Abstract
Introduction: Patients with kidney failure with replacement therapy (KFRT) suffer from a disproportionately high cardiovascular disease burden. Circulating small non-coding RNAs (c-sncRNAs) have emerged as novel epigenetic regulators and are suggested as novel biomarkers and therapeutic targets for cardiovascular disease; however, little is known about the associations of c-sncRNAs with premature cardiovascular death in KFRT. Methods: In a pilot case-control study of 50 hemodialysis patients who died of cardiovascular events as cases, and 50 matched hemodialysis controls who remained alive during a median follow-up of 2.0 years, we performed c-sncRNAs profiles using next-generation sequencing to identify differentially expressed circulating microRNAs (c-miRNAs) between the plasma of cases and that of controls. mRNA target prediction and pathway enrichment analysis were performed to examine the functional relevance of differentially expressed c-miRNAs to cardiovascular pathophysiology. The association of differentially expressed c-miRNAs with cardiovascular mortality was examined using multivariable conditional logistic regression. Results: The patient characteristics were similar between cases and controls, with a mean age of 63 years, 48% male, and 54% African American in both groups. We detected a total of 613 miRNAs in the plasma, among which five miRNAs (i.e., miR-129-1-5p, miR-500b-3p, miR-125b-1-3p, miR-3648-2-5p, and miR-3150b-3p) were identified to be differentially expressed between cases and controls with cut-offs of p < 0.05 and log2 fold-change (log2FC) > 1. When using more stringent cut-offs of p-adjusted < 0.05 and log2FC > 1, only miR-129-1-5p remained significantly differentially expressed, with higher levels of miR-129-1-5p in the cases than in the controls. The pathway enrichment analysis using predicted miR-129-1-5p mRNA targets demonstrated enrichment in adrenergic signaling in cardiomyocytes, arrhythmogenic right ventricular cardiomyopathy, and oxytocin signaling pathways. In parallel, the circulating miR-129-1-5p levels were significantly associated with the risk of cardiovascular death (adjusted OR [95% CI], 1.68 [1.01–2.81] for one increase in log-transformed miR-129-1-5p counts), independent of potential confounders. Conclusions: Circulating miR-129-1-5p may serve as a novel biomarker for premature cardiovascular death in KFRT.
1. Introduction
Kidney failure with replacement therapy (KFRT) is a condition characterized by an extremely high risk of cardiovascular morbidity and mortality, with almost half of all deaths attributable to cardiovascular disease [1]. Despite considerable efforts to improve cardiovascular outcomes, the substantial cardiovascular disease burden in KFRT remains unresolved, consuming a disproportionate amount of financial resources [2,3]. Therefore, identifying novel risk factors and promising biomarkers for cardiovascular disease in KFRT is of critical importance toward the development of novel preventives and therapeutic approaches to premature cardiovascular death in this population.
Small non-coding RNAs (sncRNAs) are a group of small (18–200 nucleotide-long) RNA molecules that function as epigenetic regulators of gene expression at the post-transcriptional level involved in intercellular communication and crosstalk between different organs [4]. As key regulators of homeostasis, their dysregulation underlies several morbidities through the combinatorial effect of gene expression changes in all related downstream targets [5,6,7,8,9]. MicroRNAs (miRNAs), for example, are the most extensively studied class of sncRNAs that negatively regulate gene expression by partially pairing to the 3′-untranslated region of their target messenger RNAs, leading to translation repression and/or transcript degradation [10]. The other major classes of sncRNAs include transfer RNA (tRNA), small interfering RNAs (siRNAs), and piwi-interacting RNAs (piRNAs) [11,12,13].
While the majority of sncRNAs exist intracellularly, they can also be found in various body fluids, including blood, through passive leakage and/or active secretion from cells [14,15,16,17,18]. Importantly, accumulating evidence indicates that the aberrant expression of these so-called circulating sncRNAs (c-sncRNAs) is associated with the risk of cardiovascular disease [19,20,21,22,23,24,25], suggesting the potential of c-sncRNAs as novel cardiovascular biomarkers and therapeutic targets [26,27]. However, the existing studies on the association of c-sncRNA with cardiovascular disease are predominantly from the non-KFRT populations, limiting the evidence among patients with KFRT who display a distinct cardiovascular phenotype [28]. Furthermore, a few studies reporting the possible involvement of circulating miRNAs (c-miRNA) in cardiovascular health in patients with KFRT have focused primarily on the association of pre-specified c-miRNAs (e.g., miR-125b and miR-133a) with cardiovascular surrogates, such as vascular calcification and cardiac hypertrophy [29,30,31]; hence, it remains unclear if these c-miRNAs are associated with hard cardiovascular outcomes, including premature cardiovascular death, in KFRT.
