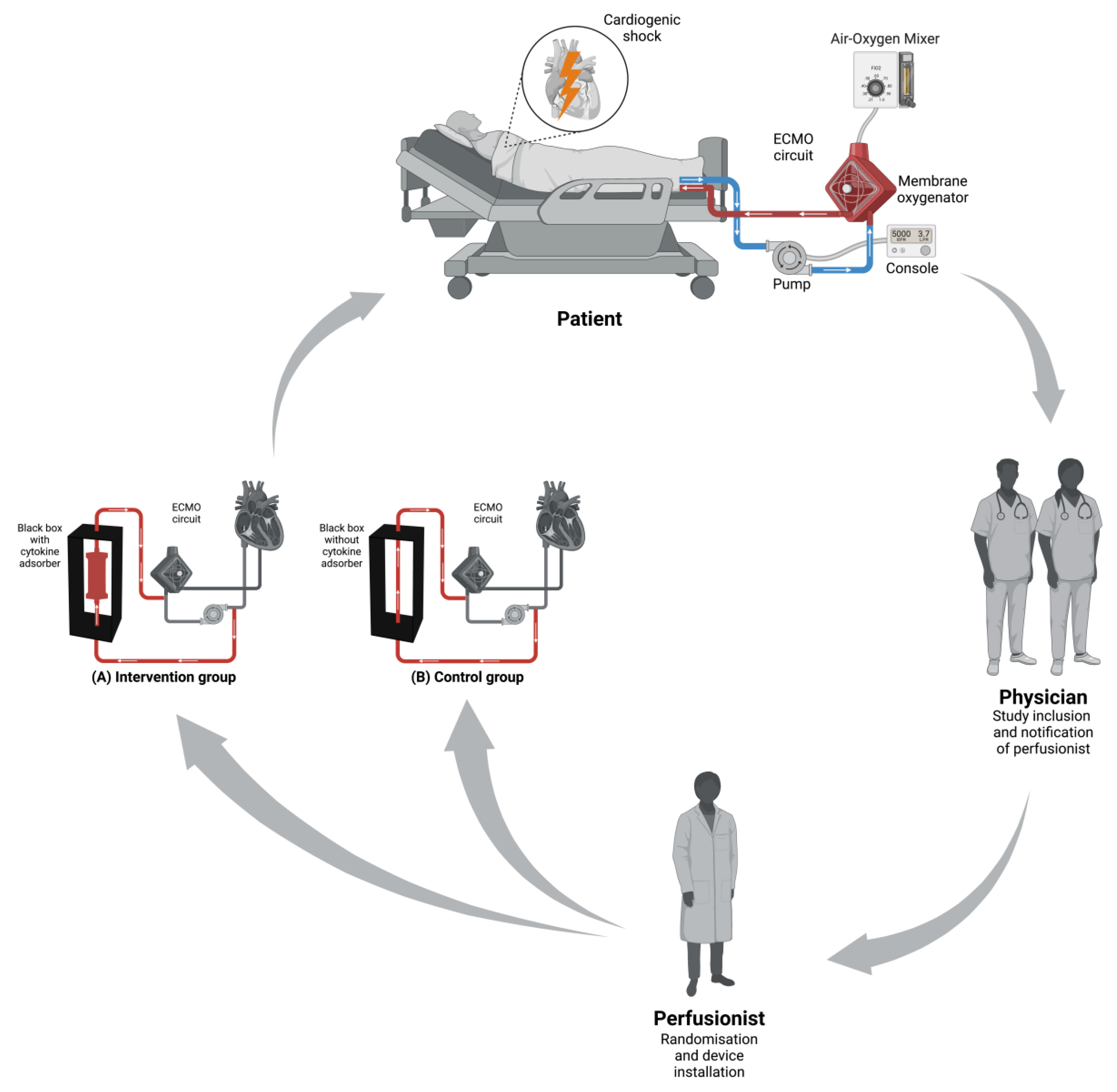
2.1. Study Design
2.2. Study Population
2.3. Time Schedule, Study Duration and Frequency of Study Visits
2.4. Screening and Randomization
2.5. Obtaining Informed Consent
2.6. Objectives
2.6.1. Primary Objective
2.6.2. Secondary Objective
2.7. Endpoints
2.7.1. Primary Endpoint
2.7.2. Secondary Endpoints of the Study Are as Follows (Selection)
2.8. Inclusion Criteria
2.9. Exclusion Criteria
2.10. Technology Used in the Trial
2.10.1. ECMO
2.10.2. VA–ECMO Weaning
2.10.3. Cytokine Adsobers
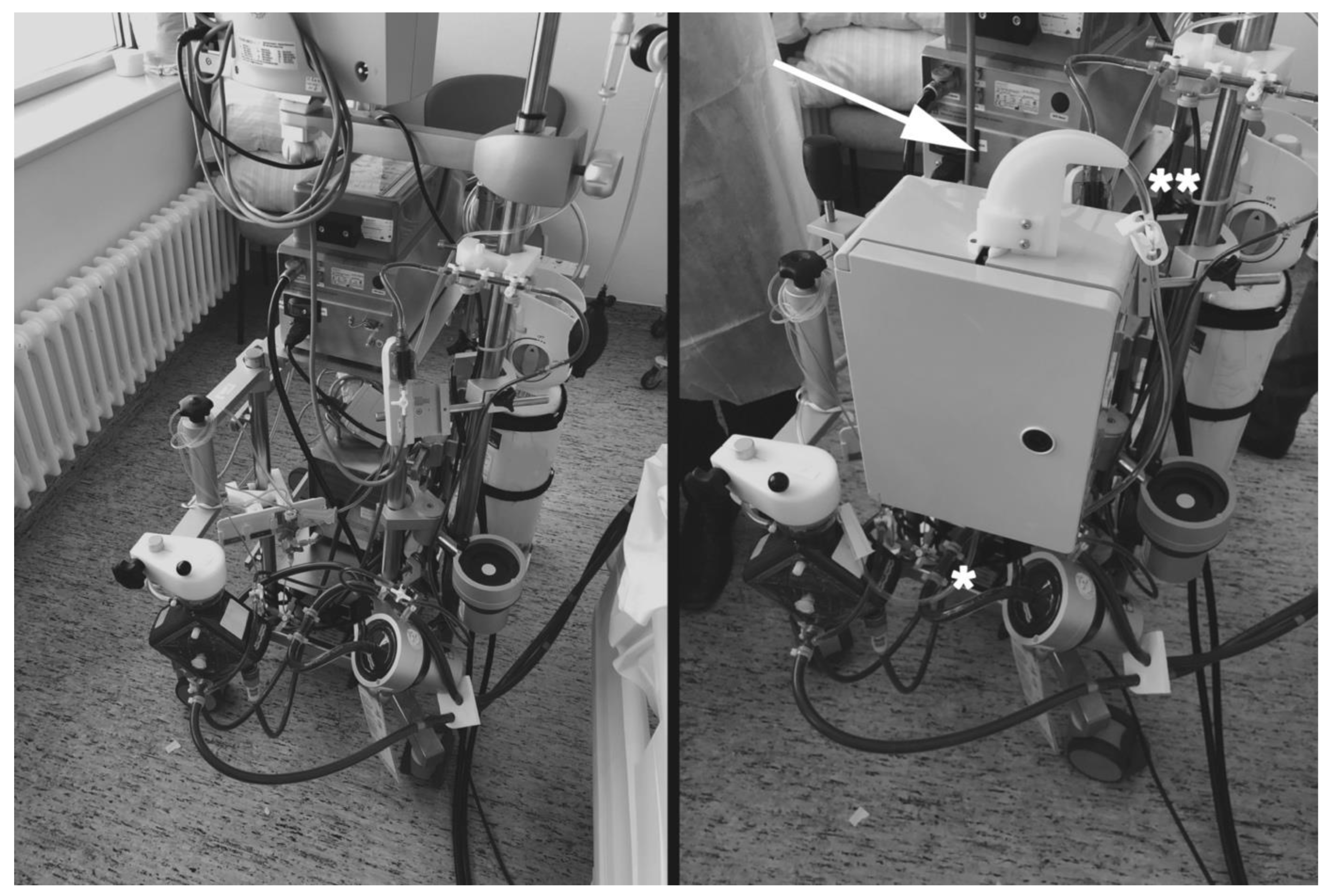
2.10.4. Black Box
2.11. Safety, Possible Complications, Risks
2.12. Statistical Considerations and Methods
2.12.1. Sample Size
2.12.2. Statistics
2.13. Addressing Bias in the Methods
2.13.1. Randomization
2.13.2. Treatment Bias
2.13.3. Measurement Bias
2.14. Data Management
2.14.1. Data Assessment/Case Report Forms
2.14.2. Ethics Approval
2.14.3. Privacy, Collection and Processing of Data
2.14.4. Dissemination
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gräsner, J.-T.; Herlitz, J.; Tjelmeland, I.B.; Wnent, J.; Masterson, S.; Lilja, G.; Bein, B.; Böttiger, B.W.; Rosell-Ortiz, F.; Nolan, J.P.; et al. European Resuscitation Council Guidelines 2021: Epidemiology of cardiac arrest in Europe. Resuscitation 2021, 161, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Soar, J.; Zideman, D.A.; Biarent, D.; Bossaert, L.L.; Deakin, C.; Koster, R.W.; Wyllie, J.; Böttiger, B. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation 2010, 81, 1219–1276. [Google Scholar] [CrossRef] [PubMed]
- Hutin, A.; Abu-Habsa, M.; Burns, B.; Bernard, S.; Bellezzo, J.; Shinar, Z.; Torres, E.C.; Gueugniaud, P.-Y.; Carli, P.; Lamhaut, L. Early ECPR for out-of-hospital cardiac arrest: Best practice in 2018. Resuscitation 2018, 130, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Michels, G.; Wengenmayer, T.; Hagl, C.; Dohmen, C.; Böttiger, B.W.; Bauersachs, J.; Markewitz, A.; Bauer, A.; Gräsner, J.-T.; Pfister, R.; et al. Empfehlungen zur extrakorporalen kardiopulmonalen Reanimation (eCPR). Der Kardiol. 2018, 12, 332–341. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.J.; Lee, H.Y.; Ahn, H.S.; Lee, S.W. Comparing extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: A meta-analysis. Resuscitation 2016, 103, 106–116. [Google Scholar] [CrossRef]
- Soar, J.; Nolan, J.P.; Böttiger, B.W.; Perkins, G.D.; Lott, C.; Carli, P.; Pellis, T.; Sandroni, C.; Skrifvars, M.B.; Smith, G.B.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support. Resuscitation 2015, 95, 100–147. [Google Scholar] [CrossRef] [Green Version]
