A Narrative Review Discussing the Obstetric Repercussions Due to Alterations of Personalized Bacterial Sites Developed within the Vagina, Cervix, and Endometrium
Abstract
:1. Introduction
2. Methodology
2.1. Academic Databases Accessed and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
2.4. Number of Entries
2.5. Number of Results
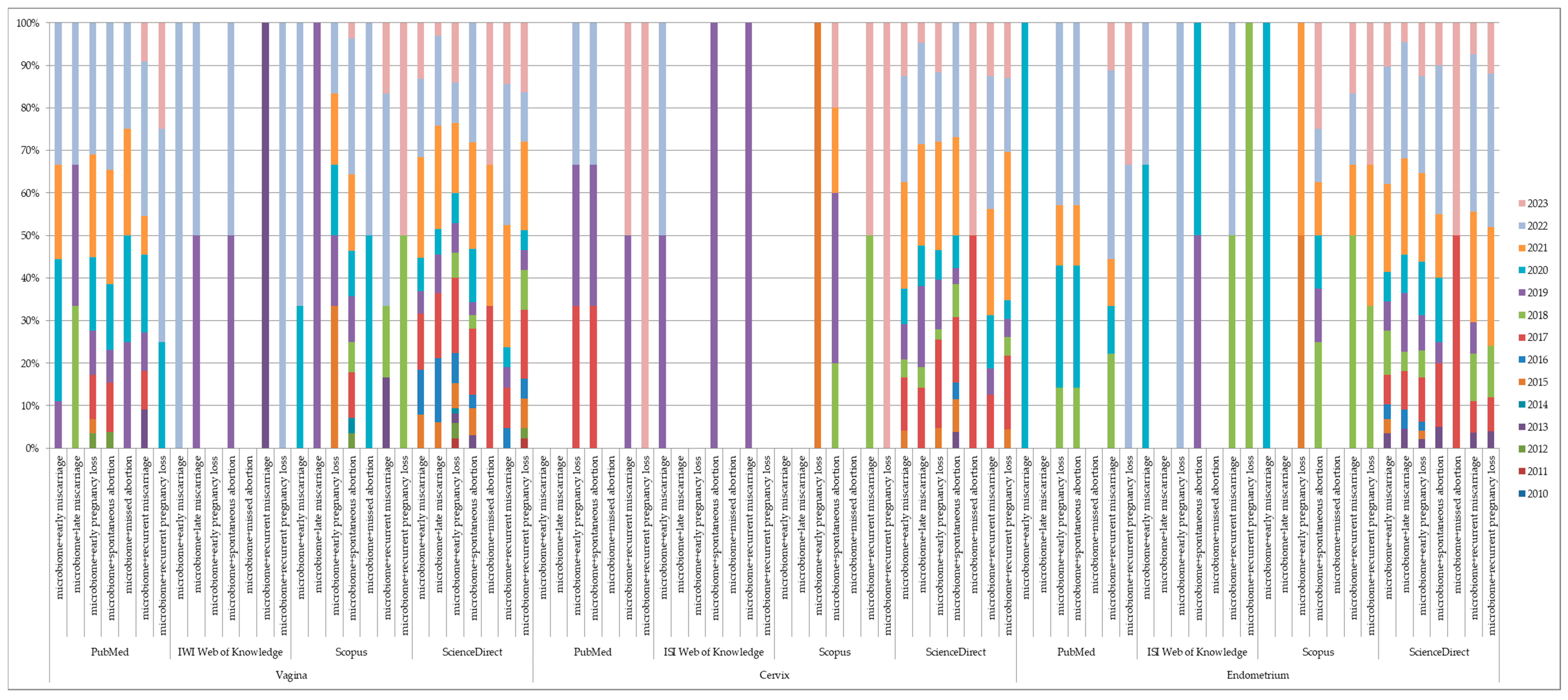
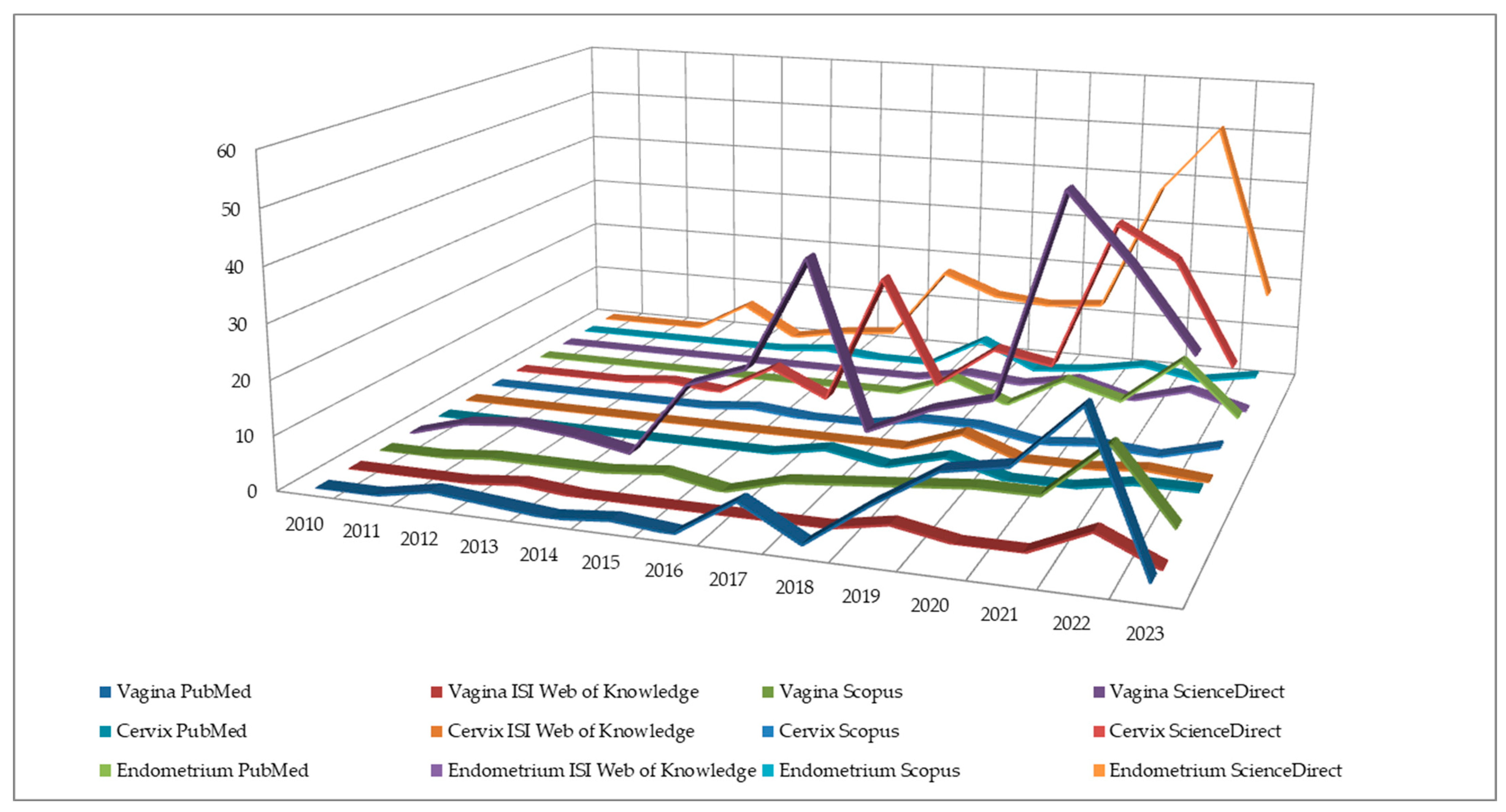
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.H. The Microbiota of the Vagina and Its Influence on Women’s Health and Disease. Am. J. Med. Sci. 2012, 343, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- van de Wijgert, J.H.H.M.; Borgdorff, H.; Verhelst, R.; Crucitti, T.; Francis, S.; Verstraelen, H.; Jespers, V. The Vaginal Microbiota: What Have We Learned after a Decade of Molecular Characterization? PLoS ONE 2014, 9, e105998. [Google Scholar] [CrossRef] [Green Version]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [Green Version]
- White, B.A.; Creedon, D.J.; Nelson, K.E.; Wilson, B.A. The vaginal microbiome in health and disease. Trends Endocrinol. Metab. 2011, 22, 389–393. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.H.; Marrazzo, J.M. The Vaginal Microbiome: Current Understanding and Future Directions. J. Infect. Dis. 2016, 214, S36–S41. [Google Scholar] [CrossRef] [Green Version]
- Theis, K.R.; Florova, V.; Romero, R.; Borisov, A.B.; Winters, A.D.; Galaz, J.; Gomez-Lopez, N. Sneathia: An emerging pathogen in female reproductive disease and adverse perinatal outcomes. Crit. Rev. Microbiol. 2021, 47, 517–542. [Google Scholar] [CrossRef]
- So, K.A.; Yang, E.J.; Kim, N.R.; Hong, S.R.; Lee, J.-H.; Hwang, C.-S.; Shim, S.-H.; Lee, S.J.; Kim, T.J. Changes of vaginal microbiota during cervical carcinogenesis in women with human papillomavirus infection. PLoS ONE 2020, 15, e0238705. [Google Scholar] [CrossRef]
- Ravel, J.; Moreno, I.; Simón, C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am. J. Obstet. Gynecol. 2021, 224, 251–257. [Google Scholar] [CrossRef]
- Kindinger, L.M.; MacIntyre, D.A.; Lee, Y.S.; Marchesi, J.R.; Smith, A.; McDonald, J.A.K.; Terzidou, V.; Cook, J.R.; Lees, C.; Israfil-Bayli, F.; et al. Relationship between vaginal microbial dysbiosis, inflammation, and pregnancy outcomes in cervical cerclage. Sci. Transl. Med. 2016, 8, 350ra102. [Google Scholar] [CrossRef] [Green Version]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Drell, T.; Lillsaar, T.; Tummeleht, L.; Simm, J.; Aaspõllu, A.; Väin, E.; Saarma, I.; Salumets, A.; Donders, G.G.G.; Metsis, M. Characterization of the Vaginal Micro- and Mycobiome in Asymptomatic Reproductive-Age Estonian Women. PLoS ONE 2013, 8, e54379. [Google Scholar] [CrossRef]
- Al-Memar, M.; Bobdiwala, S.; Fourie, H.; Mannino, R.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Timmerman, D.; Bourne, T.; Bennett, P.R.; et al. The association between vaginal bacterial composition and miscarriage: A nested case–control study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, C.K.; Brotman, R.M.; Ravel, J. Intricacies of assessing the human microbiome in epidemiologic studies. Ann. Epidemiol. 2016, 26, 311–321. [Google Scholar] [CrossRef] [Green Version]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Nikoopour, E.; Singh, B. Reciprocity in microbiome and immune system interactions and its implications in disease and health. Inflamm. Allergy Drug Targets 2014, 13, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Mazmanian, S.K. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe 2010, 7, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franasiak, J.M.; Scott, R.T.J. Reproductive tract microbiome in assisted reproductive technologies. Fertil. Steril. 2015, 104, 1364–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsonis, O.; Gkrozou, F.; Paschopoulos, M. Microbiome affecting reproductive outcome in ARTs. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102036. [Google Scholar] [CrossRef] [PubMed]
- Muzii, L.; Di Tucci, C.; Galati, G.; Mattei, G.; Pietrangeli, D.; Di Donato, V.; Perniola, G.; Palaia, I.; Benedetti Panici, P. The role of microbiota in female fertility and infertility. Minerva Obstet. Gynecol. 2022, 74, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C.M.; Haick, A.; Nkwopara, E.; Garcia, R.; Rendi, M.; Agnew, K.; Fredricks, D.N.; Eschenbach, D. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obstet. Gynecol. 2015, 212, 611.e1–611.e9. [Google Scholar] [CrossRef] [Green Version]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [Green Version]
- Franasiak, J.M.; Scott, R.T.J. Introduction: Microbiome in human reproduction. Fertil. Steril. 2015, 104, 1341–1343. [Google Scholar] [CrossRef] [Green Version]
- Moreno, I.; Franasiak, J.M. Endometrial microbiota-new player in town. Fertil. Steril. 2017, 108, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Toson, B.; Simon, C.; Moreno, I. The Endometrial Microbiome and Its Impact on Human Conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef]
- Greenbaum, S.; Greenbaum, G.; Moran-Gilad, J.; Weintraub, A.Y. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am. J. Obstet. Gynecol. 2019, 220, 324–335. [Google Scholar] [CrossRef] [PubMed]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.V.; Monteiro, P.B.; Moura, G.A.; Santos, N.O.; Fontanezi, C.T.B.; Gomes, I.A.; Teixeira, C.A. Vaginal microbioma and the presence of Lactobacillus spp. as interferences in female fertility: A review system. JBRA Assist. Reprod. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Cocomazzi, G.; De Stefani, S.; Del Pup, L.; Palini, S.; Buccheri, M.; Primiterra, M.; Sciannamè, N.; Faioli, R.; Maglione, A.; Baldini, G.M.; et al. The Impact of the Female Genital Microbiota on the Outcome of Assisted Reproduction Treatments. Microorganisms 2023, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, L.; Ala-Jaakkola, R.; Laitila, A.; Maukonen, J. Healthy Vaginal Microbiota and Influence of Probiotics Across the Female Life Span. Front. Microbiol. 2022, 13, 819958. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, W.; Yuan, Y.; Zhu, W.; Shang, A. Vaginal microecological characteristics of women in different physiological and pathological period. Front. Cell. Infect. Microbiol. 2022, 12, 959793. [Google Scholar] [CrossRef]
- Chopra, C.; Bhushan, I.; Mehta, M.; Koushal, T.; Gupta, A.; Sharma, S.; Kumar, M.; Al Khodor, S.; Sharma, S. Vaginal microbiome: Considerations for reproductive health. Future Microbiol. 2022, 17, 1501–1513. [Google Scholar] [CrossRef]