We hypothesized that c-miRNAs would be differentially expressed between patients with KFRT who died of a cardiovascular event and those without such an event and that the differentially expressed c-miRNAs would be involved in cardiovascular pathophysiology that could lead to premature cardiovascular death in patients with KFRT. In this pilot case-control study of patients with KFRT receiving hemodialysis therapy, we therefore aimed to perform comprehensive profiling of c-miRNAs and identify differentially expressed c-miRNAs associated with premature cardiovascular death in these patients.
2. Methods and Materials
2.1. Study Design
This was a case-control study sourced from a prospective study of anonymized samples and statistically de-identified clinical data (detailed below) obtained from a biorepository assembled by DaVita Clinical Research (Minneapolis, MN, USA). Anonymized samples and statistically de-identified data were made available to the authors for academic research via a grant program called BioReG.
2.2. Study Population
The DaVita Clinical Research biorepository comprises blood samples and clinical data from 4028 individuals with prevalent end-stage renal disease who received hemodialysis at a large dialysis organization (LDO) between May 2011 and October 2013, as previously described [32]. Patients with hemoglobin < 8.0 g/dL, who were <18 years of age, who were pregnant, or who had any physical, mental, or medical condition, which prohibited the ability to provide informed consent were excluded from participation. The biorepository sampling protocol was reviewed and approved by an Institutional Review Board (IRB) (Quorum IRB, Seattle, WA, USA) and patients provided written informed consent prior to the initiation of sample collection.
For the present pilot case-control study, we used plasma samples at baseline (i.e., first blood sampling date) and clinical data corresponding to the blood sampling date in a total of 100 hemodialysis patients within the repository housed at the University of Tennessee Health Science Center (UTHSC) (UT-DaVita hemodialysis cohort; n = 978) [33,34]. Cases (n = 50) were hemodialysis patients who died of a cardiovascular event, while controls (n = 50) were those who remained alive over the entire follow-up, matched 1:1 by age, sex, race, and dialysis vintage to account for major non-modifiable cardiovascular risk factors. The study was approved by the IRB at UTHSC (IRB protocol number: 17-05299-XP).
2.3. Biorepository Biospecimen and Clinical Data Collection
Under the biorepository study protocol, blood samples were collected from each subject at baseline and, thereafter, every 3 months for up to one year. Pre-dialysis blood samples were collected and processed according to a standardized protocol as previously described [32,33,34]. Briefly, anonymized plasma samples were shipped in refrigerated packs from the centralized laboratory to the researchers and stored at −80 °C. Clinical data for each biorepository subject were collected by the LDO during the course of routine care and were maintained in the LDO electronic health record. The data were then provided to the researchers by DaVita Clinical Research in a statistically de-identified form. Cardiovascular death was defined as death caused by acute myocardial infarction, atherosclerotic heart disease, cardiomyopathy, cardiac arrhythmia, cardiac arrest, or congestive heart failure [34].
2.4. RNA Purification from Plasma and cDNA Library Preparation
Total RNA from the plasma samples were purified as described before [35]. Briefly, c-sncRNAs were purified from 200 µL plasma using the Qiagen miRNeasy Serum/Plasma Advanced Kit (Cat No. 217204). The purified sncRNAs were then eluded with 15 µL RNase-free water, and 5 µL of RNA was used for c-sncRNA library using NEBNext Small RNA Library Prep Set (Cat No. E7330L) according to the manufacturer’s protocol. In total, 18 cycles of PCR amplification were performed. c-sncRNA libraries were purified by polyacrylamide gel and then subjected to Agilent bioanalyzer for quality control. Equal amounts of libraries were mixed and sequenced in NextSeq500 in Oklahoma Medical Research Foundation.
2.5. c-sncRNA Sequencing Data and Differential Expression Analysis
c-sncRNA sequencing was performed for 100 hemodialysis patients with (n = 50) and without (n = 50) a fatal cardiovascular event. Obtained reads were mapped to human genome using unitas tool [36]. We obtained around 7 million reads per sample on average and 60% of obtained reads mapped to human genome on average. Differentially expressed c-miRNAs were analyzed using DeSeq2 tool [37], and p-adjusted values for differentially expressed genes were calculated in DESeq2 using the Benjamini-Hochberg method. Detected c-miRNAs and differentially expressed c-miRNAs can be found in Supplementary Tables S1 and S2.
2.6. mRNA Target Prediction and Pathway Enrichment Analysis
mRNA targets of miR-129-1-5p were predicted using TargetScan tool [38]. In total, 732 mRNA targets with conserved sites (Supplementary Table S3) were used in downstream analysis. Pathway enrichment analysis such as for Kyoto Encyclopedia of Genes and Genomes (KEGG) and Reactome Pathways was performed using EnrichR (https://maayanlab.cloud/Enrichr/ (accessed on 15 December 2022)) [39]. Phenotype Genotype Integrator (PhenGenI) in Diseases/Drugs section in Enrich R website was used to investigate the relationship between miR-129-5p and the patient cohort using targets of miR-129-5p.