- Vdovin, N.; Günther, S.P.; de Waha, S.; Seizer, P.; Brunner, S.; Schlensak, C.; Thiele, H.; Hagl, C.; Massberg, S.; Bauer, A. Early Risk Stratification in Patients With Cardiogenic Shock Complicating Acute Myocardial Infarction Treated With Extracorporeal Life Support and Primary Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2017, 10, 2469–2471. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lin, J.-W.; Yu, H.-Y.; Ko, W.-J.; Jerng, J.-S.; Chang, W.-T.; Chen, W.-J.; Huang, S.-C.; Chi, N.-H.; Wang, C.-H.; et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet 2008, 372, 554–561. [Google Scholar] [CrossRef]
- Wengenmayer, T.; Rombach, S.; Ramshorn, F.; Biever, P.; Bode, C.; Duerschmied, D.; Staudacher, D.L. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit. Care 2017, 21, 157. [Google Scholar] [CrossRef]
- Bro-Jeppesen, J.; Kjaergaard, J.; Wanscher, M.; Nielsen, N.; Friberg, H.; Bjerre, M.; Hassager, C. Systemic Inflammatory Response and Potential Prognostic Implications After Out-of-Hospital Cardiac Arrest: A Substudy of the Target Temperature Management Trial. Crit. Care Med. 2015, 43, 1223–1232. [Google Scholar] [CrossRef]
- Kellum, J.A.; Kong, L.; Fink, M.P.; Weissfeld, L.A.; Yealy, D.M.; Pinsky, M.R.; Fine, J.; Krichevsky, A.; Delude, R.L.; Angus, D.C.; et al. Faculty Opinions recommendation of Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch. Intern. Med. 2007, 167, 1655–1663. [Google Scholar] [CrossRef] [Green Version]
- Napp, L.C.; Ziegeler, S.; Kindgen-Milles, D. Rationale of Hemoadsorption during Extracorporeal Membrane Oxygenation Support. Blood Purif. 2019, 48, 203–214. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, W.; Shi, J.; Shen, J.; Hu, Y.; Gao, T.; Zhang, J.; Xi, F.; Gong, J.; Li, J.; et al. The effect of venovenous extra-corporeal membrane oxygenation (ECMO) therapy on immune inflammatory response of cerebral tissues in porcine model. J. Cardiothorac. Surg. 2013, 8, 186. [Google Scholar] [CrossRef] [Green Version]
- Boyd, J.H.; Forbes, J.; Nakada, T.-A.; Walley, K.R.; Russell, J.A. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit. Care Med. 2011, 39, 259–265. [Google Scholar] [CrossRef]
- Dünser, M.W.; Ruokonen, E.; Pettilä, V.; Ulmer, H.; Torgersen, C.; Schmittinger, C.A.; Jakob, S.; Takala, J. Association of arterial blood pressure and vasopressor load with septic shock mortality: A post hoc analysis of a multicenter trial. Crit. Care 2009, 13, R181. [Google Scholar] [CrossRef] [Green Version]