- Kwon, M.S.; Lee, H.K. Host and Microbiome Interplay Shapes the Vaginal Microenvironment. Front. Immunol. 2022, 13, 919728. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, R.; Espinoza, J.; Mazor, M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil. Steril. 2004, 82, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.; Chaban, B.; Bocking, A.; Rocco, M.; Yang, S.; Hill, J.E.; Money, D.M.; Hemmingsen, S.; Reid, G.; Dumonceaux, T.; et al. The vaginal microbiome of pregnant women is less rich and diverse, with lower prevalence of Mollicutes, compared to non-pregnant women. Sci. Rep. 2017, 7, 9212. [Google Scholar] [CrossRef] [Green Version]
- MacIntyre, D.A.; Chandiramani, M.; Lee, Y.S.; Kindinger, L.; Smith, A.; Angelopoulos, N.; Lehne, B.; Arulkumaran, S.; Brown, R.; Teoh, T.G.; et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci. Rep. 2015, 5, 8988. [Google Scholar] [CrossRef] [Green Version]
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.-A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Van den Veyver, I.; Milosavljevic, A.; et al. A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef]
- Spear, G.T.; French, A.L.; Gilbert, D.; Zariffard, M.R.; Mirmonsef, P.; Sullivan, T.H.; Spear, W.W.; Landay, A.; Micci, S.; Lee, B.-H.; et al. Human α-amylase Present in Lower-Genital-Tract Mucosal Fluid Processes Glycogen to Support Vaginal Colonization by Lactobacillus. J. Infect. Dis. 2014, 210, 1019–1028. [Google Scholar] [CrossRef] [Green Version]
- Bradford, L.L.; Ravel, J. The vaginal mycobiome: A contemporary perspective on fungi in women’s health and diseases. Virulence 2017, 8, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, C.; Eichelberger, K. Maternal microbiome and pregnancy outcomes. Fertil. Steril. 2015, 104, 1358–1363. [Google Scholar] [CrossRef] [Green Version]
- Moosa, Y.; Kwon, D.; de Oliveira, T.; Wong, E.B. Determinants of Vaginal Microbiota Composition. Front. Cell. Infect. Microbiol. 2020, 10, 467. [Google Scholar] [CrossRef]
- García-Velasco, J.A.; Budding, D.; Campe, H.; Malfertheiner, S.F.; Hamamah, S.; Santjohanser, C.; Schuppe-Koistinen, I.; Nielsen, H.S.; Vieira-Silva, S.; Laven, J. The reproductive microbiome—Clinical practice recommendations for fertility specialists. Reprod. Biomed. Online 2020, 41, 443–453. [Google Scholar] [CrossRef]
- Heil, B.A.; Paccamonti, D.L.; Sones, J.L. Role for the mammalian female reproductive tract microbiome in pregnancy outcomes. Physiol. Genom. 2019, 51, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Grau, I.; Perez-Villaroya, D.; Bau, D.; Gonzalez-Monfort, M.; Vilella, F.; Moreno, I.; Simon, C. Taxonomical and Functional Assessment of the Endometrial Microbiota in A Context of Recurrent Reproductive Failure: A Case Report. Pathogens 2019, 8, 205. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wong, K.K.-W.; Ko, E.Y.-L.; Chen, X.; Huang, J.; Tsui, S.K.-W.; Li, T.C.; Chim, S.S.-C. Systematic Comparison of Bacterial Colonization of Endometrial Tissue and Fluid Samples in Recurrent Miscarriage Patients: Implications for Future Endometrial Microbiome Studies. Clin. Chem. 2018, 64, 1743–1752. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhang, T.; Ma, Y.; Huang, Z.; He, Y.; Pan, H.; Fang, M.; Ding, H. Alteration of vaginal microbiota in patients with unexplained recurrent miscarriage. Exp. Ther. Med. 2019, 17, 3307–3316. [Google Scholar] [CrossRef] [Green Version]
- Jiao, X.; Zhang, L.; Du, D.; Wang, L.; Song, Q.; Liu, S. Alteration of vaginal microbiota in patients with recurrent miscarriage. J. Obstet. Gynaecol. 2022, 42, 248–255. [Google Scholar] [CrossRef]
- Fen-Ting, L.; Shuo, Y.; Zi, Y.; Ping, Z.; Tianliu, P.; Jingwen, Y.; Zhenhong, Y.; Hongying, S.; Yang, Y.; Rong, L. An Altered Microbiota in the Lower and Upper Female Reproductive Tract of Women with Recurrent Spontaneous Abortion. Microbiol. Spectr. 2022, 10, e00462-22. [Google Scholar] [CrossRef]
- Vomstein, K.; Reider, S.; Böttcher, B.; Watschinger, C.; Kyvelidou, C.; Tilg, H.; Moschen, A.R.; Toth, B. Uterine microbiota plasticity during the menstrual cycle: Differences between healthy controls and patients with recurrent miscarriage or implantation failure. J. Reprod. Immunol. 2022, 151, 103634. [Google Scholar] [CrossRef]
- Soyer Caliskan, C.; Yurtcu, N.; Celik, S.; Sezer, O.; Kilic, S.S.; Cetin, A. Derangements of vaginal and cervical canal microbiota determined with real-time PCR in women with recurrent miscarriages. J. Obstet. Gynaecol. 2022, 42, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, L.; Lian, C.; Xue, H.; Lu, Y.; Chen, X.; Xia, Y. Vaginal Microbiota Diversity of Patients with Embryonic Miscarriage by Using 16S rDNA High-Throughput Sequencing. Int. J. Genom. 2020, 2020, 1764959. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, Y.; Xie, X.; Qin, X.; He, X.; Shi, C.; Zeng, W.; Guo, Y.; Lin, Y. Association between vaginal microbiota and risk of early pregnancy miscarriage. Comp. Immunol. Microbiol. Infect. Dis. 2021, 77, 101669. [Google Scholar] [CrossRef]
- Fernández, L.; Castro, I.; Arroyo, R.; Alba, C.; Beltrán, D.; Rodríguez, J.M. Application of Ligilactobacillus salivarius CECT5713 to Achieve Term Pregnancies in Women with Repetitive Abortion or Infertility of Unknown Origin by Microbiological and Immunological Modulation of the Vaginal Ecosystem. Nutrients 2021, 13, 162. [Google Scholar] [CrossRef]
- Fan, T.; Zhong, X.-M.; Wei, X.-C.; Miao, Z.-L.; Luo, S.-Y.; Cheng, H.; Xiao, Q. The alteration and potential relationship of vaginal microbiota and chemokines for unexplained recurrent spontaneous abortion. Medicine 2020, 99, e23558. [Google Scholar] [CrossRef]
- Kyono, K.; Hashimoto, T.; Nagai, Y.; Sakuraba, Y. Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: A single-center pilot study. Reprod. Med. Biol. 2018, 17, 297–306. [Google Scholar] [CrossRef]
- Shi, Y.; Yamada, H.; Sasagawa, Y.; Tanimura, K.; Deguchi, M. Uterine endometrium microbiota and pregnancy outcome in women with recurrent pregnancy loss. J. Reprod. Immunol. 2022, 152, 103653. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S.; Saqib, S.; Kanerva, T.; Nieminen, P.; Kalliala, I.; Salonen, A. Metagenome-validated Parallel Amplicon Sequencing and Text Mining-based Annotations for Simultaneous Profiling of Bacteria and Fungi: Vaginal Microbiome and Mycobiota in Healthy Women. 2021. Available online: https://assets.researchsquare.com/files/rs-321778/v1/6c64918d-8904-429f-81da-c72b17a8e7e4.pdf?c=1631879415 (accessed on 30 May 2023).