2.7. Statistical Analysis
Baseline patient characteristics by cardiovascular case status were presented as numbers (percentages) for categorical variables and mean (standard deviation [SD]) for continuous variables with a normal distribution or median (interquartile interval [IQI]) for those with a skewed distribution. Differences between groups were assessed using Chi-square test or Fisher’s exact test (for categorical variables) and t-test or Wilcoxon rank-sum test (for continuous variables), as appropriate.
In order to examine the risk of cardiovascular death associated with differentially expressed miR-129-1-5p, we applied multivariable conditional logistic regression models. Given the limited sample size of this study, the exposure (i.e., the log-transformed miR-129-1-5p counts) was treated as a continuous variable, and the following incremental models were used to account for potential confounders on top of the matching factors (i.e., age, sex, race, and dialysis vintage) based on theoretical consideration, data availability, and residual imbalance after matching: Model 1 was unadjusted; Model 2 included body mass index, diabetes mellitus, congestive heart failure, and ischemic heart disease; and Model 3 was additionally adjusted for serum alkaline phosphatase and phosphorus levels. All analyses were performed in patients with complete data available using STATA/MP Version 17 (STATA Corporation, College Station, TX, USA). A threshold of statistical significance was set at the level of p < 0.05 for these association analyses.
3. Results
3.1. Baseline Characteristics
Patients’ baseline characteristics by cardiovascular case status are presented in Table 1. Cases and controls were of similar age at baseline (means of 63.1 ± 11.0 and 63.1 ± 11.2 years, respectively) and, by design, did not differ for other matching factors, including sex (48.0% male in both), self-reported race/ethnicity (54.0% African American in both), and dialysis vintage (4.1 ± 3.4 and 4.4 ± 3.2 years, respectively). Compared with controls, cases had a lower prevalence of erythropoiesis-stimulating agent use and tended to have higher serum alkaline phosphatase levels and a higher prevalence of statin use.

Table 1.
Baseline patient characteristics by cardiovascular case status.
3.2. c-sncRNA Profiles in Hemodialysis Patients with and without Cardiovascular Death
Using the plasma of 50 hemodialysis patients who died of cardiovascular events as cases and that of 50 matched hemodialysis controls, we detected 613 unique c-miRNAs (Supplementary Table S1). Among these, miR-423-5p, miR-122-5p, and miR-486-5p were the most abundant c-miRNAs in these patients (Supplementary Table S1). We identified five differentially expressed c-miRNAs, including miR-129-1-5p, miR-500b-3p, miR-125b-3p, miR3648-2-5p, and miR3150b-3p, with cut-offs of p < 0.05 and log2 fold-change (log2FC) > 1 (Figure 1A and Supplementary Table S2). Interestingly, all these miRNAs were upregulated in the cases compared to the controls. When we applied a more stringent cut-off of p-adjusted < 0.05 and log2FC > 1, miR-129-1-5p was the only miRNA significantly differentially expressed between groups (Figure 1B and Supplementary Table S2).
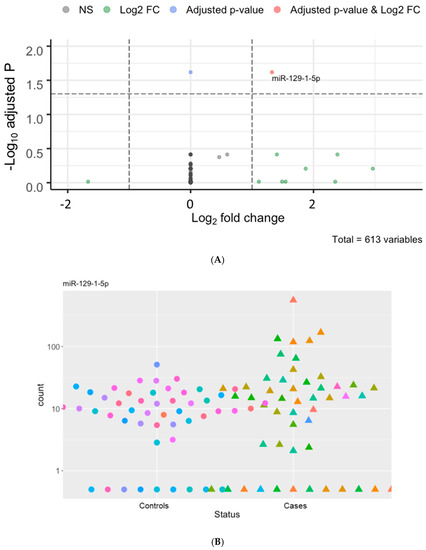
Figure 1.
miR-129-1-5p was overexpressed in the plasma of hemodialysis patients with a fatal cardiovascular death (cases) compared to those without such an event (controls). (A): Volcano plot showing differentially expressed miRNAs. (B): Read counts for miR-129-1-5p in every patient grouped by cases (triangles) vs. controls (circles). Abbreviations: log2 FC = log2 fold-change; miRNA = microRNA.
3.3. Pathway Enrichment Analysis of Circulating miR-129-5p
To understand the functional role of c-miRNAs in premature cardiovascular death in patients with KFRT, we performed a pathway analysis using the predicted targets of miR-129-5p. The top three enriched KEGG pathways in miR-129-5p targets were adrenergic signaling in cardiomyoctes, arrhythmogenic right ventricular cardiomyopathy, and oxytocin signaling pathway (Figure 2). Intriguingly, all these pathways were reported to be directly related to heart function [40,41,42]. Moreover, the most enriched Reactome pathway in miR-129-5p targets was transcriptional regulation by Methyl-CpG-Binding Protein 2 (MECP2), which was shown to have a critical role in heart failure [43,44,45] (Figure 3). In parallel, atherosclerosis was the most enriched phenotype based on the Phenotype Genotype Integrator Enrichment using Enrich R (Figure 4).