- Tarvasmaki, T.; Lassus, J.; Varpula, M.; Sionis, A.; Sund, R.; Kober, L.; Spinar, J.; Parissis, J.; Banaszewski, M.; Silva Cardoso, J.; et al. Current real-life use of vasopressors and inotropes in cardiogenic shock—Adrenaline use is associated with excess organ injury and mortality. Crit. Care 2016, 20, 208. [Google Scholar] [CrossRef] [Green Version]
- Samuels, L.E.; Kaufman, M.S.; Thomas, M.P.; Holmes, E.C.; Brockman, S.K.; Wechsler, A.S. Pharmacological Criteria for Ventricular Assist Device Insertion Following Postcardiotomy Shock: Experience with the Abiomed BVS System. J. Card. Surg. 1999, 14, 288–293. [Google Scholar] [CrossRef]
- Kellum, J.A. Immunomodulation in sepsis: The role of hemofiltration. Minerva Anestesiol. 1999, 65, 410–418. [Google Scholar]
- Kellum, J.A. Hemoadsorption therapy for sepsis syndromes. Crit. Care Med. 2003, 31, 323–324. [Google Scholar] [CrossRef]
- Kellum, J.A.; Song, M.; Venkataraman, R. Hemoadsorption removes tumor necrosis factor, interleukin-6, and interleukin-10, reduces nuclear factor-κB DNA binding, and improves short-term survival in lethal endotoxemia. Crit. Care Med. 2004, 32, 801–805. [Google Scholar] [CrossRef]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, E.; Houston, B.L.; Kumar, A.; Abou-Setta, A.M.; Friesen, C.; Marshall, J.C.; Rock, G.; Turgeon, A.F.; Cook, D.J.; Houston, D.S.; et al. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: A systematic review and meta-analysis. Crit. Care 2014, 18, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opal, S.M.; Laterre, P.-F.; Francois, B.; LaRosa, S.P.; Angus, D.C.; Mira, J.-P.; Wittebole, X.; Dugernier, T.; Perrotin, D.; Tidswell, M.; et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: The ACCESS randomized trial. JAMA 2013, 309, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, A.; Lorente, J.A.; Steingrub, J.; Bakker, J.; McLuckie, A.; Willatts, S.; Brockway, M.; Anzueto, A.; Holzapfel, L.; Breen, D.; et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: Effect on survival in patients with septic shock. Crit. Care Med. 2004, 32, 21–30. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Privalle, C.T.; Singer, M.; Lorente, J.A.; Boehm, E.; Meier-Hellmann, A.; Darius, H.; Ferrer, R.; Sirvent, J.-M.; Marx, G.; et al. Multicenter, Randomized, Placebo-Controlled Phase III Study of Pyridoxalated Hemoglobin Polyoxyethylene in Distributive Shock (PHOENIX). Crit. Care Med. 2015, 43, 57–64. [Google Scholar] [CrossRef]