- Peuranpää, P.; Holster, T.; Saqib, S.; Kalliala, I.; Tiitinen, A.; Salonen, A.; Hautamäki, H. Female reproductive tract microbiota and recurrent pregnancy loss: A nested case-control study. Reprod. Biomed. Online 2022, 45, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Guang, Y.; Shen, X.; Tan, Y.; Tang, S.; Chen, J.; Zhang, L.; Wang, B.; Ye, S.; Chen, X.; Yang, C.; et al. Systematic analysis of microbiota in pregnant Chinese women and its association with miscarriage. Ann. Transl. Med. 2022, 10, 1099. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhao, X.; Pan, Q.; Li, F.; Gao, B.; Zhang, A.; Huang, H.; Xu, D.; Cheng, C. The association between vaginal microbiota disorders and early missed abortion: A prospective study. Acta Obstet. Gynecol. Scand. 2022, 101, 960–971. [Google Scholar] [CrossRef]
- Mori, R.; Hayakawa, T.; Hirayama, M.; Ozawa, F.; Yoshihara, H.; Goto, S.; Kitaori, T.; Ozaki, Y.; Sugiura-Ogasawara, M. Cervicovaginal microbiome in patients with recurrent pregnancy loss. J. Reprod. Immunol. 2023, 157, 103944. [Google Scholar] [CrossRef]
- Teixeira Oliveira, C.N.; Oliveira, M.T.S.; Martins Oliveira, H.B.; Coelho Silva, L.S.; Santos Júnior, M.N.; Almeida, C.F.; Amorim, A.T.; Oliveira, M.V.; Timenetsky, J.; Campos, G.B.; et al. Ureaplasma parvum alters the immune tolerogenic state in placental tissue and could cause miscarriage. Fertil. Steril. 2021, 116, 1030–1039. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, Y.; Gao, J.; Wu, M.; Li, C.; Wang, Z.; Huang, N.; Cui, L.; Du, M.; Ying, C. Characterization of Vaginal Microbiota in Women with Recurrent Spontaneous Abortion That Can Be Modified by Drug Treatment. Front. Cell. Infect. Microbiol. 2021, 11, 680643. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.S.; Arokiyaraj, S.; Kim, M.K.; Oh, H.Y.; Kwon, M.; Kong, J.S.; Shin, M.K.; Yu, Y.L.; Lee, J.K. High Prevalence of Leptotrichia amnionii, Atopobium vaginae, Sneathia sanguinegens, and Factor 1 Microbes and Association of Spontaneous Abortion among Korean Women. Biomed Res. Int. 2017, 2017, 5435089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Xue, X.; Zhang, Y.; Zhang, H.; Huang, X.; Chen, X.; Deng, G.; Luo, S.; Gao, J. Vaginal Atopobium is Associated with Spontaneous Abortion in the First Trimester: A Prospective Cohort Study in China. Microbiol. Spectr. 2022, 10, e0203921. [Google Scholar] [CrossRef] [PubMed]
- Grewal, K.; Lee, Y.S.; Smith, A.; Brosens, J.J.; Bourne, T.; Al-Memar, M.; Kundu, S.; MacIntyre, D.A.; Bennett, P.R. Chromosomally normal miscarriage is associated with vaginal dysbiosis and local inflammation. BMC Med. 2022, 20, 38. [Google Scholar] [CrossRef]
- Kuon, R.J.; Togawa, R.; Vomstein, K.; Weber, M.; Goeggl, T.; Strowitzki, T.; Markert, U.R.; Zimmermann, S.; Daniel, V.; Dalpke, A.H.; et al. Higher prevalence of colonization with Gardnerella vaginalis and gram-negative anaerobes in patients with recurrent miscarriage and elevated peripheral natural killer cells. J. Reprod. Immunol. 2017, 120, 15–19. [Google Scholar] [CrossRef]
- Pelzer, E.S.; Willner, D.; Buttini, M.; Huygens, F. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Van Leeuwenhoek 2018, 111, 933–943. [Google Scholar] [CrossRef]
- Kadogami, D.; Nakaoka, Y.; Morimoto, Y. Use of a vaginal probiotic suppository and antibiotics to influence the composition of the endometrial microbiota. Reprod. Biol. 2020, 20, 307–314. [Google Scholar] [CrossRef]
- Craciunas, L.; Gallos, I.; Chu, J.; Bourne, T.; Quenby, S.; Brosens, J.J.; Coomarasamy, A. Conventional and modern markers of endometrial receptivity: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 202–223. [Google Scholar] [CrossRef]
- Onogi, S.; Ezoe, K.; Nishihara, S.; Fukuda, J.; Kobayashi, T.; Kato, K. Endometrial thickness on the day of the LH surge: An effective predictor of pregnancy outcomes after modified natural cycle-frozen blastocyst transfer. Hum. Reprod. Open 2020, 2020, hoaa060. [Google Scholar] [CrossRef]
- Nasioudis, D.; Forney, L.J.; Schneider, G.M.; Gliniewicz, K.; France, M.; Boester, A.; Sawai, M.; Scholl, J.; Witkin, S.S. Influence of Pregnancy History on the Vaginal Microbiome of Pregnant Women in their First Trimester. Sci. Rep. 2017, 7, 10201. [Google Scholar] [CrossRef]
- Carosso, A.; Revelli, A.; Gennarelli, G.; Canosa, S.; Cosma, S.; Borella, F.; Tancredi, A.; Paschero, C.; Boatti, L.; Zanotto, E.; et al. Controlled ovarian stimulation and progesterone supplementation affect vaginal and endometrial microbiota in IVF cycles: A pilot study. J. Assist. Reprod. Genet. 2020, 37, 2315–2326. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Verhelst, R.; De Sutter, P.; Pieper, D.H.; Van De Wiele, T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 2016, 4, e1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, T.; Kyono, K. Does dysbiotic endometrium affect blastocyst implantation in IVF patients? J. Assist. Reprod. Genet. 2019, 36, 2471–2479. [Google Scholar] [CrossRef] [Green Version]
- Franasiak, J.M.; Werner, M.D.; Juneau, C.R.; Tao, X.; Landis, J.; Zhan, Y.; Treff, N.R.; Scott, R.T. Endometrial microbiome at the time of embryo transfer: Next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Liu, Y.; Ko, E.Y.-L.; Wong, K.K.-W.; Chen, X.; Cheung, W.-C.; Law, T.S.-M.; Chung, J.P.-W.; Tsui, S.K.-W.; Li, T.-C.; Chim, S.S.-C. Endometrial microbiota in infertile women with and without chronic endometritis as diagnosed using a quantitative and reference range-based method. Fertil. Steril. 2019, 112, 707–717.e1. [Google Scholar] [CrossRef]
- Kitaya, K.; Matsubayashi, H.; Yamaguchi, K.; Nishiyama, R.; Takaya, Y.; Ishikawa, T.; Yasuo, T.; Yamada, H. Chronic Endometritis: Potential Cause of Infertility and Obstetric and Neonatal Complications. Am. J. Reprod. Immunol. 2016, 75, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Matsubayashi, H.; Takaya, Y.; Nishiyama, R.; Yamaguchi, K.; Takeuchi, T.; Ishikawa, T. Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am. J. Reprod. Immunol. 2017, 78, e12719. [Google Scholar] [CrossRef]
- Cicinelli, E.; Trojano, G.; Mastromauro, M.; Vimercati, A.; Marinaccio, M.; Mitola, P.C.; Resta, L.; de Ziegler, D. Higher prevalence of chronic endometritis in women with endometriosis: A possible etiopathogenetic link. Fertil. Steril. 2017, 108, 289–295.e1. [Google Scholar] [CrossRef] [Green Version]
- Cicinelli, E.; Matteo, M.; Trojano, G.; Mitola, P.C.; Tinelli, R.; Vitagliano, A.; Crupano, F.M.; Lepera, A.; Miragliotta, G.; Resta, L. Chronic endometritis in patients with unexplained infertility: Prevalence and effects of antibiotic treatment on spontaneous conception. Am. J. Reprod. Immunol. 2018, 79, e12782. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, C.; Yang, W.; Wei, X.; Wu, K.; Huang, D. The Unique Microbiome and Innate Immunity During Pregnancy. Front. Immunol. 2019, 10, 2886. [Google Scholar] [CrossRef] [PubMed]
- Song, S.D.; Acharya, K.D.; Zhu, J.E.; Deveney, C.M.; Walther-Antonio, M.R.S.; Tetel, M.J.; Chia, N. Daily Vaginal Microbiota Fluctuations Associated with Natural Hormonal Cycle, Contraceptives, Diet, and Exercise. mSphere 2020, 5, 00593-20. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Merchant, M.; Haque, M.M.; Mande, S.S. Crosstalk Between Female Gonadal Hormones and Vaginal Microbiota Across Various Phases of Women’s Gynecological Lifecycle. Front. Microbiol. 2020, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.-L.; Chen, L.-X.; Shu, W.-S.; Yao, S.-Z.; Wang, S.-W.; Chen, Y.-Q. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am. J. Transl. Res. 2016, 8, 1581–1592. [Google Scholar]
- Churchill, S.J.; Moreno, I.; Simón, C.; Lathi, R. The uterine microbiome in recurrent pregnancy loss. Fertil. Steril. 2018, 109, e12. [Google Scholar] [CrossRef] [Green Version]
- Krog, M.C.; Hugerth, L.W.; Fransson, E.; Bashir, Z.; Nyboe Andersen, A.; Edfeldt, G.; Engstrand, L.; Schuppe-Koistinen, I.; Nielsen, H.S. The healthy female microbiome across body sites: Effect of hormonal contraceptives and the menstrual cycle. Hum. Reprod. 2022, 37, 1525–1543. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Ge, Y.; Cen, J.; Liao, Y.; Xu, G. Reproductive outcomes and reproductive tract microbiota shift in women with moderate-to-severe intrauterine adhesions following 30-day post-hysteroscopic placement of balloon stents or intrauterine contraceptive devices: A randomized controlled trial. EClinicalMedicine 2022, 43, 101200. [Google Scholar] [CrossRef]
