Effect of Injury Patterns on the Development of Complications and Trauma-Induced Mortality in Patients Suffering Multiple Trauma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion criteria
2.1.1. Inclusion Criteria and Ethics
2.1.2. Exclusion Criteria
2.2. Data Collection
2.2.1. Demographics
2.2.2. Clinical Course
2.2.3. Status at Admission and Scoring Systems
2.3. Endpoints
2.3.1. Mortality
2.3.2. Complications
2.4. Statistics
3. Results
3.1. Demographics
3.2. Status at Admission and Clinical Course
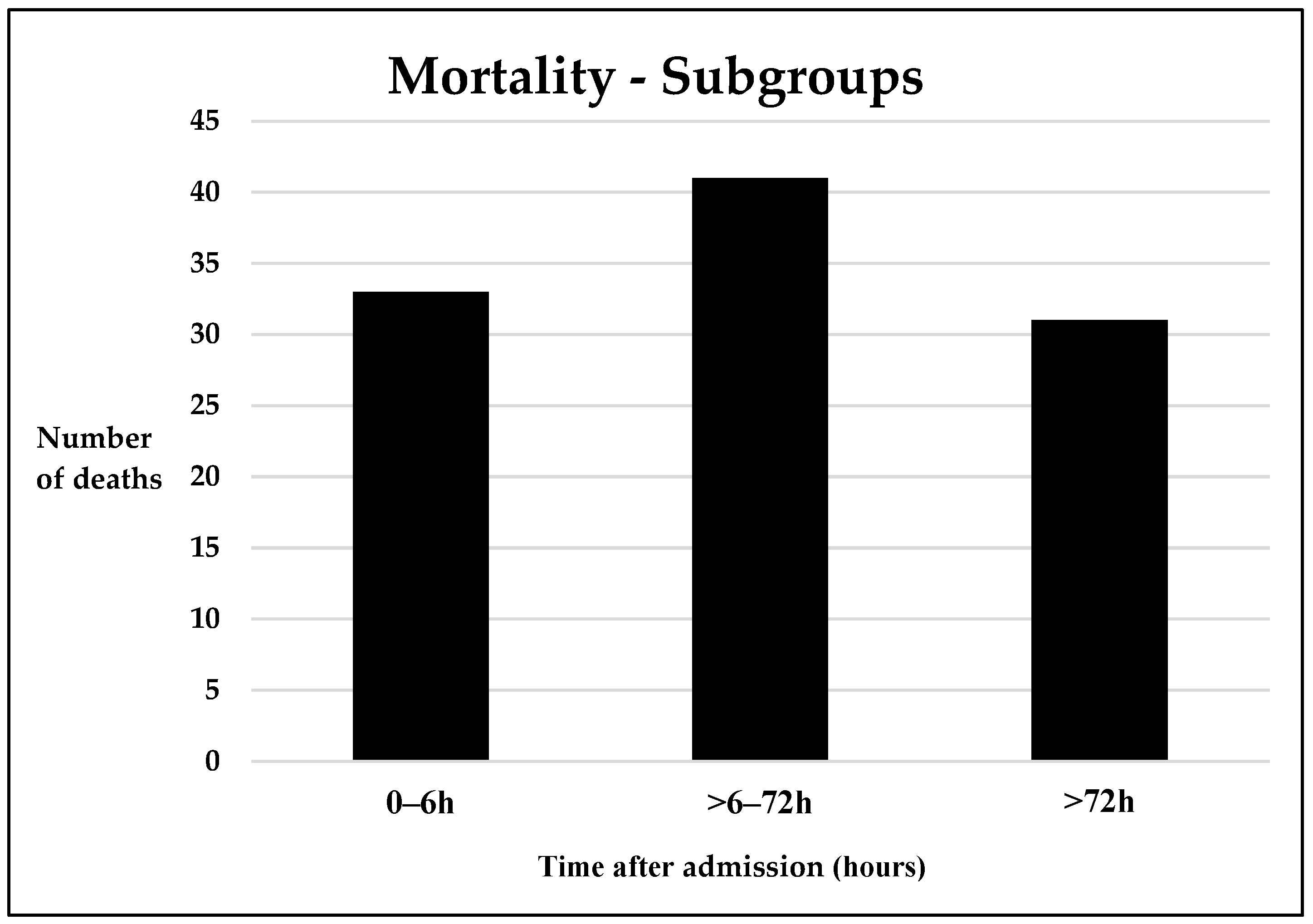
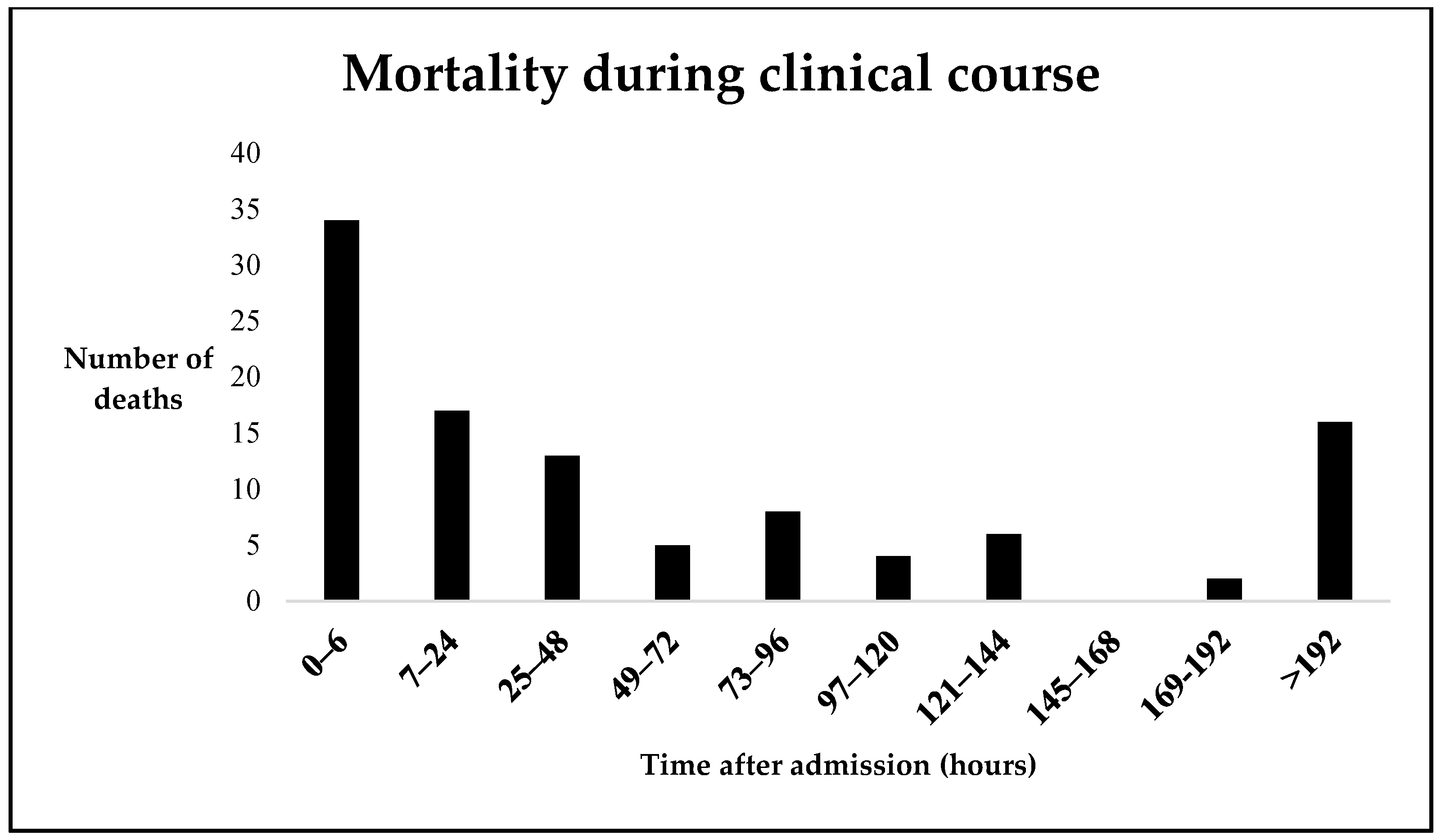
3.3. Mortality
3.3.1. Cox Hazard Regression Analysis for In-Hospital Mortality after Trauma
3.3.2. Logistic Regression Analysis for In-Hospital Mortality after Trauma
Early Mortality
Late Mortality
3.4. Complication Rates and Characteristics
3.5. Complication Cluster Specifics
3.6. Individual Risk Factors
4. Discussion
4.1. Trauma-Associated Mortality
4.2. The Development of Posttraumatic Complications
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Mestoui, Z.; Jalalzadeh, H.; Giannakopoulos, G.F.; Zuidema, W.P. Incidence and etiology of mortality in polytrauma patients in a Dutch level I trauma center. Eur. J. Emerg. Med. 2017, 24, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, R.; Teuben, M.; Andruszkow, H.; Barkatali, B.M.; Pape, H.C. Mortality Patterns in Patients with Multiple Trauma: A Systematic Review of Autopsy Studies. PLoS ONE 2016, 11, e0148844. [Google Scholar] [CrossRef] [Green Version]
- Demetriades, D.; Kimbrell, B.; Salim, A.; Velmahos, G.; Rhee, P.; Preston, C.; Gruzinski, G.; Chan, L. Trauma deaths in a mature urban trauma system: Is “trimodal” distribution a valid concept? J. Am. Coll. Surg. 2005, 201, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Lefering, R.; Gruber-Rathmann, M.; Rueger, J.M.; Lehmann, W.; Wolfgang Lehmann Trauma Registry of the German Society for Trauma Surgery. The impact of BMI on polytrauma outcome. Injury 2012, 43, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Drost, S.; Finkbeiner, R.; Lefering, R.; Grosso, M.; Krinner, S.; Langenbach, A.; The TraumaRegister DGU. Lung Contusion in Polytrauma: An Analysis of the TraumaRegister DGU. Thorac. Cardiovasc. Surg. 2021, 69, 735–748. [Google Scholar] [CrossRef]
- Chrysou, K.; Halat, G.; Hoksch, B.; Schmid, R.A.; Kocher, G.J. Lessons from a large trauma center: Impact of blunt chest trauma in polytrauma patients-still a relevant problem? Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 42. [Google Scholar] [CrossRef]
- Moore, T.A.; Simske, N.M.; Vallier, H.A. Fracture fixation in the polytrauma patient: Markers that matter. Injury 2020, 51, S10–S14. [Google Scholar] [CrossRef]
- van Breugel, J.M.M.; Niemeyer, M.J.S.; Houwert, R.M.; Groenwold, R.H.H.; Leenen, L.P.H.; van Wessem, K.J.P. Global changes in mortality rates in polytrauma patients admitted to the ICU-a systematic review. World J. Emerg. Surg. 2020, 15, 55. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Welch, K.; Siddiqui, J.; Remick, D.G. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 2006, 177, 1967–1974. [Google Scholar] [CrossRef]