- Ziegler, E.J.; Fisher, C.J.; Sprung, C.L.; Straube, R.C.; Sadoff, J.C.; Foulke, G.E.; Wortel, C.H.; Fink, M.P.; Dellinger, R.P.; Teng, N.N.; et al. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N. Engl. J. Med. 1991, 324, 429–436. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Biston, P.; Devriendt, J.; Madl, C.; Chochrad, D.; Aldecoa, C.; Brasseur, A.; Defrance, P.; Gottignies, P.; Vincent, J.-L.; et al. Comparison of dopamine and norepinephrine in the treatment of shock. N. Engl. J. Med. 2010, 362, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Scharf, C.; Liebchen, U.; Paal, M.; Irlbeck, M.; Zoller, M.; Schroeder, I. Blood purification with a cytokine adsorber for the elimination of myoglobin in critically ill patients with severe rhabdomyolysis. Crit. Care 2021, 25, 41. [Google Scholar] [CrossRef]
- Napp, L.C.; Kühn, C.; Bauersachs, J. ECMO in cardiac arrest and cardiogenic shock. Herz 2017, 42, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Napp, L.C.; Kühn, C.; Hoeper, M.M.; Vogel-Claussen, J.; Haverich, A.; Schäfer, A.; Bauersachs, J. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin. Res. Cardiol. 2016, 105, 283–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napp, L.C.; Vogel-Claussen, J.; Schäfer, A.; Haverich, A.; Bauersachs, J.; Kühn, C.; Tongers, J. First-in-Man Fully Percutaneous Complete Bypass of Heart and Lung. JACC Cardiovasc. Interv. 2017, 10, e231–e233. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Lang, H.; Barbosa, C.V.; Tian, Z.; Melk, A.; Schmidt, B.M.W. Efficacy of CytoSorb(R): A systematic review and meta-analysis. Crit. Care 2023, 27, 215. [Google Scholar] [CrossRef]


| Protocol Items | Time Point | ||||||
|---|---|---|---|---|---|---|---|
| S | V0 | V1 | V2 | V3 | |||
| Routine Exami-nation | Informed Consent Process | Screening | Baseline | Intervention | Post Intervention | Post Intervention | |
| Day 0–30 | Day 0 | 0–72 h | 7 Days after Intervention | 30 Days after Intervention | |||
| Trial related procedures | |||||||
| Inclusion/exclusion criteria | X | ||||||
| Informed consent | X | ||||||
| Patient registration | X | ||||||
| Randomization | X | ||||||
| Study device † initiation | X 1 | X 2 | |||||
| General examination | |||||||
| Medical history, comorbidities ∆ | X | X | |||||
| Physical examination ∆ | X | X | X | ||||
| Prior/concomitant medication ∆ | X | X | X | ||||
| Neurological exam (incl. mRS) ∆ | X | X | X | X | X | X | |
| Quality of Life (EQ -5D-3L including EQ VAS) | X | X | |||||