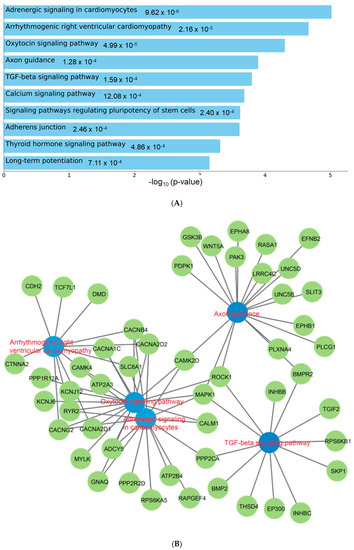
Figure 2.
Pathway enrichment analysis for miR-129-5p targets. Enriched KEGG pathways indicated a potential relation to cardiovascular disease through cardiomyoctes. (A): Bar chart showing the enriched KEGG pathways. (B): Network depicting the miR-129-5p targets in the enriched pathways.
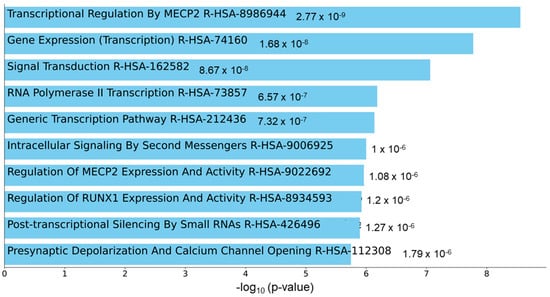
Figure 3.
Enriched Reactome pathways hinted contribution of MECP2 dependent transcriptional regulation to fatal cardiovascular events. Bar chart highlighting the enriched pathways in Reactome pathway enrichment analysis with miR-129-5p targets. Abbreviation: MECP2 = methyl-CpG binding protein 2.
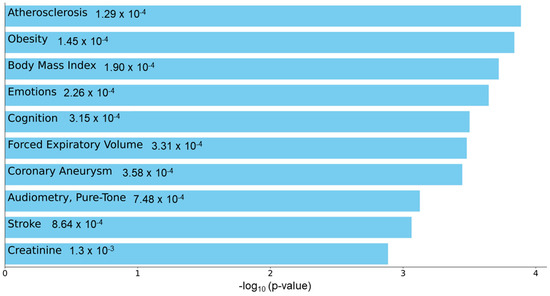
Figure 4.
Enrichment analysis for miR-129-5p targets using Enrich R Disease/Ontologies tools. Enrichment in Phenotype-Genotype Integrator (PhenGenI), which merges genome-wide association studies (GWAS) with several other catalog databases including Gene, the database of Genotypes and Phenotypes (dbGAP), Online Mendelian Inheritance in Man (OMIM), expression quantitative trait loci (eQTL) and Single Nucleotide Polymorphism Database (dbSNP) showed potential relationship to atherosclerosis and obesity. Bar chart highlighting the enriched phenotypes in Phenotype Genotype Integrator enrichment analysis with miR-129-5p targets.
3.4. Association of Circulating miR-129-1-5p with Cardiovascular Death
Since miRNAs were the most abundant c-sncRNA species in the sequencing results, and only miR-129-1-5p was significantly differentially expressed between cases and controls when using stringent significance thresholds (i.e., p-adjusted < 0.05 and log2FC > 1), only miR-129-1-5p was considered in the association analysis with cardiovascular death. Table 2 shows the association of circulating miR-129-1-5p with cardiovascular death using univariable and multivariable conditional logistic regression analyses. In the univariable model, circulating miR-129-1-5p was associated with cardiovascular death, albeit without reaching statistical significance (odds ratio [OR] [95% confidence interval (CI)] for 1-unit higher log-transformed circulating miR-129-1-5p counts: 1.28 [0.89–1.86], in Model 1). This association was slightly modified after multivariable adjustment, with a statistically significant association observed in a fully adjusted model accounting for body mass index, diabetes mellitus, congestive heart failure, ischemic heart disease, and serum alkaline phosphatase and phosphorus levels, on top of the matching factors (i.e., age, sex, race, and dialysis vintage) (adjusted OR [95% CI]: 1.68 [1.01–2.81], in Model 3; Table 2).

Table 2.
Odds ratios and 95% confidence interval for cardiovascular death associated with circulating miR-129-1-5p expression in 100 hemodialysis patients.