- de Vries, R.; Reininga, I.H.F.; de Graaf, M.W.; Heineman, E.; El Moumni, M.; Wendt, K.W. Older polytrauma: Mortality and complications. Injury 2019, 50, 1440–1447. [Google Scholar] [CrossRef]
- Colnaric, J.M.; El Sibai, R.H.; Bachir, R.H.; El Sayed, M.J. Injury severity score as a predictor of mortality in adult trauma patients by injury mechanism types in the United States: A retrospective observational study. Medicine 2022, 101, e29614. [Google Scholar] [CrossRef]
- Steinhausen, E.; Lefering, R.; Tjardes, T.; Neugebauer, E.A.; Bouillon, B.; Rixen, D.; The Committee on Emergency Medicine, Intensive and Trauma Care (Sektion NIS) of the German Society for Trauma Surgery (DGU). A risk-adapted approach is beneficial in the management of bilateral femoral shaft fractures in multiple trauma patients: An analysis based on the trauma registry of the German Trauma Society. J. Trauma Acute Care Surg. 2014, 76, 1288–1293. [Google Scholar] [CrossRef] [Green Version]
- Pape, H.C.; Halvachizadeh, S.; Leenen, L.; Velmahos, G.D.; Buckley, R.; Giannoudis, P.V. Timing of major fracture care in polytrauma patients—An update on principles, parameters and strategies for 2020. Injury 2019, 50, 1656–1670. [Google Scholar] [CrossRef]
- Wu, J.; Sheng, L.; Ma, Y.; Gu, J.; Zhang, M.; Gan, J.; Xu, S.; Jiang, G. The analysis of risk factors of impacting mortality rate in severe multiple trauma patients with posttraumatic acute respiratory distress syndrome. Am. J. Emerg. Med. 2008, 26, 419–424. [Google Scholar] [CrossRef]
- Meyer-Zehnder, B.; Tobias, E.E.; Pargger, H. Mortality 7 years after prolonged treatment on a surgical intensive care unit. Swiss Med. Wkly. 2022, 152, w30144. [Google Scholar] [CrossRef]
- Bouillon, B.; Probst, C.; Maegele, M.; Wafaisade, A.; Helm, P.; Mutschler, M.; Brockamp, T.; Shafizadeh, S.; Paffrath, T. Emergency room management of multiple trauma: ATLS(R) and S3 guidelines. Chirurg 2013, 84, 745–752. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Rating the severity of tissue damage: I. The abbreviated scale. JAMA 1971, 215, 277–280. [CrossRef]
- Girshausen, R.; Horst, K.; Herren, C.; Blasius, F.; Hildebrand, F.; Andruszkow, H. Polytrauma scoring revisited: Prognostic validity and usability in daily clinical practice. Eur. J. Trauma Emerg. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensiv. Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Sauaia, A.; Moore, F.A.; Moore, E.E. Postinjury Inflammation and Organ Dysfunction. Crit. Care Clin. 2017, 33, 167–191. [Google Scholar] [CrossRef] [Green Version]
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 1992, 20, 864–874. [CrossRef]
- Rozenfeld, M.; Radomislensky, I.; Freedman, L.; Givon, A.; Novikov, I.; Peleg, K. ISS groups: Are we speaking the same language? Inj. Prev. 2014, 20, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Legall, J.R.; Morris, A.; Spragg, R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994, 149, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N.; Van Biesen, W.; Vanholder, R. Acute renal failure. Lancet 2005, 365, 417–430. [Google Scholar] [CrossRef]