| SCOREs (SAPS II, APACHE II, SOFA) ∆ | X | X | X | X | |||
| Adverse events/complications ∆ | X * | X | X | X | |||
| Diagnostic procedures | |||||||
| 12-lead surface ECG ∆ | X | X | X | X | |||
| TTE ∆ | X | X | X | X | X | ||
| Technical data from ECMO ∆ | X | X | X | X | |||
| Laboratory evaluation ∆ | X | X | X | X | X | ||
| Local blood chemistry ∆ | X | X | X | X | |||
| PAC–Hemodynamics ∆ | X | X | X | ||||
| Measurement of parameters of hemodynamics ∆ | X | X | X | X | X | ||
| Measurement of mortality ∆ | X | X | X | ||||
| Measurement of duration of ECMO, ventilation, dialysis, inotropic therapy ∆ | X | X | X | X | X | ||
| Measurement of the neurological outcome ∆ | X | X | X | X | X | ||
| VA–ECMO Implantation | X | X | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haertel, F.; Lehmann, T.; Heller, T.; Fritzenwanger, M.; Pfeifer, R.; Kretzschmar, D.; Otto, S.; Bogoviku, J.; Westphal, J.; Bruening, C.; et al. Impact of a VA–ECMO in Combination with an Extracorporeal Cytokine Hemadsorption System in Critically Ill Patients with Cardiogenic Shock–Design and Rationale of the ECMOsorb Trial. J. Clin. Med. 2023, 12, 4893. https://doi.org/10.3390/jcm12154893
Haertel F, Lehmann T, Heller T, Fritzenwanger M, Pfeifer R, Kretzschmar D, Otto S, Bogoviku J, Westphal J, Bruening C, et al. Impact of a VA–ECMO in Combination with an Extracorporeal Cytokine Hemadsorption System in Critically Ill Patients with Cardiogenic Shock–Design and Rationale of the ECMOsorb Trial. Journal of Clinical Medicine. 2023; 12(15):4893. https://doi.org/10.3390/jcm12154893
Chicago/Turabian StyleHaertel, Franz, Thomas Lehmann, Tabitha Heller, Michael Fritzenwanger, Ruediger Pfeifer, Daniel Kretzschmar, Sylvia Otto, Jurgen Bogoviku, Julian Westphal, Christiane Bruening, and et al. 2023. "Impact of a VA–ECMO in Combination with an Extracorporeal Cytokine Hemadsorption System in Critically Ill Patients with Cardiogenic Shock–Design and Rationale of the ECMOsorb Trial" Journal of Clinical Medicine 12, no. 15: 4893. https://doi.org/10.3390/jcm12154893
APA StyleHaertel, F., Lehmann, T., Heller, T., Fritzenwanger, M., Pfeifer, R., Kretzschmar, D., Otto, S., Bogoviku, J., Westphal, J., Bruening, C., Gecks, T., Kaluza, M., Moebius-Winkler, S., & Schulze, P. C. (2023). Impact of a VA–ECMO in Combination with an Extracorporeal Cytokine Hemadsorption System in Critically Ill Patients with Cardiogenic Shock–Design and Rationale of the ECMOsorb Trial. Journal of Clinical Medicine, 12(15), 4893. https://doi.org/10.3390/jcm12154893