4. Discussion
In this pilot case-control study of 100 patients with KFRT receiving maintenance hemodialysis, we detected a total of 613 miRNAs in the plasma, among which five miRNAs (i.e., miR-129-1-5p, miR-500b-3p, miR-125b-1-3p, miR-3648-2-5p, and miR-3150b-3p) were differentially expressed between those who died of a cardiovascular event and those who remained alive. When using stringent statistical thresholds of p-adjusted < 0.05 and log2FC > 1, only miR-129-1-5p remained differentially expressed, with significantly higher expression levels seen in the cardiovascular cases than in the controls. Pathway enrichment analysis using predicted miR-129-1-5p mRNA targets demonstrated enrichment in adrenergic signaling in cardiomyocytes, arrhythmogenic right ventricular cardiomyopathy, and oxytocin signaling pathways. Furthermore, we found that higher circulating miR-129-1-5p levels were significantly associated with a higher risk of cardiovascular death, independent of potential confounders.
Over the past decade, an increasing number of c-sncRNAs have been identified as biomarker candidates in cardiovascular disease [46]; however, their biomarker potential has been poorly understood among patients with KFRT who display a unique cardiovascular phenotype [28]. In an earlier study of 64 maintenance hemodialysis patients and 18 healthy controls, researchers compared the plasma concentrations of circulating miR-133a between hemodialysis patients and healthy controls and examined their association with cardiac hypertrophy among those on hemodialysis. They demonstrated that the circulating miR-133a levels were lower in the hemodialysis patients than in the healthy controls, and they were negatively associated with left ventricular mass index and interventricular septum thickness in hemodialysis patients [30]. Similarly, the levels of circulating miR-125b and miR-206 and their association with vascular calcification among hemodialysis patients have been reported in subsequent studies [29,47]. In a recent study evaluating the association of circulating levels of a selected miRNAs panel (30a-5p, 23a-3p, 451a, and let7d-5p) with a composite of all-cause and cardiovascular mortality in 74 maintenance hemodialysis patients, lower (vs. higher) levels of circulating miRNA 30-5p, 23a-3p, and 451a were significantly associated with a higher risk of the composite outcome [48]. Importantly, however, all these previous studies assessed pre-specified miRNAs and their associations with surrogate measures or composite outcomes of cardiovascular disease, limiting the ability to identify previously unknown or under-recognized circulating miRNAs that may be involved in the pathogenesis of premature cardiovascular death in KFRT. In this context, our unbiased (hypothesis-free), comprehensive profiles of c-miRNAs using a matched case-control design would be of particular value, allowing the identification of novel c-miRNAs associated with premature cardiovascular death in patients with KFRT. And in fact, among 613 c-miRNAs detected in the plasma of 50 cardiovascular cases and 50 controls in our study, we found that circulating miR-129-1-5p was the only c-miRNA that was significantly differentially expressed between groups (Figure 1) and was also independently associated with premature cardiovascular death in these patients (Table 2).
MiR-129-1-5p is a 5′-prime product of a member of the miR-129 family, miR-129-1, which is located on chromosome 7q32.1 and has been shown to be involved in various oncological and non-oncological diseases, including cardiovascular disease [49]. In previous experimental studies in heart failure, for example, miRNA-129-5p (same as miRNA-129-1-5p) [50] has been shown to play a protective role in myocardial cell injury through targeting high-mobility group box-1 (HMGB1) and tumor necrosis factor receptor-associated factor 3 (TRAF3) and thereby ameliorating oxidative stress and inflammatory responses in cardiomyocytes [51,52]. Although these observations seemingly contradict what we have found in the present study, the significantly higher expression of circulating miR-129-1-5p among patients with (vs. without) a fatal cardiovascular event in our study may reflect the physiological process of upregulating miR-129-1-5p in response to damaged cardiomyocytes that eventually led to their fatal cardiovascular events. Nonetheless, it is important to note that the upregulation of miR-129-5p has also been implicated in the development of atherosclerosis by impairing the protective effects of endothelial cell autophagy through miR-129-5p-mediated Beclin-1 suppression [53]. In line with this observation, our enrichment analysis using the Phenotype Genotype Integrator demonstrated that atherosclerosis was the phenotype most significantly associated with miR-129-5p (Figure 4). In addition, the top three enriched pathways in KEGG pathway enrichment suggested that miR-129-5p is directly related to cardiomyocytes and cardiomyopathy (Figure 2). Moreover, Reactome pathways enrichment analysis demonstrated that MECP2-dependent transcriptional regulation might play an important role in the pathogenesis of premature cardiovascular death in patients with KFRT (Figure 3). Interestingly, it has been shown that Mecp2 mutant mice developed lethal cardiac arrhythmias [43]. Most importantly, these findings provide novel insights into the mechanisms underlying premature cardiovascular death in KFRT and could lead to the discovery of novel molecular-based biomarkers and potential therapeutic strategies for premature cardiovascular death in this relevant population.