- Pape, H.C.; Grimme, K.; Van Griensven, M.; Sott, A.H.; Giannoudis, P.; Morley, J.; Roise, O.; Ellingsen, E.; Hildebrand, F.; Wiese, B.; et al. Impact of intramedullary instrumentation versus damage control for femoral fractures on immunoinflammatory parameters: Prospective randomized analysis by the EPOFF Study Group. J. Trauma 2003, 55, 7–13. [Google Scholar] [CrossRef]
- van Wessem, K.J.P.; Leenen, L.P.H. Incidence of acute respiratory distress syndrome and associated mortality in a polytrauma population. Trauma Surg. Acute Care Open 2018, 3, e000232. [Google Scholar] [CrossRef] [Green Version]
- Ciesla, D.J.; Moore, E.E.; Johnson, J.L.; Burch, J.M.; Cothren, C.C.; Sauaia, A. A 12-year prospective study of postinjury multiple organ failure: Has anything changed? Arch. Surg. 2005, 140, 432–438; discussion 438–440. [Google Scholar] [CrossRef]
- Trunkey, D.D. Trauma. Accidental and intentional injuries account for more years of life lost in the U.S. than cancer and heart disease. Among the prescribed remedies are improved preventive efforts, speedier surgery and further research. Sci. Am. 1983, 249, 28–35. [Google Scholar] [CrossRef]
- de Knegt, C.; Meylaerts, S.A.; Leenen, L.P. Applicability of the trimodal distribution of trauma deaths in a Level I trauma centre in the Netherlands with a population of mainly blunt trauma. Injury 2008, 39, 993–1000. [Google Scholar] [CrossRef]
- Farzan, N.; Foroghi Ghomi, S.Y.; Mohammadi, A.R. A retrospective study on evaluating GAP, MGAP, RTS and ISS trauma scoring system for the prediction of mortality among multiple trauma patients. Ann. Med. Surg. 2022, 76, 103536. [Google Scholar] [CrossRef] [PubMed]
- Leijdesdorff, H.A.; Gillissen, S.; Schipper, I.B.; Krijnen, P. Injury Pattern and Injury Severity of In-Hospital Deceased Road Traffic Accident Victims in The Netherlands: Dutch Road Traffic Accidents Fatalities. World J. Surg. 2020, 44, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M.; Adina, H.; Schmidt, J. Correlation of procalcitonin and C-reactive protein to inflammation, complications, and outcome during the intensive care unit course of multiple-trauma patients. Crit. Care 2006, 10, R12006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerovic, O.; Golubovic, V.; Spec-Marn, A.; Kremzar, B.; Vidmar, G. Relationship between injury severity and lactate levels in severely injured patients. Intensiv. Care Med. 2003, 29, 1300–1305. [Google Scholar] [CrossRef]
- Odom, S.R.; Howell, M.D.; Silva, G.S.; Nielsen, V.M.; Gupta, A.; Shapiro, N.I.; Talmor, D. Lactate clearance as a predictor of mortality in trauma patients. J. Trauma Acute Care Surg. 2013, 74, 999–1004. [Google Scholar] [CrossRef]
- Qi, J.; Bao, L.; Yang, P.; Chen, D. Comparison of base excess, lactate and pH predicting 72-h mortality of multiple trauma. BMC Emerg. Med. 2021, 21, 80. [Google Scholar] [CrossRef]