- Liu, N.-N.; Zhao, X.; Tan, J.-C.; Liu, S.; Li, B.-W.; Xu, W.-X.; Peng, L.; Gu, P.; Li, W.; Shapiro, R.; et al. Mycobiome Dysbiosis in Women with Intrauterine Adhesions. Microbiol. Spectr. 2022, 10, e0132422. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [Green Version]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and Inputs from Lactic Acid Bacteria and Their Bacteriocins as Alternatives to Antibiotic Growth Promoters During Food-Animal Production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Leoni, C.; Ceci, O.; Manzari, C.; Fosso, B.; Volpicella, M.; Ferrari, A.; Fiorella, P.; Pesole, G.; Cicinelli, E.; Ceci, L.R. Human Endometrial Microbiota at Term of Normal Pregnancies. Genes 2019, 10, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-R.; Dong, Y.-H.; Liu, C.-J.; Tang, X.-D.; Zhang, N.-N.; Shen, J.; Wu, Z.; Li, X.-R.; Shao, J.-Y. Microbiological composition of follicular fluid in patients undergoing IVF and its association with infertility. Am. J. Reprod. Immunol. 2023, 89, e13652. [Google Scholar] [CrossRef]
- Matsumoto, A.; Yamagishi, Y.; Miyamoto, K.; Oka, K.; Takahashi, M.; Mikamo, H. Characterization of the vaginal microbiota of Japanese women. Anaerobe 2018, 54, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Allotey, J.; Drymoussi, Z.; Wilks, M.; Fernandez-Felix, B.M.; Whiley, A.; Dodds, J.; Thangaratinam, S.; McCourt, C.; Prosdocimi, E.M.; et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: A randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, Y.; Nakanishi, T.; Ozaki, Y.; Nozawa, K.; Sato, T.; Sugiura-Ogasawara, M. Uterine Cervical Inflammatory Cytokines, Interleukin-6 and -8, as Predictors of Miscarriage in Recurrent Cases. Am. J. Reprod. Immunol. 2007, 58, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Coomarasamy, A.; Devall, A.J.; Cheed, V.; Harb, H.; Middleton, L.J.; Gallos, I.D.; Williams, H.; Eapen, A.K.; Roberts, T.; Ogwulu, C.C.; et al. A Randomized Trial of Progesterone in Women with Bleeding in Early Pregnancy. N. Engl. J. Med. 2019, 380, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, S.; Terao, A.; Yamamoto, Y.; Mukai, T.; Miura, T.; Shoji, T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 2016, 6, 31208. [Google Scholar] [CrossRef]
- Guan, S.-M.; Shu, L.; Fu, S.-M.; Liu, B.; Xu, X.-L.; Wu, J.-Z. Prevotella intermedia induces matrix metalloproteinase-9 expression in human periodontal ligament cells. FEMS Microbiol. Lett. 2008, 283, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Guan, S.-M.; Shu, L.; Fu, S.-M.; Liu, B.; Xu, X.-L.; Wu, J.-Z. Prevotella intermedia upregulates MMP-1 and MMP-8 expression in human periodontal ligament cells. FEMS Microbiol. Lett. 2009, 299, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Borgdorff, H.; Gautam, R.; Armstrong, S.D.; Xia, D.; Ndayisaba, G.F.; van Teijlingen, N.H.; Geijtenbeek, T.B.H.; Wastling, J.M.; van de Wijgert, J.H.H.M. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 2016, 9, 621–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozos, I. Mechanisms linking red blood cell disorders and cardiovascular diseases. Biomed Res. Int. 2015, 2015, 682054. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G. Iron metabolism in the anemia of chronic disease. Biochim. Biophys. Acta 2009, 1790, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Yamasaki, M.; Iwata, T.; Suzuki, K.; Nakane, A.; Nakamura, H. Anaerobic bacterial extracts influence production of matrix metalloproteinases and their inhibitors by human dental pulp cells. J. Endod. 2000, 26, 410–413. [Google Scholar] [CrossRef]
- Melnick, A.P.; Pereira, N.; Murphy, E.M.; Rosenwaks, Z.; Spandorfer, S.D. How low is too low? Cycle day 28 estradiol levels and pregnancy outcomes. Fertil. Steril. 2016, 105, 905–909.e1. [Google Scholar] [CrossRef] [Green Version]
- Arck, P.C.; Rücke, M.; Rose, M.; Szekeres-Bartho, J.; Douglas, A.J.; Pritsch, M.; Blois, S.M.; Pincus, M.K.; Bärenstrauch, N.; Dudenhausen, J.W.; et al. Early risk factors for miscarriage: A prospective cohort study in pregnant women. Reprod. Biomed. Online 2008, 17, 101–113. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Feng, L.; Zhang, J. Interactions between gut microbiota and metabolites modulate cytokine network imbalances in women with unexplained miscarriage. NPJ Biofilms Microbiomes 2021, 7, 24. [Google Scholar] [CrossRef]
- Yang, H.; Guo, R.; Li, S.; Liang, F.; Tian, C.; Zhao, X.; Long, Y.; Liu, F.; Jiang, M.; Zhang, Y.; et al. Systematic analysis of gut microbiota in pregnant women and its correlations with individual heterogeneity. NPJ Biofilms Microbiomes 2020, 6, 32. [Google Scholar] [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahçeci, M.; Barrionuevo, M.J.; Taguchi, S.; Puente, E.; Dimattina, M.; Lim, M.W.; et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef]
- Riganelli, L.; Iebba, V.; Piccioni, M.; Illuminati, I.; Bonfiglio, G.; Neroni, B.; Calvo, L.; Gagliardi, A.; Levrero, M.; Merlino, L.; et al. Structural Variations of Vaginal and Endometrial Microbiota: Hints on Female Infertility. Front. Cell. Infect. Microbiol. 2020, 10, 350. [Google Scholar] [CrossRef]
- Moore, D.E.; Soules, M.R.; Klein, N.A.; Fujimoto, V.Y.; Agnew, K.J.; Eschenbach, D.A. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil. Steril. 2000, 74, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Selman, H.; Mariani, M.; Barnocchi, N.; Mencacci, A.; Bistoni, F.; Arena, S.; Pizzasegale, S.; Brusco, G.F.; Angelini, A. Examination of bacterial contamination at the time of embryo transfer, and its impact on the IVF/pregnancy outcome. J. Assist. Reprod. Genet. 2007, 24, 395–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindinger, L.M.; Bennett, P.R.; Lee, Y.S.; Marchesi, J.R.; Smith, A.; Cacciatore, S.; Holmes, E.; Nicholson, J.K.; Teoh, T.G.; MacIntyre, D.A. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Lopez, N.; Romero, R.; Panaitescu, B.; Leng, Y.; Xu, Y.; Tarca, A.L.; Faro, J.; Pacora, P.; Hassan, S.S.; Hsu, C.-D. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am. J. Reprod. Immunol. 2018, 80, e13049. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, N.; Eren, A.M.; Barreiro, L.B.; Yotova, V.; Dumaine, A.; Allard, C.; Fraser, W.D. Vaginal microbiome in early pregnancy and subsequent risk of spontaneous preterm birth: A case-control study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Leizer, J.; Nasioudis, D.; Forney, L.J.; Schneider, G.M.; Gliniewicz, K.; Boester, A.; Witkin, S.S. Properties of Epithelial Cells and Vaginal Secretions in Pregnant Women when Lactobacillus crispatus or Lactobacillus iners Dominate the Vaginal Microbiome. Reprod. Sci. 2018, 25, 854–860. [Google Scholar] [CrossRef]
- Nasioudis, D.; Forney, L.J.; Schneider, G.M.; Gliniewicz, K.; France, M.T.; Boester, A.; Sawai, M.; Scholl, J.; Witkin, S.S. The composition of the vaginal microbiome in first trimester pregnant women influences the level of autophagy and stress in vaginal epithelial cells. J. Reprod. Immunol. 2017, 123, 35–39. [Google Scholar] [CrossRef]
- Saxtorph, M.H.; Hallager, T.; Persson, G.; Petersen, K.B.; Eriksen, J.O.; Larsen, L.G.; Hviid, T.V.; Macklon, N. Assessing endometrial receptivity after recurrent implantation failure: A prospective controlled cohort study. Reprod. Biomed. Online 2020, 41, 998–1006. [Google Scholar] [CrossRef]
- Koedooder, R.; Maghdid, D.M.; Beckers, N.G.M.; Schoenmakers, S.; Kok, D.J.; Laven, J.S.E. Dynamics of the urinary microbiome in pregnancy and the coincidental predictive value of the microbiota for IVF/IVF-ICSI outcome. Reprod. Biomed. Online 2021, 43, 871–879. [Google Scholar] [CrossRef]