Despite the strengths of our study, including a well-designed matched case-control cohort nested from a nationwide prospective hemodialysis cohort, the study results must be interpreted in light of several limitations. Given the substantial heterogeneity of hemodialysis patients with various etiologies and comorbidities, our study population was not representative of all patients with KFRT. Although we adjusted for various potential confounders in the analysis to examine the association of miR-129-1-5p with cardiovascular death, due to the small sample size of this pilot case-control study, we were unable to fully account for potential confounders and hence cannot eliminate the possibility of unmeasured confounders that might have affected the association. Similarly, due to the small sample size of this study, we were unable to examine specific causes of CV death. Despite efforts to perform pathway enrichment analyses, we cannot infer any causal relationship between miR-129-1-5p and cardiovascular death, which needs to be examined in more in-depth experimental and clinical studies.
In conclusion, in this pilot case-control study of prevalent hemodialysis patients, we detected a total of 613 miRNAs in the plasma and found that circulating miR-129-1-5p was significantly upregulated (p-adjusted < 0.05 and log2FC > 1) among those with (vs. without) a fatal cardiovascular event. We also demonstrated in the pathway enrichment analysis that miR-129-1-5p is closely involved in cardiovascular pathophysiology. Although these findings should be validated in future larger studies, our results suggest a biomarker potential of miR-129-1-5p for premature cardiovascular death in patients with KFRT.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12155010/s1, Table S1. List of detected miRNAs in the plasma of 100 hemodialysis patients. Table S2. Summary table of differentially expressed miRNAs between cardiovascular cases and controls. Table S3. Targets of miR-129-5p predicted using TargetScan.
Author Contributions
Conceptualization, C.K. (Canan Kuscu), C.P.K. and K.S.; methodology, C.K. (Canan Kuscu), S.N., C.P.K. and K.S.; software, Y.M. and K.S.; formal analysis, C.K. (Canan Kuscu), S.N. and K.S.; resources, Z.H., C.P.K. and K.S.; data curation, C.K. (Canan Kuscu), Y.M., C.P.K. and K.S.; writing—original draft preparation, C.K. (Canan Kuscu), Y.M., C.J.B. and K.S.; writing—review and editing, C.K. (Canan Kuscu), Y.M., S.N., Z.H., C.J.B., C.K. (Cem Kuscu), C.P.K. and K.S.; visualization, C.K. (Canan Kuscu), Y.M., S.N. and K.S.; supervision, C.P.K.; project administration, C.K. (Canan Kuscu) and K.S.; funding acquisition, C.K. (Canan Kuscu) and K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Tennessee Clinical and Translational Science Institute (TN-CTSI).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Tennessee Health Science Center (IRB protocol number: 17-05299-XP; 23 June 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used in these analyses were provided by DaVita Clinical Research. Requests for access to data can be made in writing to DaVita Clinical Research.
Acknowledgments
Kovesdy is an employee of the US Department of Veterans affairs. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of Veterans Affairs or the US government. The results of this paper have not been published previously in whole or part.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Bragg-Gresham, J.; Balkrishnan, R.; Bhave, N.; Dietrich, X.; Ding, Z.; Eggers, P.W.; et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2019, 73, A7–A8. [Google Scholar] [CrossRef]
- Levey, A.S.; Atkins, R.; Coresh, J.; Cohen, E.P.; Collins, A.J.; Eckardt, K.U.; Nahas, M.E.; Jaber, B.L.; Jadoul, M.; Levin, A.; et al. Chronic kidney disease as a global public health problem: Approaches and initiatives—A position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007, 72, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Kottgen, A.; Levey, A.S.; Levin, A. Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 2013, 382, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, A.; Lee, I.; Hood, L.; Galas, D.; Wang, K. Extracellular microRNA: A new source of biomarkers. Mutat. Res. 2011, 717, 85–90. [Google Scholar] [CrossRef]
- Cortez, M.A.; Calin, G.A. MicroRNA identification in plasma and serum: A new tool to diagnose and monitor diseases. Expert Opin. Biol. Ther. 2009, 9, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Stachurska, A.; Zorro, M.M.; van der Sijde, M.R.; Withoff, S. Small and Long Regulatory RNAs in the Immune System and Immune Diseases. Front. Immunol. 2014, 5, 513. [Google Scholar] [CrossRef]