- Gustafson, M.L.; Hollosi, S.; Chumbe, J.T.; Samanta, D.; Modak, A.; Bethea, A. The effect of ethanol on lactate and base deficit as predictors of morbidity and mortality in trauma. Am. J. Emerg. Med. 2015, 33, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Vella, M.A.; Crandall, M.L.; Patel, M.B. Acute Management of Traumatic Brain Injury. Surg. Clin. N. Am. 2017, 97, 1015–1030. [Google Scholar] [CrossRef]
- Maegele, M.; Schochl, H.; Menovsky, T.; Marechal, H.; Marklund, N.; Buki, A.; Stanworth, S. Coagulopathy and haemorrhagic progression in traumatic brain injury: Advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017, 16, 630–647. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, S. Recent Advances in Pathophysiology of Traumatic Brain Injury. Curr. Neuropharmacol. 2018, 16, 1224–1238. [Google Scholar] [CrossRef]
- Frohlich, M.; Lefering, R.; Probst, C.; Paffrath, T.; Schneider, M.M.; Maegele, M.; Sakka, S.G.; Bouillon, B.; Wafaisade, A.; Committee on Emergency Medicine, Intensive Care and Trauma Management of the German Trauma Society (Sektion NIS). Epidemiology and risk factors of multiple-organ failure after multiple trauma: An analysis of 31,154 patients from the TraumaRegister DGU. J. Trauma Acute Care Surg. 2014, 76, 921–927; discussion 927–928. [Google Scholar] [CrossRef] [PubMed]
- Ingraham, A.M.; Xiong, W.; Hemmila, M.R.; Shafi, S.; Goble, S.; Neal, M.L.; Nathens, A.B. The attributable mortality and length of stay of trauma-related complications: A matched cohort study. Ann. Surg. 2010, 252, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Komori, A.; Shiraishi, A.; Sugiyama, T.; Iriyama, H.; Kainoh, T.; Saitoh, D. Trauma complications and in-hospital mortality: Failure-to-rescue. Crit. Care 2020, 24, 223. [Google Scholar] [CrossRef]
- Halvachizadeh, S.; Baradaran, L.; Cinelli, P.; Pfeifer, R.; Sprengel, K.; Pape, H.C. How to detect a polytrauma patient at risk of complications: A validation and database analysis of four published scales. PLoS ONE 2020, 15, e0228082. [Google Scholar] [CrossRef]
- Poole, G.V.; Tinsley, M.; Tsao, A.K.; Thomae, K.R.; Martin, R.W.; Hauser, C.J. Abbreviated Injury Scale does not reflect the added morbidity of multiple lower extremity fractures. J. Trauma 1996, 40, 951–954; discussion 954–955. [Google Scholar] [CrossRef]
- Zeelenberg, M.L.; Den Hartog, D.; Halvachizadeh, S.; Pape, H.C.; Verhofstad, M.H.J.; Van Lieshout, E.M.M. The impact of upper-extremity injuries on polytrauma patients at a level 1 trauma center. J. Shoulder Elb. Surg. 2022, 31, 914–922. [Google Scholar] [CrossRef]
- Namas, R.; Ghuma, A.; Hermus, L.; Zamora, R.; Okonkwo, D.O.; Billiar, T.R.; Vodovotz, Y. The acute inflammatory response in trauma/hemorrhage and traumatic brain injury: Current state and emerging prospects. Libyan J. Med. 2009, 4, 97–103. [Google Scholar] [CrossRef]