- Iwami, N.; Kawamata, M.; Ozawa, N.; Yamamoto, T.; Watanabe, E.; Mizuuchi, M.; Moriwaka, O.; Kamiya, H. Therapeutic intervention based on gene sequencing analysis of microbial 16S ribosomal RNA of the intrauterine microbiome improves pregnancy outcomes in IVF patients: A prospective cohort study. J. Assist. Reprod. Genet. 2023, 40, 125–135. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, X.; Chen, P.; Wang, Y.; Li, W.; Huang, R. The endometrial microbiota profile influenced pregnancy outcomes in patients with repeated implantation failure: A retrospective study. J. Reprod. Immunol. 2023, 155, 103782. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, P.; Guo, Y.; Fang, C.; Li, T. Interaction Between Chronic Endometritis Caused Endometrial Microbiota Disorder and Endometrial Immune Environment Change in Recurrent Implantation Failure. Front. Immunol. 2021, 12, 748447. [Google Scholar] [CrossRef] [PubMed]
- Lozano, F.M.; Bernabeu, A.; Lledo, B.; Morales, R.; Diaz, M.; Aranda, F.I.; Llacer, J.; Bernabeu, R. Characterization of the vaginal and endometrial microbiome in patients with chronic endometritis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 263, 25–32. [Google Scholar] [CrossRef]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef]
- Campisciano, G.; Iebba, V.; Zito, G.; Luppi, S.; Martinelli, M.; Fischer, L.; De Seta, F.; Basile, G.; Ricci, G.; Comar, M. Lactobacillus iners and gasseri, Prevotella bivia and HPV Belong to the Microbiological Signature Negatively Affecting Human Reproduction. Microorganisms 2020, 9, 39. [Google Scholar] [CrossRef]
- Haahr, T.; Zacho, J.; Bräuner, M.; Shathmigha, K.; Skov Jensen, J.; Humaidan, P. Reproductive outcome of patients undergoing in vitro fertilisation treatment and diagnosed with bacterial vaginosis or abnormal vaginal microbiota: A systematic PRISMA review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Leitich, H.; Kiss, H. Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 375–390. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Ma, X.; Du, L.; Jia, Z.; Cui, X.; Yu, L.; Yang, J.; Xiao, L.; Zhang, B.; et al. Translocation of vaginal microbiota is involved in impairment and protection of uterine health. Nat. Commun. 2021, 12, 4191. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Ambrosino, E.; Schlaepfer, M.; Morré, S.A.; Wieten, L.; Voncken, J.W.; Spinelli, M.; Mueller, M.; Kramer, B.W. The Interplay Between Reproductive Tract Microbiota and Immunological System in Human Reproduction. Front. Immunol. 2020, 11, 378. [Google Scholar] [CrossRef]
- Bardos, J.; Fiorentino, D.; Longman, R.E.; Paidas, M. Immunological Role of the Maternal Uterine Microbiome in Pregnancy: Pregnancies Pathologies and Alterated Microbiota. Front. Immunol. 2019, 10, 2823. [Google Scholar] [CrossRef] [Green Version]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef] [Green Version]
- Shiroda, M.; Manning, S.D. Lactobacillus strains vary in their ability to interact with human endometrial stromal cells. PLoS ONE 2020, 15, e0238993. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D.; Dunk, C.E.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am. J. Pathol. 2009, 174, 1959–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moffett, A.; Shreeve, N. First do no harm: Uterine natural killer (NK) cells in assisted reproduction. Hum. Reprod. 2015, 30, 1519–1525. [Google Scholar] [CrossRef]
- Parazzini, F.; Chatenoud, L.; Tozzi, L.; Di Cintio, E.; Benzi, G.; Fedele, L. Induced abortion in the first trimester of pregnancy and risk of miscarriage. Br. J. Obstet. Gynaecol. 1998, 105, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Conde-Ferráez, L.; May, A.D.A.C.; Carrillo-Martínez, J.R.; Ayora-Talavera, G.; del Refugio González-Losa, M. Human papillomavirus infection and spontaneous abortion: A case–control study performed in Mexico. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 468–473. [Google Scholar] [CrossRef]
- Liu, Y.; You, H.; Chiriva-Internati, M.; Korourian, S.; Lowery, C.L.; Carey, M.J.; Smith, C.V.; Hermonat, P.L. Display of Complete Life Cycle of Human Papillomavirus Type 16 in Cultured Placental Trophoblasts. Virology 2001, 290, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, H.; Liu, Y.; Agrawal, N.; Prasad, C.K.; Chiriva-Internati, M.; Lowery, C.L.; Kay, H.H.; Hermonat, P.L. Infection, replication, and cytopathology of human papillomavirus type 31 in trophoblasts. Virology 2003, 316, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Gomez, L.M.; Ma, Y.; Ho, C.; McGrath, C.M.; Nelson, D.B.; Parry, S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum. Reprod. 2008, 23, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.A.; Banwatt, D.; Croy, B.A. Murine Trophoblast Failure and Spontaneous Abortion. Am. J. Reprod. Immunol. 1993, 29, 199–205. [Google Scholar] [CrossRef]
- Martín, R.; Jiménez, E.; Olivares, M.; Marín, M.L.; Fernández, L.; Xaus, J.; Rodríguez, J.M. Lactobacillus salivarius CECT 5713, a potential probiotic strain isolated from infant feces and breast milk of a mother–child pair. Int. J. Food Microbiol. 2006, 112, 35–43. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Aldunate, M.; Srbinovski, D.; Hearps, A.C.; Latham, C.F.; Ramsland, P.A.; Gugasyan, R.; Cone, R.A.; Tachedjian, G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, D.E.; Come, R.A.; Moench, T.R. Vaginal pH measured in vivo: Lactobacilli determine pH and lactic acid concentration. BMC Microbiol. 2019, 19, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, V.; Cárdenas, N.; Ocaña, S.; Marín, M.; Arroyo, R.; Beltrán, D.; Badiola, C.; Fernández, L.; Rodríguez, J.M. Rectal and Vaginal Eradication of Streptococcus agalactiae (GBS) in Pregnant Women by Using Lactobacillus salivarius CECT 9145, A Target-specific Probiotic Strain. Nutrients 2019, 11, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boskey, E.R.; Cone, R.A.; Whaley, K.J.; Moench, T.R. Origins of vaginal acidity: High D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001, 16, 1809–1813. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect. Dis. 2011, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef]
- Ruíz, F.O.; Gerbaldo, G.; García, M.J.; Giordano, W.; Pascual, L.; Barberis, I.L. Synergistic effect between two bacteriocin-like inhibitory substances produced by Lactobacilli Strains with inhibitory activity for Streptococcus agalactiae. Curr. Microbiol. 2012, 64, 349–356. [Google Scholar] [CrossRef]
- Aldunate, M.; Tyssen, D.; Johnson, A.; Zakir, T.; Sonza, S.; Moench, T.; Cone, R.; Tachedjian, G. Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 2013, 68, 2015–2025. [Google Scholar] [CrossRef]
- Tyssen, D.; Wang, Y.-Y.; Hayward, J.A.; Agius, P.A.; DeLong, K.; Aldunate, M.; Ravel, J.; Moench, T.R.; Cone, R.A.; Tachedjian, G. Anti-HIV-1 Activity of Lactic Acid in Human Cervicovaginal Fluid. mSphere 2018, 3, e00055-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, S.Y.; Cheah, Y.K.; Seow, H.F.; Sandai, D.; Than, L.T.L. In vitro modulation of probiotic bacteria on the biofilm of Candida glabrata. Anaerobe 2015, 34, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, V.H.; Wang, Y.; Bandara, H.M.H.N.; Mayer, M.P.A.; Samaranayake, L.P. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl. Microbiol. Biotechnol. 2016, 100, 6415–6426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, S.Y.; Cheah, Y.K.; Seow, H.F.; Sandai, D.; Than, L.T.L. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J. Appl. Microbiol. 2015, 118, 1180–1190. [Google Scholar] [CrossRef] [Green Version]
- Aarti, C.; Khusro, A.; Varghese, R.; Arasu, M.V.; Agastian, P.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C. In vitro investigation on probiotic, anti-Candida, and antibiofilm properties of Lactobacillus pentosus strain LAP1. Arch. Oral Biol. 2018, 89, 99–106. [Google Scholar] [CrossRef]
- Hütt, P.; Lapp, E.; Štšepetova, J.; Smidt, I.; Taelma, H.; Borovkova, N.; Oopkaup, H.; Ahelik, A.; Rööp, T.; Hoidmets, D.; et al. Characterisation of probiotic properties in human vaginal lactobacilli strains. Microb. Ecol. Health Dis. 2016, 27, 30484. [Google Scholar] [CrossRef]
- Cárdenas, N.; Martín, V.; Arroyo, R.; López, M.; Carrera, M.; Badiola, C.; Jiménez, E.; Rodríguez, J.M. Prevention of Recurrent Acute Otitis Media in Children Through the Use of Lactobacillus salivarius PS7, a Target-Specific Probiotic Strain. Nutrients 2019, 11, 376. [Google Scholar] [CrossRef] [Green Version]