- Guay, C.; Roggli, E.; Nesca, V.; Jacovetti, C.; Regazzi, R. Diabetes mellitus, a microRNA-related disease? Transl. Res. J. Lab. Clin. Med. 2011, 157, 253–264. [Google Scholar] [CrossRef]
- Romano, G.; Veneziano, D.; Acunzo, M.; Croce, C.M. Small non-coding RNA and cancer. Carcinogenesis 2017, 38, 485–491. [Google Scholar] [CrossRef]
- Van Rooij, E.; Purcell, A.L.; Levin, A.A. Developing microRNA therapeutics. Circ. Res. 2012, 110, 496–507. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Mattick, J.S. The genetic signatures of noncoding RNAs. PLoS Genet. 2009, 5, e1000459. [Google Scholar] [CrossRef]
- Dozmorov, M.G.; Giles, C.B.; Koelsch, K.A.; Wren, J.D. Systematic classification of non-coding RNAs by epigenomic similarity. BMC Bioinform. 2013, 14 (Suppl. S14), S2. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.M.; Bhakta, N.R.; Barczak, A.J.; Cakmak, H.; Fisher, S.; MacKenzie, T.C.; Patel, T.; Price, R.W.; Smith, J.F.; Woodruff, P.G.; et al. Large Differences in Small RNA Composition Between Human Biofluids. Cell Rep. 2018, 25, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, A.F.; Lee, E.S. Non-coding RNA: What is functional and what is junk? Front. Genet. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, G.; Cordero, F.; Tarallo, S.; Arigoni, M.; Riccardo, F.; Gallo, G.; Ronco, G.; Allasia, M.; Kulkarni, N.; Matullo, G.; et al. Small non-coding RNA profiling in human biofluids and surrogate tissues from healthy individuals: Description of the diverse and most represented species. Oncotarget 2018, 9, 3097–3111. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Cheng, G. Circulating miRNAs: Roles in cancer diagnosis, prognosis and therapy. Adv. Drug Deliv. Rev. 2015, 81, 75–93. [Google Scholar] [CrossRef]
- Cheng, Y.; Tan, N.; Yang, J.; Liu, X.; Cao, X.; He, P.; Dong, X.; Qin, S.; Zhang, C. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin. Sci. 2010, 119, 87–95. [Google Scholar] [CrossRef]
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506. [Google Scholar] [CrossRef]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef]
- Fichtlscherer, S.; De Rosa, S.; Fox, H.; Schwietz, T.; Fischer, A.; Liebetrau, C.; Weber, M.; Hamm, C.W.; Roxe, T.; Muller-Ardogan, M.; et al. Circulating microRNAs in patients with coronary artery disease. Circ. Res. 2010, 107, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; de Windt, L.J.; van der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p as a circulating biomarker for heart failure. Circ. Res. 2010, 106, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Cambier, L.; de Couto, G.; Ibrahim, A.; Echavez, A.K.; Valle, J.; Liu, W.; Kreke, M.; Smith, R.R.; Marban, L.; Marban, E. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol. Med. 2017, 9, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.S.; Ramasamy, S.; George-William, J.N.; Rajendhran, J. Emerging cardiac non-coding landscape: The importance of meta-analysis. Biochimie 2017, 133, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lv, Y.; Duan, Y.; Li, G.; Zhang, Z. Circulating Non-coding RNAs and Cardiovascular Diseases. Adv. Exp. Med. Biol. 2020, 1229, 357–367. [Google Scholar] [CrossRef]
- Viereck, J.; Thum, T. Circulating Noncoding RNAs as Biomarkers of Cardiovascular Disease and Injury. Circ. Res. 2017, 120, 381–399. [Google Scholar] [CrossRef]
- Baigent, C.; Burbury, K.; Wheeler, D. Premature cardiovascular disease in chronic renal failure. Lancet 2000, 356, 147–152. [Google Scholar] [CrossRef]
- Chao, C.T.; Liu, Y.P.; Su, S.F.; Yeh, H.Y.; Chen, H.Y.; Lee, P.J.; Chen, W.J.; Lee, Y.M.; Huang, J.W.; Chiang, C.K.; et al. Circulating MicroRNA-125b Predicts the Presence and Progression of Uremic Vascular Calcification. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1402–1414. [Google Scholar] [CrossRef]
- Wen, P.; Song, D.; Ye, H.; Wu, X.; Jiang, L.; Tang, B.; Zhou, Y.; Fang, L.; Cao, H.; He, W.; et al. Circulating MiR-133a as a biomarker predicts cardiac hypertrophy in chronic hemodialysis patients. PLoS ONE 2014, 9, e103079. [Google Scholar] [CrossRef]
- Shang, F.; Wang, S.C.; Hsu, C.Y.; Miao, Y.; Martin, M.; Yin, Y.; Wu, C.C.; Wang, Y.T.; Wu, G.; Chien, S.; et al. MicroRNA-92a Mediates Endothelial Dysfunction in CKD. J. Am. Soc. Nephrol. JASN 2017, 28, 3251–3261. [Google Scholar] [CrossRef]