- Yi, J.; Slaughter, A.; Kotter, C.V.; Moore, E.E.; Hauser, C.J.; Itagaki, K.; Wohlauer, M.; Frank, D.N.; Silliman, C.; Banerjee, A.; et al. A “Clean Case” of Systemic Injury: Mesenteric Lymph after Hemorrhagic Shock Elicits a Sterile Inflammatory Response. Shock 2015, 44, 336–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halbgebauer, R.; Braun, C.K.; Denk, S.; Mayer, B.; Cinelli, P.; Radermacher, P.; Wanner, G.A.; Simmen, H.P.; Gebhard, F.; Rittirsch, D.; et al. Hemorrhagic shock drives glycocalyx, barrier and organ dysfunction early after polytrauma. J. Crit. Care 2018, 44, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, R.; Lichte, P.; Schreiber, H.; Sellei, R.M.; Dienstknecht, T.; Sadeghi, C.; Pape, H.C.; Kobbe, P. Models of hemorrhagic shock: Differences in the physiological and inflammatory response. Cytokine 2013, 61, 585–590. [Google Scholar] [CrossRef]
- Zang, K.; Chen, B.; Wang, M.; Chen, D.; Hui, L.; Guo, S.; Ji, T.; Shang, F. The effect of early mobilization in critically ill patients: A meta-analysis. Nurs. Crit. Care 2020, 25, 360–367. [Google Scholar] [CrossRef]
- Allemann, F.; Heining, S.; Zelle, B.; Probst, C.; Pape, H.C. Risk factors for complications and adverse outcomes in polytrauma patients with associated upper extremity injuries. Patient Saf. Surg. 2019, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Seibold, T.; Schonfelder, J.; Weeber, F.; Lechel, A.; Armacki, M.; Waldenmaier, M.; Wille, C.; Palmer, A.; Halbgebauer, R.; Karasu, E.; et al. Small Extracellular Vesicles Propagate the Inflammatory Response After Trauma. Adv. Sci. 2021, 8, e2102381. [Google Scholar] [CrossRef]
- Grubmuller, M.; Kerschbaum, M.; Diepold, E.; Angerpointner, K.; Nerlich, M.; Ernstberger, A. Severe thoracic trauma—Still an independent predictor for death in multiple injured patients? Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelet, P.; Couret, D.; Bregeon, F.; Perrin, G.; D’Journo, X.B.; Pequignot, V.; Vig, V.; Auffray, J.P. Early onset pneumonia in severe chest trauma: A risk factor analysis. J. Trauma 2010, 68, 395–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muldowney, M.; Bhalla, P.I. Pain management in thoracic trauma. Int. Anesthesiol. Clin. 2021, 59, 40–47. [Google Scholar] [CrossRef]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.K.; Obremskey, W.T.; Hsu, J.R.; Andersen, R.C.; Calhoun, J.H.; Clasper, J.C.; Whitman, T.J.; Curry, T.K.; Fleming, M.E.; Wenke, J.C.; et al. Prevention of infections associated with combat-related extremity injuries. J. Trauma 2011, 71, S235–S257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wutzler, S.; Blasius, F.M.; Stormann, P.; Lustenberger, T.; Frink, M.; Maegele, M.; Weuster, M.; Bayer, J.; Caspers, M.; Seekamp, A.; et al. Pneumonia in severely injured patients with thoracic trauma: Results of a retrospective observational multi-centre study. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 31. [Google Scholar] [CrossRef] [Green Version]
- Kohlenberg, A.; Schwab, F.; Behnke, M.; Geffers, C.; Gastmeier, P. Pneumonia associated with invasive and noninvasive ventilation: An analysis of the German nosocomial infection surveillance system database. Intensiv. Care Med. 2010, 36, 971–978. [Google Scholar] [CrossRef]