- Boris, S.; Suárez, J.E.; Vázquez, F.; Barbés, C. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect. Immun. 1998, 66, 1985–1989. [Google Scholar] [CrossRef]
- Bogado Pascottini, O.; Spricigo, J.F.W.; Van Schyndel, S.J.; Mion, B.; Rousseau, J.; Weese, J.S.; LeBlanc, S.J. Effects of parity, blood progesterone, and non-steroidal anti-inflammatory treatment on the dynamics of the uterine microbiota of healthy postpartum dairy cows. PLoS ONE 2021, 16, e0233943. [Google Scholar] [CrossRef]
- Gärtner, M.A.; Peter, S.; Jung, M.; Drillich, M.; Einspanier, R.; Gabler, C. Increased mRNA expression of selected pro-inflammatory factors in inflamed bovine endometrium in vivo as well as in endometrial epithelial cells exposed to Bacillus pumilus in vitro. Reprod. Fertil. Dev. 2016, 28, 982–994. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitz Gerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- O’Hanlon, D.E.; Lanier, B.R.; Moench, T.R.; Cone, R.A. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect. Dis. 2010, 10, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macklaim, J.M.; Clemente, J.C.; Knight, R.; Gloor, G.B.; Reid, G. Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb. Ecol. Health Dis. 2015, 26, 27799. [Google Scholar] [CrossRef]
- Martín, R.; Soberón, N.; Vaneechoutte, M.; Flórez, A.B.; Vázquez, F.; Suárez, J.E. Characterization of indigenous vaginal lactobacilli from healthy women as probiotic candidates. Int. Microbiol. Off. J. Spanish Soc. Microbiol. 2008, 11, 261–266. [Google Scholar] [CrossRef]
- Mendes-Soares, H.; Suzuki, H.; Hickey, R.J.; Forney, L.J. Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. J. Bacteriol. 2014, 196, 1458–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- France, M.T.; Mendes-Soares, H.; Forney, L.J. Genomic Comparisons of Lactobacillus crispatus and Lactobacillus iners Reveal Potential Ecological Drivers of Community Composition in the Vagina. Appl. Environ. Microbiol. 2016, 82, 7063–7073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macklaim, J.M.; Gloor, G.B.; Anukam, K.C.; Cribby, S.; Reid, G. At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB-1. Proc. Natl. Acad. Sci. USA 2011, 108, 4688–4695. [Google Scholar] [CrossRef]
- Vaneechoutte, M. Lactobacillus iners, the unusual suspect. Res. Microbiol. 2017, 168, 826–836. [Google Scholar] [CrossRef]
- Borgdorff, H.; Armstrong, S.D.; Tytgat, H.L.P.; Xia, D.; Ndayisaba, G.F.; Wastling, J.M.; van de Wijgert, J.H.H.M. Unique Insights in the Cervicovaginal Lactobacillus iners and L. crispatus Proteomes and Their Associations with Microbiota Dysbiosis. PLoS ONE 2016, 11, e0150767. [Google Scholar] [CrossRef]
- Petricevic, L.; Domig, K.J.; Nierscher, F.J.; Sandhofer, M.J.; Fidesser, M.; Krondorfer, I.; Husslein, P.; Kneifel, W.; Kiss, H. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci. Rep. 2014, 4, 5136. [Google Scholar] [CrossRef]
- Lepargneur, J.-P. Lactobacillus crispatus as biomarker of the healthy vaginal tract. Ann. Biol. Clin. 2016, 74, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Anton, L.; Sierra, L.-J.; DeVine, A.; Barila, G.; Heiser, L.; Brown, A.G.; Elovitz, M.A. Common Cervicovaginal Microbial Supernatants Alter Cervical Epithelial Function: Mechanisms by Which Lactobacillus crispatus Contributes to Cervical Health. Front. Microbiol. 2018, 9, 2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Yao, Z.; Klionsky, D.J. How to control self-digestion: Transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 2015, 25, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, S.; Liu, C.; Mitchell, C.M.; Fiedler, T.L.; Thomas, K.K.; Agnew, K.J.; Marrazzo, J.M.; Fredricks, D.N. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS ONE 2010, 5, e10197. [Google Scholar] [CrossRef] [Green Version]
- Zheng, N.; Guo, R.; Yao, Y.; Jin, M.; Cheng, Y.; Ling, Z. Lactobacillus iners Is Associated with Vaginal Dysbiosis in Healthy Pregnant Women: A Preliminary Study. Biomed Res. Int. 2019, 2019, 6079734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myziuk, L.; Romanowski, B.; Johnson, S.C. BVBlue test for diagnosis of bacterial vaginosis. J. Clin. Microbiol. 2003, 41, 1925–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mårdh, P.-A.; Novikova, N.; Niklasson, O.; Bekassy, Z.; Skude, L. Leukocyte esterase activity in vaginal fluid of pregnant and non-pregnant women with vaginitis/vaginosis and in controls. Infect. Dis. Obstet. Gynecol. 2003, 11, 19–26. [Google Scholar] [CrossRef]
- Lee, Y.S.; Koo, K.-H.; Kim, H.J.; Tian, S.; Kim, T.-Y.; Maltenfort, M.G.; Chen, A.F. Synovial Fluid Biomarkers for the Diagnosis of Periprosthetic Joint Infection: A Systematic Review and Meta-Analysis. J. Bone Joint Surg. Am. 2017, 99, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Fredricks, D.N.; Fiedler, T.L.; Marrazzo, J.M. Molecular Identification of Bacteria Associated with Bacterial Vaginosis. N. Engl. J. Med. 2005, 353, 1899–1911. [Google Scholar] [CrossRef] [Green Version]
- Culhane, J.F.; Nyirjesy, P.; McCollum, K.; Goldenberg, R.L.; Gelber, S.E.; Cauci, S. Variation in vaginal immune parameters and microbial hydrolytic enzymes in bacterial vaginosis positive pregnant women with and without Mobiluncus species. Am. J. Obstet. Gynecol. 2006, 195, 516–521. [Google Scholar] [CrossRef]
- Afolabi, B.B.; Moses, O.E.; Oduyebo, O.O. Bacterial Vaginosis and Pregnancy Outcome in Lagos, Nigeria. Open Forum Infect. Dis. 2016, 3, ofw030. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, A.; Khodabandehloo, M.; Ramazanzadeh, R.; Farhadifar, F.; Nikkhoo, B.; Soofizade, N.; Rezaii, M. Association between Ureaplasma urealyticum endocervical infection and spontaneous abortion. Iran. J. Microbiol. 2014, 6, 392–397. [Google Scholar]
- Mercer, B.M.; Goldenberg, R.L.; Meis, P.J.; Moawad, A.H.; Shellhaas, C.; Das, A.; Menard, M.K.; Caritis, S.N.; Thurnau, G.R.; Dombrowski, M.P.; et al. The Preterm Prediction Study: Prediction of preterm premature rupture of membranes through clinical findings and ancillary testing. Am. J. Obstet. Gynecol. 2000, 183, 738–745. [Google Scholar] [CrossRef]
- Dasari, S.; Anandan, S.K.; Rajendra, W.; Valluru, L. Role of microbial flora in female genital tract: A comprehensive review. Asian Pacific J. Trop. Dis. 2016, 6, 909–917. [Google Scholar] [CrossRef]
- Koumans, E.H.; Markowitz, L.E.; Hogan, V. Indications for therapy and treatment recommendations for bacterial vaginosis in nonpregnant and pregnant women: A synthesis of data. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2002, 35, S152–S172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradshaw, C.S.; Morton, A.N.; Hocking, J.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 2006, 193, 1478–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradshaw, C.S.; Tabrizi, S.N.; Fairley, C.K.; Morton, A.N.; Rudland, E.; Garland, S.M. The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. J. Infect. Dis. 2006, 194, 828–836. [Google Scholar] [CrossRef] [Green Version]
- De Backer, E.; Verhelst, R.; Verstraelen, H.; Claeys, G.; Verschraegen, G.; Temmerman, M.; Vaneechoutte, M. Antibiotic susceptibility of Atopobium vaginae. BMC Infect. Dis. 2006, 6, 51. [Google Scholar] [CrossRef]
- MacPhee, R.A.; Hummelen, R.; Bisanz, J.E.; Miller, W.L.; Reid, G. Probiotic strategies for the treatment and prevention of bacterial vaginosis. Expert Opin. Pharmacother. 2010, 11, 2985–2995. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Simopoulou, M.; Nikas, Y.; Rapani, A.; Nitsos, N.; Pierouli, K.; Pappas, A.; Pantou, A.; Markomichali, C.; Koutsilieris, M.; et al. Efficient treatment of chronic endometritis through a novel approach of intrauterine antibiotic infusion: A case series. BMC Womens. Health 2018, 18, 197. [Google Scholar] [CrossRef] [Green Version]