- Han, Z.; Xiao, Z.; Kalantar-Zadeh, K.; Moradi, H.; Shafi, T.; Waikar, S.S.; Quarles, L.D.; Yu, Z.; Tin, A.; Coresh, J.; et al. Validation of a Novel Modified Aptamer-Based Array Proteomic Platform in Patients with End-Stage Renal Disease. Diagnostics 2018, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Han, Z.; Dashputre, A.A.; Potukuchi, P.K.; Kovesdy, C.P. Association between Nrf2 and CDKN2A expression in patients with end-stage renal disease: A pilot study. Aging 2020, 12, 16357–16367. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Pierre, J.F.; Han, Z.; Mims, T.S.; Potukuchi, P.K.; Yuzefpolskaya, M.; Colombo, P.C.; Demmer, R.T.; Datta, S.; Kovesdy, C.P. Circulating Microbial Signatures and Cardiovascular Death in Patients With ESRD. Kidney Int. Rep. 2021, 6, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- Kuscu, C.; Kiran, M.; Mohammed, A.; Kuscu, C.; Satpathy, S.; Wolen, A.; Bardhi, E.; Bajwa, A.; Eason, J.D.; Maluf, D.; et al. Integrative Analyses of Circulating Small RNAs and Kidney Graft Transcriptome in Transplant Glomerulopathy. Int. J. Mol. Sci. 2021, 22, 6218. [Google Scholar] [CrossRef]
- Gebert, D.; Hewel, C.; Rosenkranz, D. Unitas: The universal tool for annotation of small RNAs. BMC Genom. 2017, 18, 644. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Santulli, G.; Iaccarino, G. Adrenergic signaling in heart failure and cardiovascular aging. Maturitas 2016, 93, 65–72. [Google Scholar] [CrossRef]
- Austin, K.M.; Trembley, M.A.; Chandler, S.F.; Sanders, S.P.; Saffitz, J.E.; Abrams, D.J.; Pu, W.T. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat. Rev. Cardiol. 2019, 16, 519–537. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.; Broderick, T.L.; Gutkowska, J. The Role of Oxytocin in Cardiovascular Protection. Front. Psychol. 2020, 11, 2139. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.D.; Wang, T.; Mike, E.; Herrera, J.; Beavers, D.L.; Huang, T.W.; Ward, C.S.; Skinner, S.; Percy, A.K.; Glaze, D.G.; et al. Pathogenesis of lethal cardiac arrhythmias in Mecp2 mutant mice: Implication for therapy in Rett syndrome. Sci. Transl. Med. 2011, 3, 113ra125. [Google Scholar] [CrossRef]
- Hara, M.; Takahashi, T.; Mitsumasu, C.; Igata, S.; Takano, M.; Minami, T.; Yasukawa, H.; Okayama, S.; Nakamura, K.; Okabe, Y.; et al. Disturbance of cardiac gene expression and cardiomyocyte structure predisposes Mecp2-null mice to arrhythmias. Sci. Rep. 2015, 5, 11204. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Cao, Q.; Li, Z.; Huang, L.; Chen, S. The Effect of Mecp2 on Heart Failure. Cell. Physiol. Biochem. 2018, 47, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Barwari, T.; Joshi, A.; Zeller, T.; Mayr, M. Noncoding RNAs versus Protein Biomarkers in Cardiovascular Disease. Trends Mol. Med. 2020, 26, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Rabajdova, M.; Spakova, I.; Zelko, A.; Rosenberger, J.; Kolarcik, P.; Sobolova, V.; Pella, D.; Marekova, M.; Madarasova Geckova, A. The role of physical activity and miRNAs in the vascular aging and cardiac health of dialysis patients. Physiol. Rep. 2021, 9, e14879. [Google Scholar] [CrossRef]
- Bolignano, D.; Greco, M.; Presta, P.; Duni, A.; Vita, C.; Pappas, E.; Mirabelli, M.; Lakkas, L.; Naka, K.K.; Brunetti, A.; et al. A small circulating miRNAs signature predicts mortality and adverse cardiovascular outcomes in chronic hemodialysis patients. Clin. Kidney J. 2023, 16, 868–878. [Google Scholar] [CrossRef]
- Deng, B.; Tang, X.; Wang, Y. Role of microRNA-129 in cancer and non-cancerous diseases (Review). Exp. Ther. Med. 2021, 22, 918. [Google Scholar] [CrossRef]
- Tang, X.; Tang, J.; Liu, X.; Zeng, L.; Cheng, C.; Luo, Y.; Li, L.; Qin, S.L.; Sang, Y.; Deng, L.M.; et al. Downregulation of miR-129-2 by promoter hypermethylation regulates breast cancer cell proliferation and apoptosis. Oncol. Rep. 2016, 35, 2963–2969. [Google Scholar] [CrossRef]
- Xiao, N.; Zhang, J.; Chen, C.; Wan, Y.; Wang, N.; Yang, J. miR-129-5p improves cardiac function in rats with chronic heart failure through targeting HMGB1. Mamm. Genome 2019, 30, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Cui, W.; Chen, Z. Mesenchymal Stem Cell-Derived Exosome-Loaded microRNA-129-5p Inhibits TRAF3 Expression to Alleviate Apoptosis and Oxidative Stress in Heart Failure. Cardiovasc. Toxicol. 2022, 22, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Xu, F.; Zhang, Y. MiR-129-5p-mediated Beclin-1 suppression inhibits endothelial cell autophagy in atherosclerosis. Am. J. Transl. Res. 2016, 8, 1886–1894. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).