- Mangram, A.J.; Sohn, J.; Zhou, N.; Hollingworth, A.K.; Ali-Osman, F.R.; Sucher, J.F.; Moyer, M.; Dzandu, J.K. Trauma-associated pneumonia: Time to redefine ventilator-associated pneumonia in trauma patients. Am. J. Surg. 2015, 210, 1056–1061; discussion 1061-1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahill, L.A.; Guo, F.; Nguyen, J.; Zhang, F.; Seshadri, A.; Keegan, J.; Hauser, C.J.; Otterbein, L.E.; Robson, S.; Shaefi, S.; et al. Circulating Factors in Trauma Plasma Activate Specific Human Immune Cell Subsets. Injury 2020, 51, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, J.T.; Klingebiel, F.; Blasius, F.M.; Greven, J.; Bolierakis, E.; Janicova, A.; Dunay, I.R.; Hildebrand, F.; Marzi, I.; Relja, B. Alterations of Phagocytic Activity and Capacity in Granulocytes and Monocytes Depend on the Pathogen Strain in Porcine Polytrauma. Front. Med. 2021, 8, 645589. [Google Scholar] [CrossRef] [PubMed]
- Muller-Heck, R.M.; Bosken, B.; Michiels, I.; Dudda, M.; Jager, M.; Flohe, S.B. Major Surgical Trauma Impairs the Function of Natural Killer Cells but Does Not Affect Monocyte Cytokine Synthesis. Life 2021, 12, 13. [Google Scholar] [CrossRef]
- Leonard, J.M.; Zhang, C.X.; Lu, L.; Hoofnagle, M.H.; Fuchs, A.; Clemens, R.A.; Ghosh, S.; Hughes, S.W.; Bochicchio, G.V.; Hotchkiss, R.; et al. Extrathoracic multiple trauma dysregulates neutrophil function and exacerbates pneumonia-induced lung injury. J. Trauma Acute Care Surg. 2021, 90, 924–934. [Google Scholar] [CrossRef]
- Turnbull, I.R.; Ghosh, S.; Fuchs, A.; Hilliard, J.; Davis, C.G.; Bochicchio, G.V.; Southard, R.E. Polytrauma Increases Susceptibility to Pseudomonas Pneumonia in Mature Mice. Shock 2016, 45, 555–563. [Google Scholar] [CrossRef]

| Parameters | Mean/Median (±SD/IQR) | |
|---|---|---|
| Epidemiology | Age (years) | 51.5 (±20.4 *) |
| BMI | 25.9 (±4.1 *) | |
| Gender (% male) | 71% | |
| Injury mechanism | Road traffic car (n; %) | 60 (15.7) |
| Road traffic motorcycle (n; %) | 45 (11.7) | |
| Road traffic bike (n; %) | 27 (7) | |
| Road traffic pedestrian (n; %) | 34 (8.9) | |
| Fall > 3 m height (n; %) | 61 (15.9) | |
| Fall < 3 m height (n; %) | 92 (24) | |
| Explosion trauma (n; %) | 10 (2.6) | |
| Others (n; %) | 54 (14.1) | |
| Trauma severity (median) | ISS | 24 (17–27 #) |
| AIS head | 3 (0–4 #)) | |
| AIS face | 0 (0–0 #) | |
| AIS thorax | 2 (0–3 #) | |
| AIS abdomen | 0 (0–2 #) | |
| AIS extremities | 2 (0–3 #) | |
| AIS external | 0 (0–1 #) | |
| Parameters on admission | GCS | 7 (3–15 #) |
| SOFA | 6 (4–8 #) | |
| Lactate (mmol/L) | 2.92 (±2.65 *) | |
| Onset parameters | Duration of ventilation (h) | 290.5 (±483.8 *) |
| ICU stay (d) | 13.2 (±21.4 *) | |
| Length of emergency surgery (min) | 146.8 (±116.3 *) | |
| Mortality (n; %) | 106 (27.7) | |
| Parameter | Death Overall (n = 106) | Death Immediate | Death Early | Death Late | ||
|---|---|---|---|---|---|---|
| Yes | No | p | ||||
| Age (years) | 61.0 | 47.9 | <0.001 | 59.5 | 58.3 | 66.1 |
| SD | 21.1 | 18.9 | 20.7 | 23.9 | 19.2 | |
| BMI | 26.1 | 25.9 | 0.751 | 25.8 | 26.7 | 25.4 |
| SD | 3.9 | 4.2 | 3.2 | 4.0 | 4.0 | |
| Gender (% male) | 67.0 | 72.6 | 0.272 | 60.6 | 70.7 | 67.7 |
| AIS head (% severe) | 77.4 | 51.6 | <0.001 | 66.7 | 78.0 | 83.9 |
| AIS face (% severe) | 7.5 | 9.4 | 0.571 | 3.0 | 14.6 | 3.2 |
| AIS abdomen (% severe) | 12.3 | 14.8 | 0.523 | 21.2 | 14.6 | 3.2 |