- Turovskiy, Y.; Sutyak Noll, K.; Chikindas, M.L. The aetiology of bacterial vaginosis. J. Appl. Microbiol. 2011, 110, 1105–1128. [Google Scholar] [CrossRef] [Green Version]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.S.; Eschenbach, D.; Holmes, K.K. Nonspecific vaginitis: Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.E. Bacterial vaginosis and miscarriage. Curr. Opin. Infect. Dis. 2004, 17, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Sebire, N.J. Choriodecidual inflammatory syndrome (CoDIS) is the leading, and under recognised, cause of early preterm delivery and second trimester miscarriage. Med. Hypotheses 2001, 56, 497–500. [Google Scholar] [CrossRef]
- U.S. Preventive Services Task Force. Screening for bacterial vaginosis in pregnancy to prevent preterm delivery: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2008, 148, 214–219. [Google Scholar] [CrossRef]
- Giakoumelou, S.; Wheelhouse, N.; Cuschieri, K.; Entrican, G.; Howie, S.E.M.; Horne, A.W. The role of infection in miscarriage. Hum. Reprod. Update 2016, 22, 116–133. [Google Scholar] [CrossRef] [Green Version]
- Yudin, M.H.; Money, D.M. No. 211-Screening and Management of Bacterial Vaginosis in Pregnancy. J. Obstet. Gynaecol. Can. JOGC J. Obstet. Gynaecol. Can. 2017, 39, e184–e191. [Google Scholar] [CrossRef]
- Donders, G.G.; Van Calsteren, K.; Bellen, G.; Reybrouck, R.; Van den Bosch, T.; Riphagen, I.; Van Lierde, S. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 1315–1324. [Google Scholar] [CrossRef]
- du Fossé, N.A.; Lashley, E.E.L.O.; van Beelen, E.; Meuleman, T.; le Cessie, S.; van Lith, J.M.M.; Eikmans, M.; van der Hoorn, M.L.P. Identification of distinct seminal plasma cytokine profiles associated with male age and lifestyle characteristics in unexplained recurrent pregnancy loss. J. Reprod. Immunol. 2021, 147, 103349. [Google Scholar] [CrossRef]
- Baqui, A.H.; Lee, A.C.C.; Koffi, A.K.; Khanam, R.; Mitra, D.K.; Dasgupta, S.K.; Uddin, J.; Ahmed, P.; Rafiqullah, I.; Rahman, M.; et al. Prevalence of and risk factors for abnormal vaginal flora and its association with adverse pregnancy outcomes in a rural district in north-east Bangladesh. Acta Obstet. Gynecol. Scand. 2019, 98, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Bonneton, M.; Huynh, B.-T.; Seck, A.; Bercion, R.; Sarr, F.D.; Delarocque-Astagneau, E.; Vray, M. Bacterial vaginosis and other infections in pregnant women in Senegal. BMC Infect. Dis. 2021, 21, 1090. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.A.; John, S.; Sobel, J.D.; Akins, R.A. Longitudinal analysis of vaginal microbiome dynamics in women with recurrent bacterial vaginosis: Recognition of the conversion process. PLoS ONE 2013, 8, e82599. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.Y.; Seo, S.-S.; Kong, J.-S.; Lee, J.-K.; Kim, M.K. Association between Obesity and Cervical Microflora Dominated by Lactobacillus iners in Korean Women. J. Clin. Microbiol. 2015, 53, 3304–3309. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.C.; McAndrew, T.; Chen, Z.; Harari, A.; Barris, D.M.; Viswanathan, S.; Rodriguez, A.C.; Castle, P.; Herrero, R.; Schiffman, M.; et al. The cervical microbiome over 7 years and a comparison of methodologies for its characterization. PLoS ONE 2012, 7, e40425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Brown, C.J.; Abdo, Z.; Davis, C.C.; Hansmann, M.A.; Joyce, P.; Foster, J.A.; Forney, L.J. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007, 1, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Bobdiwala, S.; Al-Memar, M.; Lee, Y.; Smith, A.; Marchesi, J.; Bennett, P.; Bourne, T.; MacIntyre, D. OC08.06: *Association of vaginal microbiota composition and outcomes in women found to have a pregnancy of unknown location (PUL) on an initial early pregnancy ultrasound scan. Ultrasound Obstet. Gynecol. 2017, 50, 16. [Google Scholar] [CrossRef]
- Mangalam, A.; Shahi, S.K.; Luckey, D.; Karau, M.; Marietta, E.; Luo, N.; Choung, R.S.; Ju, J.; Sompallae, R.; Gibson-Corley, K.; et al. Human Gut-Derived Commensal Bacteria Suppress CNS Inflammatory and Demyelinating Disease. Cell Rep. 2017, 20, 1269–1277. [Google Scholar] [CrossRef] [Green Version]
- Liblau, R. Glatiramer acetate for the treatment of multiple sclerosis: Evidence for a dual anti-inflammatory and neuroprotective role. J. Neurol. Sci. 2009, 287, S17–S23. [Google Scholar] [CrossRef]
- Nelson, D.B.; Bellamy, S.; Nachamkin, I.; Ness, R.B.; Macones, G.A.; Allen-Taylor, L. First trimester bacterial vaginosis, individual microorganism levels, and risk of second trimester pregnancy loss among urban women. Fertil. Steril. 2007, 88, 1396–1403. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Murugesan, S.; Singh, P.; Saadaoui, M.; Elhag, D.A.; Terranegra, A.; Kabeer, B.S.A.; Marr, A.K.; Kino, T.; Brummaier, T.; et al. Vaginal Microbiota and Cytokine Levels Predict Preterm Delivery in Asian Women. Front. Cell. Infect. Microbiol. 2021, 11, 639665. [Google Scholar] [CrossRef]
- Sharma, H.; Tal, R.; Clark, N.A.; Segars, J.H. Microbiota and pelvic inflammatory disease. Semin. Reprod. Med. 2014, 32, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaer, A.; Doğan, B.; Günal, S.; Tuncay, G.; Arda Düz, S.; Ünver, T.; Tecellioğlu, N. The vaginal microbiota composition of women undergoing assisted reproduction: A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Reynolds, S.; Stern, V.L.; Parker, J.L.; Stafford, G.P.; Paley, M.N.; Anumba, D.O.C. Identifying metabolite markers for preterm birth in cervicovaginal fluid by magnetic resonance spectroscopy. Metabolomics 2016, 12, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosca, A.S.; Castro, J.; Sousa, L.G.V.; Cerca, N. Gardnerella and vaginal health: The truth is out there. FEMS Microbiol. Rev. 2020, 44, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Odogwu, N.M.; Chen, J.; Onebunne, C.A.; Jeraldo, P.; Yang, L.; Johnson, S.; Ayeni, F.A.; Walther-Antonio, M.R.S.; Olayemi, O.O.; Chia, N.; et al. Predominance of Atopobium vaginae at Midtrimester: A Potential Indicator of Preterm Birth Risk in a Nigerian Cohort. mSphere 2021, 6, 01261-20. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hočevar, K.; Maver, A.; Vidmar Šimic, M.; Hodžić, A.; Haslberger, A.; Premru Seršen, T.; Peterlin, B. Vaginal Microbiome Signature Is Associated with Spontaneous Preterm Delivery. Front. Med. 2019, 6, 201. [Google Scholar] [CrossRef]
- Larsen, B.; Hwang, J. Mycoplasma, Ureaplasma, and adverse pregnancy outcomes: A fresh look. Infect. Dis. Obstet. Gynecol. 2010, 2010, 521921. [Google Scholar] [CrossRef] [Green Version]
- Pace, R.M.; Chu, D.M.; Prince, A.L.; Ma, J.; Seferovic, M.D.; Aagaard, K.M. Complex species and strain ecology of the vaginal microbiome from pregnancy to postpartum and association with preterm birth. Med 2021, 2, 1027–1049. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Bieda, J.; Chaemsaithong, P.; Miranda, J.; Chaiworapongsa, T.; Ravel, J. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2014, 2, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elovitz, M.A.; Gajer, P.; Riis, V.; Brown, A.G.; Humphrys, M.S.; Holm, J.B.; Ravel, J. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat. Commun. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Chen, L.; Chen, Z.; Jiang, X.; Pan, M. Association of the vaginal microbiota with pregnancy outcomes in Chinese women after cervical cerclage. Reprod. Biomed. Online 2020, 41, 698–706. [Google Scholar] [CrossRef]
- Brown, R.G.; Chan, D.; Terzidou, V.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; MacIntyre, D.A.; Bennett, P.R. Prospective observational study of vaginal microbiota pre- and post-rescue cervical cerclage. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 916–925. [Google Scholar] [CrossRef] [Green Version]
- Garry, N.; Keenan, O.; Lindow, S.W.; Darcy, T. Pregnancy outcomes following elective abdominal cerclage following cervical excision surgery for neoplastic disease. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 225–229. [Google Scholar] [CrossRef]
- Vomstein, K.; Egerup, P.; Kolte, A.M.; Behrendt-Møller, I.; Boje, A.D.; Bertelsen, M.-L.; Eiken, C.S.; Reiersen, M.R.; Toth, B.; la Cour Freiesleben, N.; et al. Biopsy-free profiling of the uterine immune system in patients with recurrent pregnancy loss and unexplained infertility. Reprod. Biomed. Online 2023, in press. 103207. [Google Scholar] [CrossRef]