| AIS thorax (% severe) | 35.8 | 45.5 | 0.088 | 51.5 | 24.4 | 35.5 |
| AIS extremity (% severe) | 22.6 | 30.3 | 0.135 | 30.3 | 22.0 | 12.9 |
| AIS external (% severe) | 5.7 | 2.5 | 0.130 | 6.1 | 2.4 | 9.7 |
| ISS | 25 | 22 | <0.001 | 26 | 25 | 25 |
| IQR | 20–30 | 17–27 | 23–34 | 24–29 | 18–27 | |
| SOFA | 7 | 5 | <0.001 | 7 | 8 | 6 |
| IQR | 6–9 | 4–8 | 6–8 | 7–9 | 4–8 | |
| GCS | 3 | 11 | <0.001 | 3 | 3 | 3 |
| IQR | 3–9 | 3–15 | 3–4 | 3–9 | 3–11 | |
| Lactate (mmol/L) | 4.7 | 2.3 | <0.001 | 6.8 | 3.7 | 3.9 |
| SD | 4.0 | 1.5 | 4.7 | 2.6 | 3.5 | |
| Ventilation duration (h) | 138 | 357 | <0.001 | 3 | 32 | 337 |
| SD | 302.0 | 532.2 | 1.4 | 22.9 | 434.2 | |
| ICU stay (d) | 5.7 | 16 | <0.001 | 1 | 2 | 15 |
| SD | 12.2 | 23.4 | 0.4 | 1.1 | 19.7 | |
| Emergency operation duration (min) | 126 | 153 | 0.143 | 54.0 | 135.9 | 140 |
| SD | 97.5 | 120.8 | 30.5 | 98.5 | 95.9 | |
| Complication rate (%) | 28.3 | 54.9 | <0.001 | 6.1 | 19.5 | 64.5 |
| Cluster Infection (%) | 17.0 | 42.6 | <0.001 | 0.0 | 2.4 | 54.8 |
| Cluster Thromboemolism (%) | 2.8 | 6.5 | 0.16 | 0.0 | 0.0 | 9.7 |
| Cluster Surgery (%) | 6.6 | 15.9 | 0.017 | 0.0 | 2.4 | 19.4 |
| Cluster Organ failure (%) | 19.8 | 19.5 | 0.944 | 6.1 | 17.1 | 38.7 |
| Mortality | ISS | Severe Head Injury | Severe Thorax Injury | Age | Complication |
|---|---|---|---|---|---|
| OR | 1.048 | 1.664 | 0.751 | 1.027 | 0.268 |
| 95%-CI | 1.028; 1.068 | 0.994; 2.783 | 0.474; 1.188 | 1.017; 1.038 | 0.172; 0.418 |
| p | <0.001 | 0.053 | 0.221 | <0.001 | <0.001 |
| Mortality Early | ISS | Severe Head Injury | Age | Lactate | SOFA Score |
|---|---|---|---|---|---|
| OR | 1.043 | 1.367 | 1.001 | 1.217 | 1.196 |
| 95%-CI | 1.000; 1.088 | 0.503; 3.720 | 0.978; 1.025 | 1.034; 1.433 | 0.976; 1.466 |
| p | 0.052 | 0.540 | 0.921 | 0.018 | 0.085 |
| Mortality Late | ISS | Severe Head Injury | Severe Thorax Injury | Age | Complication |
|---|---|---|---|---|---|
| OR | 1.028 | 3.557 | 0.998 | 1.041 | 2.773 |
| 95%-CI | 0.987; 1.071 | 1.218; 10.387 | 0.401; 2.482 | 1.019; 1.064 | 1.231; 6.246 |
| p | 0.128 | 0.020 | 0.997 | <0.001 | 0.014 |
| Mortality Late | ISS | Severe Head Injury | Severe Thorax Injury | Age | Complication |
|---|---|---|---|---|---|
| OR | 1.039 | 3.326 | 0.944 | 1.050 | 1.364 |
| 95%-CI | 0.995; 1.085 | 1.114; 9.929 | 0.369; 2.414 | 1.025; 1.075 | 0.592; 3.146 |
| p | 0.081 | 0.031 | 0.904 | <0.001 | 0.466 |
| Mortality Late | ISS | Severe head injury | Age | Organ failure | |
| OR | 1.039 | 3.485 | 1.050 | 2.419 | |
| 95%-CI | 0.997; 1.082 | 1.242; 9.780 | 1.025; 1.076 | 1.040; 5.630 | |
| p | 0.067 | 0.018 | <0.001 | 0.040 |
| Cluster of Complication | Complication | Quantity (% of All Patients; % of All Complications) |
|---|---|---|
| Infection | Pneumonia | 83 (21.7%; 23.6%) |
| Urinary tract infection | 19 (5%; 5.4%) | |
| Wound infection | 34 (8.9%; 9.7%) | |
| Sepsis [22] | 53 (13.8%; 15.1%) | |
| Thromboembolism | Myocardial infarction | 2 (0.5%; 0.6%) |
| Brain infarction | 8 (2.1%; 2.3%) | |
| Thrombosis | 7 (1.8%; 2%) | |
| Pulmonary embolism | 5 (1.3%; 1.4%) | |
| Surgical treatment associated (Surgery) | Compartment syndrome | 5 (1.3%; 1.4%) |
| Hematoma/Seroma | 8 (2.1%; 2.3%) | |
| Wound-healing disorders | 12 (3.1%; 3.4%) | |
| Nerve damage | 20 (5.2%; 5.7%) | |
| Implant-associated complications | 13 (3.4%; 3.7%) | |
| Organ failure | Acute respiratory distress syndrome (ARDS) [24] | 51 (13.3%; 14.5%) |
| Acute renal failure [25] | 32 (8.5%; 9.1%) |
| Complication | ISS | Age | Severe Thorax Injury | Severe Extremity Injury | Severe External Injury | Mechanical Ventilation Duration | ICU Stay | |
|---|---|---|---|---|---|---|---|---|
| Overall | OR | 0.987 | 1.008 | 1.754 | 3.359 | 4.028 | 1.002 | 1.061 |
| 95%-CI | 0.952; 1.023 | 0.993; 1.023 | 0.924; 3.331 | 1.555; 7.257 | 0.597; 27.156 | 1.000; 1.005 | 1.014; 1.111 | |
| p | 0.462 | 0.291 | 0.086 | 0.002 | 0.152 | 0.038 | 0.010 | |
| Cluster Infection | OR | 0.992 | 1.010 | 0.928 | 2.947 | 2.965 | 1.004 | 1.047 |
| 95%-CI | 0.956; 1.030 | 0.994; 1.025 | 0.531; 2.004 | 1.278; 6.303 | 0.447; 19.657 | 1.001; 1.006 | 1.003; 1.093 | |
| p | 0.677 | 0.220 | 0.928 | 0.005 | 0.260 | 0.002 | 0.037 | |
| Cluster Thrombo-embolism | OR | 0.998 | 0.996 | 1.223 | 0.918 | 0.000 | 1.003 | 0.976 |
| 95%-CI | 0.931; 1.069 | 0.970; 1.021 | 0.398; 3.756 | 0.257; 3.284 | 0.000 | 1.000; 1.006 | 0.925; 1.030 | |
| p | 0.950 | 0.732 | 0.725 | 0.895 | 0.999 | 0.054 | 0.375 | |
| Cluster Surgery | OR | 0.986 | 0.996 | 2.478 | 3.280 | 0.000 | 1.001 | 1.004 |
| 95%-CI | 0.942; 1.032 | 0.978; 1.015 | 1.136; 5.405 | 1.487; 7.234 | 0.000 | 0.999; 1.003 | 0.960; 1.051 | |
| p | 0.549 | 0.693 | 0.023 | 0.003 | 0.999 | 0.456 | 0.849 | |
| Cluster Organ failure | OR | 0.980 | 1.013 | 2.031 | 1.522 | 3.710 | 1.001 | 0.996 |
| 95%-CI | 0.946; 1.014 | 0.998; 1.028 | 1.085; 3.803 | 0.751; 3.087 | 0.875; 15.726 | 0.999; 1.003 | 0.961; 1.033 | |
| p | 0.246 | 0.095 | 0.027 | 0.244 | 0.075 | 0.178 | 0.835 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, N.; Hammen, A.; Bläsius, F.; Weber, C.D.; Hildebrand, F.; Horst, K. Effect of Injury Patterns on the Development of Complications and Trauma-Induced Mortality in Patients Suffering Multiple Trauma. J. Clin. Med. 2023, 12, 5111. https://doi.org/10.3390/jcm12155111
Becker N, Hammen A, Bläsius F, Weber CD, Hildebrand F, Horst K. Effect of Injury Patterns on the Development of Complications and Trauma-Induced Mortality in Patients Suffering Multiple Trauma. Journal of Clinical Medicine. 2023; 12(15):5111. https://doi.org/10.3390/jcm12155111
Chicago/Turabian StyleBecker, Nils, Antonia Hammen, Felix Bläsius, Christian David Weber, Frank Hildebrand, and Klemens Horst. 2023. "Effect of Injury Patterns on the Development of Complications and Trauma-Induced Mortality in Patients Suffering Multiple Trauma" Journal of Clinical Medicine 12, no. 15: 5111. https://doi.org/10.3390/jcm12155111
APA StyleBecker, N., Hammen, A., Bläsius, F., Weber, C. D., Hildebrand, F., & Horst, K. (2023). Effect of Injury Patterns on the Development of Complications and Trauma-Induced Mortality in Patients Suffering Multiple Trauma. Journal of Clinical Medicine, 12(15), 5111. https://doi.org/10.3390/jcm12155111