- Miles, S.M.; Hardy, B.L.; Merrell, D.S. Investigation of the microbiota of the reproductive tract in women undergoing a total hysterectomy and bilateral salpingo-oopherectomy. Fertil. Steril. 2017, 107, 813–820.e1. [Google Scholar] [CrossRef] [Green Version]
- Moreno, I.; Cicinelli, E.; Garcia-Grau, I.; Gonzalez-Monfort, M.; Bau, D.; Vilella, F.; De Ziegler, D.; Resta, L.; Valbuena, D.; Simon, C. The diagnosis of chronic endometritis in infertile asymptomatic women: A comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am. J. Obstet. Gynecol. 2018, 218, 602.e1–602.e16. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Franasiak, J.M.; Zhan, Y.; Scott, R.T.; Rajchel, J.; Bedard, J.; Newby, R.; Scott, R.T.; Treff, N.R.; Chu, T. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: Next generation sequencing (NGS) analysis of the 16S ribosomal gene. Hum. Microbiome J. 2017, 3, 15–21. [Google Scholar] [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Bau, D.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Vilella, F.; Romero, R.; Simón, C. The first glimpse of the endometrial microbiota in early pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 296–305. [Google Scholar] [CrossRef]
- Chang, D.-H.; Shin, J.; Rhee, M.-S.; Park, K.-R.; Cho, B.-K.; Lee, S.-K.; Kim, B.-C. Vaginal Microbiota Profiles of Native Korean Women and Associations with High-Risk Pregnancy. J. Microbiol. Biotechnol. 2020, 30, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.D.; Romero, R.; Gervasi, M.T.; Gomez-Lopez, N.; Tran, M.R.; Garcia-Flores, V.; Pacora, P.; Jung, E.; Hassan, S.S.; Hsu, C.-D.; et al. Does the endometrial cavity have a molecular microbial signature? Sci. Rep. 2019, 9, 9905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyman, R.W.; Fukushima, M.; Jiang, H.; Fung, E.; Rand, L.; Johnson, B.; Vo, K.C.; Caughey, A.B.; Hilton, J.F.; Davis, R.W.; et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod. Sci. 2014, 21, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Tanimura, K.; Sasagawa, Y.; Yamada, H. Vaginal microbiota associated with preterm delivery. J. Infect. Chemother. Off. J. Japan Soc. Chemother. 2020, 26, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.S.; Newnham, J.P.; Doherty, D.A.; Furfaro, L.L.; Pendal, N.L.; Loh, D.E.; Keelan, J.A. A specific bacterial DNA signature in the vagina of Australian women in midpregnancy predicts high risk of spontaneous preterm birth (the Predict1000 study). Am. J. Obstet. Gynecol. 2021, 224, 206.e1–206.e23. [Google Scholar] [CrossRef]
- Brown, R.G.; Al-Memar, M.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Chan, D.; Lewis, H.; Kindinger, L.; Terzidou, V.; Bourne, T.; et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl. Res. 2019, 207, 30–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, C.M.; Mazzoni, C.; Hogstrom, L.; Bryant, A.; Bergerat, A.; Cher, A.; Pochan, S.; Herman, P.; Carrigan, M.; Sharp, K.; et al. Delivery Mode Affects Stability of Early Infant Gut Microbiota. Cell Rep. Med. 2020, 1, 100156. [Google Scholar] [CrossRef]

| Total Number of Patients | Allocation of Patients | Hypervariable Region | Primers | Sequencing Platform | Reference |
|---|---|---|---|---|---|
| n = 1 patients | n = 1 RRF | V2–4–8 V3–6, 7–9 | NS | Ion S5 XL | [53] |
| n = 25 patients | n = 25 RM | V4 | NS | MiSeq | [54] |
| n = 36 patients | n = 20 Control n = 16 RM | V3–V4 | 341F [55] 5′-barcode-CCTACGGGNGGCWGCAG-3′ 805R [55] 5′-barcode-GACTACHVGGGTATCTAATCC-3′ | MiSeq | [56] |
| n = 50 patients | n = 25 Control n = 25 RSA | V3–V4 | 338F 5′-ACTCCTACGGGAGGCAGCA-3′ 806R 5′-GGACTACHVGGGTWTCTAAT-3′ | NovaSeq | [57] |
| n = 50 patients | n = 10 Control n = 20 RM n = 20 RIF | V3–V4 | 341F 5′-CCTACGGGNGGCWGCAG-3′ 805R 5′-GACTACHVGGGTATCTAATCC-3′ | MiSeq | [58] |
| n = 50 patients | n = 25 Control n = 25 RM | NS | NS | NS | [59] |
| n = 50 | n = 25 Control n = 25 Embryonic miscarriage | V4 | 515F GTGCCAGCMGCCGCGGTAA 806R GGACTACHVGGGTWTCTAAT | PGM Ion Torrent HiSeq3000/4000 | [60] |
| n = 50 | n = 15 Control n = 13 Empty-sac miscarriage n = 22 Missed miscarriage | V4 | 520F 5′-AYTGGGYDTAAAGNG-3′ 802R 5′-TACNVGGGTATCTAATCC-3′ | MiSeq | [61] |
| n = 58 patients | n = 14 Control n = 21 RA n = 23 INF | V3–V4 | S-D-Bact-0341-b-S-17 CCTACGGGNGGCWGCAG S-D-Bact-1290785-a-A-21 GACTACHVGGGTATCTAATCC | MiSeq | [62] |
| n = 58 patients | n = 27 Normal Induced Abortion n = 31 Unexplained RSA | V3–V4 | 338F 5′-ACTCCTACGGGAGGCAGCA-3′ 806R 5′-GGACTACHVGGGTWTCTAAT-3′ | MiSeq | [63] |
| n = 63 patients | n = 44 Became pregnant n = 19 Did not become pregnant | V4 | 515F [64] 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGYCAGCMGCCGCGGTAA-3′ 806rB [64] 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACNVGGGTWTCTAAT-3′ | MiSeq | [65] |
| n = 85 patients | n = 39 Control n = 46 RPL | V3–V4 | 341F [66] 5′-CCTACGGGNGGCWGCAG-3′ ITS1F [66] 5′-GGTCATTTAGAGGAAGTAA-3′ 785R [66] 5′-GACTACHVGGGTATCTAATCC-3′ ITS2 [66] 5′-GCTGCGTTCTTCATCGATGC-3′ | MiSeq | [67] |
| n = 87 patients | n = 24 Elective Abortion n = 63 Missed Miscarriage | V4 | 515F 5′-GTGCCAGCMGCCGCGGTAA-3′ 806R 5′-GGACTACHVGGGTWTCTAAT-3′ | Mini Seq | [68] |
| n = 104 patients | n = 50 Control n = 54 Missed abortion | V1–V9 | F44 RGTTYGATYMTGGCTCAG R1543 GGNTACCTTKTTACGACTT | Bacterial chip | [69] |
| n = 105 patients | n = 17 Control n = 88 Unexplained RPL | V3–V4 | S-D-Bact-0341-b-S-17 5′-CCTACGGGNGGCWGCAG-3′ S-D-Bact-0785-a-A-21 5′-GACTACHVGGGTATCTAATCC-3′ | MiSeq | [70] |
| n = 109 patients | n = 20 Control n = 89 SA | NS | NS | NS | [71] |
| n = 126 patients | n = 18 Control n = 108 RSA | V3–V4 | 338F 5′-ACTCCTACGGGAGGCAGCAG-3′ 806R 5′-GGACTACHVGGGTWTCTAAT-3′ | MiSeq | [72] |
| n = 147 patients | n = 23 SA n = 36 Nonabortion n = 88 Induced Abortion | V1–V3 | NS | Roche/454 GS Junior | [73] |
| n = 161 pregnancies | n = 83 Control n = 64 1st Trimester Miscarriage n = 14 2nd Trimester Miscarriage | V1–V2 | Illumina i5 adapter 5′-AATGATACGGCGACCACCGAGATCTACAC-3′ 8–bp bar code 5′-TATGGTAATT-3′ 28F 5′-GAGTTTGATCNTGGCTCAG-3′ Illumina i7 adapter 5′-CAAGCAGAAGACGGCATACGAGAT-3′ 8-bp bar code 5′-AGTCAGTCAG-3′ 388R 5′-TGCTGCCTCCCGTAGGAGT-3′ | MiSeq | [18] |
| n = 164 patients | n = 48 SA n = 116 NP | V3–V4 | 343F 5′TACGGRAGGCAGCAG-3 798R 5-AGGGTATCTAATCCT3′ | MiSeq | [74] |
| n = 167 patients | n = 74 Control n = 39 Aneuploid miscarriage n = 54 Euploid miscarriage | V1–V2 | 28F-YM GAGTTTGATYMTGGCTCAG 28F-Borrellia GAGTTTGATCCTGGCTTAG 28F-Chloroflex GAATTTGATCTTGGTTCAG 28F-Bifdo GGGTTCGATTCTGGCTCAG 388R TGCTGCCTCCCGTAGGAGT | MiSeq | [75] |
| n = 243 patients | n = 243 RM | NS | NS | NS | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doroftei, B.; Ilie, O.-D.; Armeanu, T.; Stoian, I.-L.; Anton, N.; Babici, R.-G.; Ilea, C. A Narrative Review Discussing the Obstetric Repercussions Due to Alterations of Personalized Bacterial Sites Developed within the Vagina, Cervix, and Endometrium. J. Clin. Med. 2023, 12, 5069. https://doi.org/10.3390/jcm12155069
Doroftei B, Ilie O-D, Armeanu T, Stoian I-L, Anton N, Babici R-G, Ilea C. A Narrative Review Discussing the Obstetric Repercussions Due to Alterations of Personalized Bacterial Sites Developed within the Vagina, Cervix, and Endometrium. Journal of Clinical Medicine. 2023; 12(15):5069. https://doi.org/10.3390/jcm12155069
Chicago/Turabian StyleDoroftei, Bogdan, Ovidiu-Dumitru Ilie, Theodora Armeanu, Irina-Liviana Stoian, Nicoleta Anton, Ramona-Geanina Babici, and Ciprian Ilea. 2023. "A Narrative Review Discussing the Obstetric Repercussions Due to Alterations of Personalized Bacterial Sites Developed within the Vagina, Cervix, and Endometrium" Journal of Clinical Medicine 12, no. 15: 5069. https://doi.org/10.3390/jcm12155069
APA StyleDoroftei, B., Ilie, O.-D., Armeanu, T., Stoian, I.-L., Anton, N., Babici, R.-G., & Ilea, C. (2023). A Narrative Review Discussing the Obstetric Repercussions Due to Alterations of Personalized Bacterial Sites Developed within the Vagina, Cervix, and Endometrium. Journal of Clinical Medicine, 12(15), 5069. https://doi.org/10.3390/jcm12155069







