Switching to Dupilumab from Other Biologics without a Treatment Interval in Patients with Severe Asthma: A Multi-Center Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Assessments
2.3. Statistical Analysis
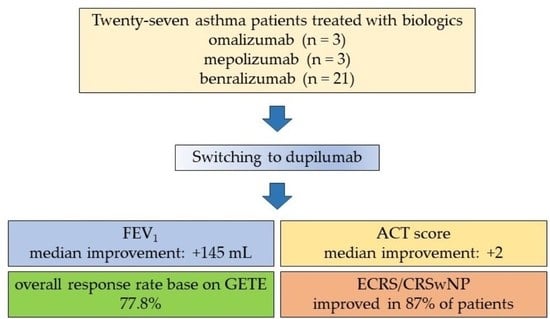
3. Results
3.1. Clinical Characteristics
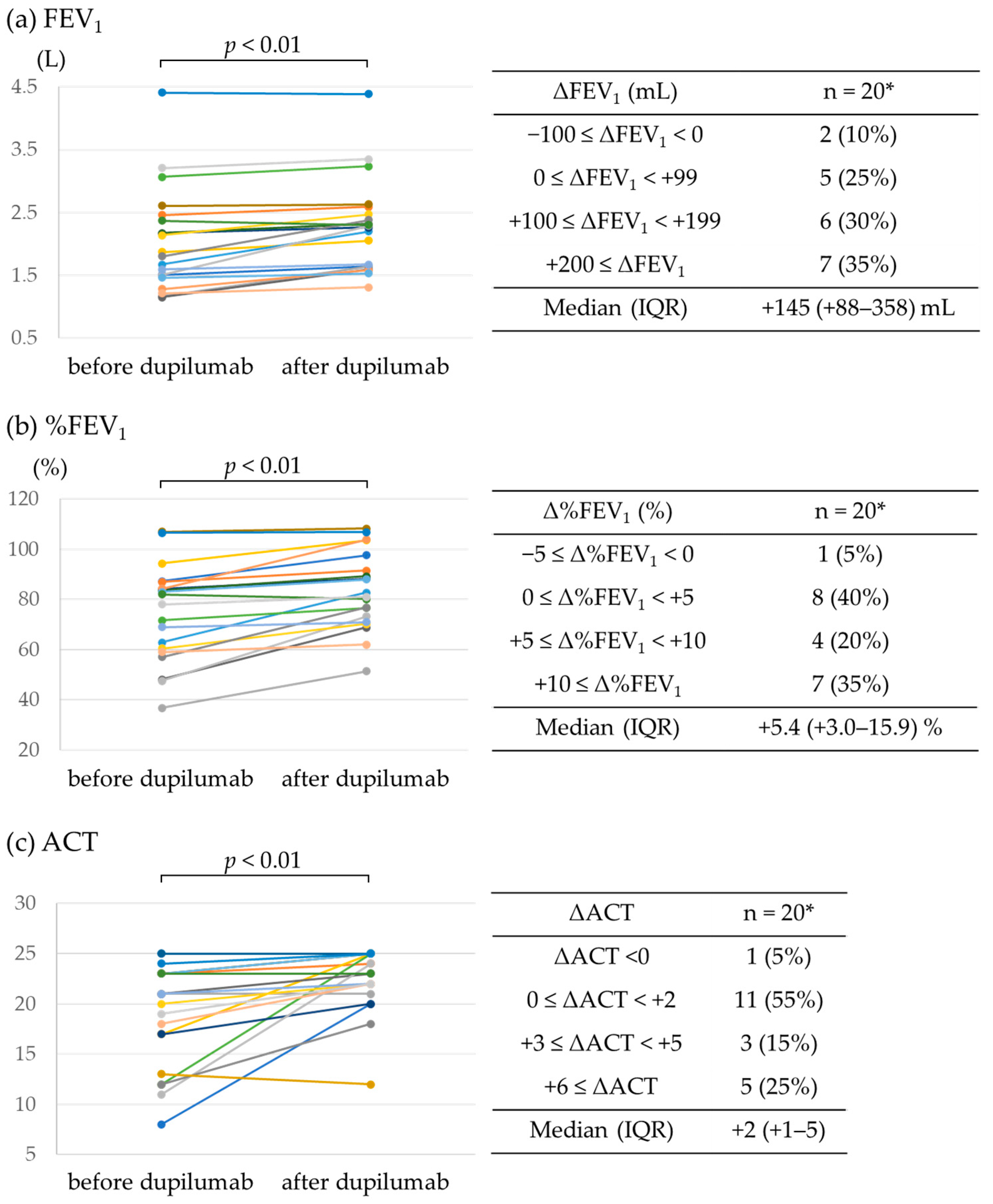
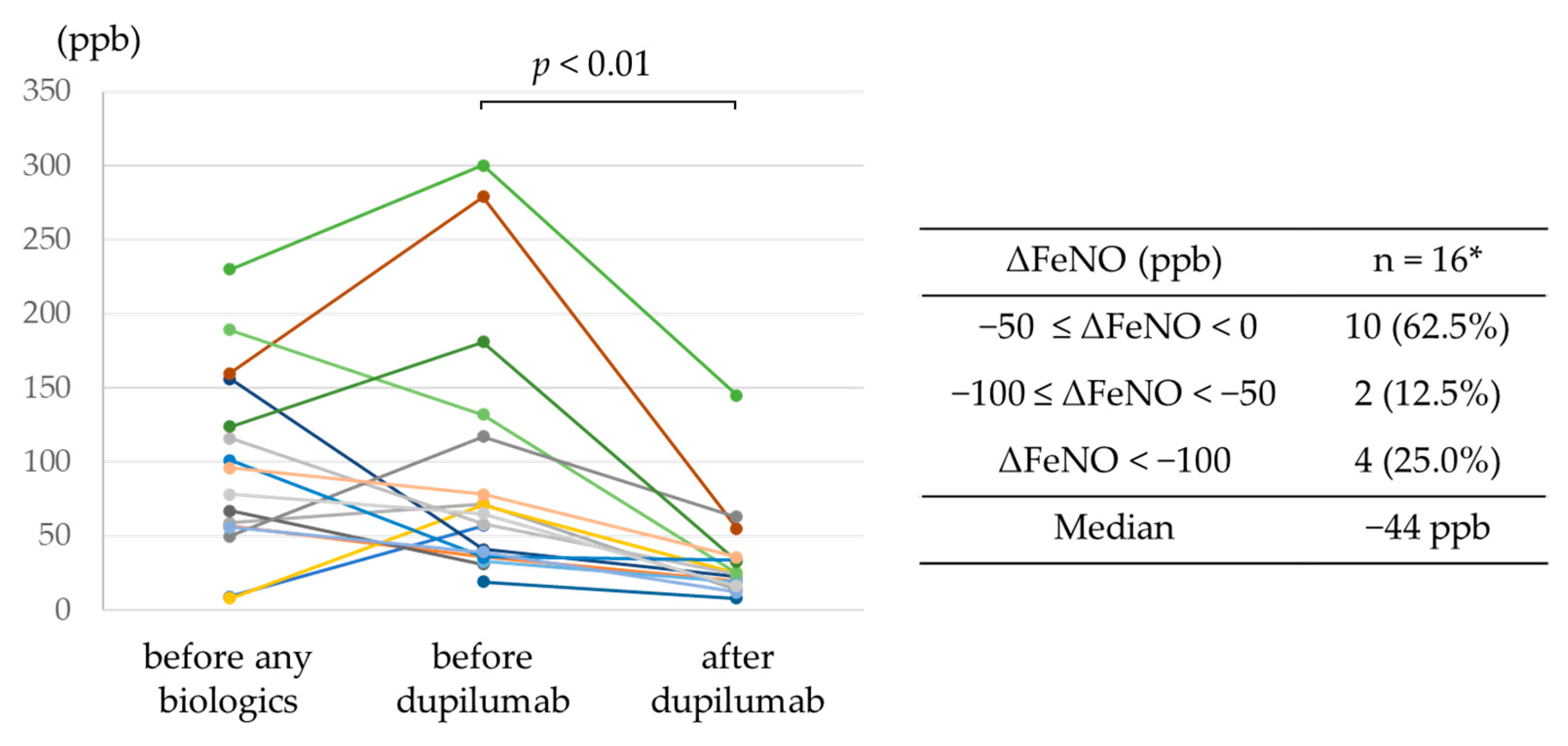
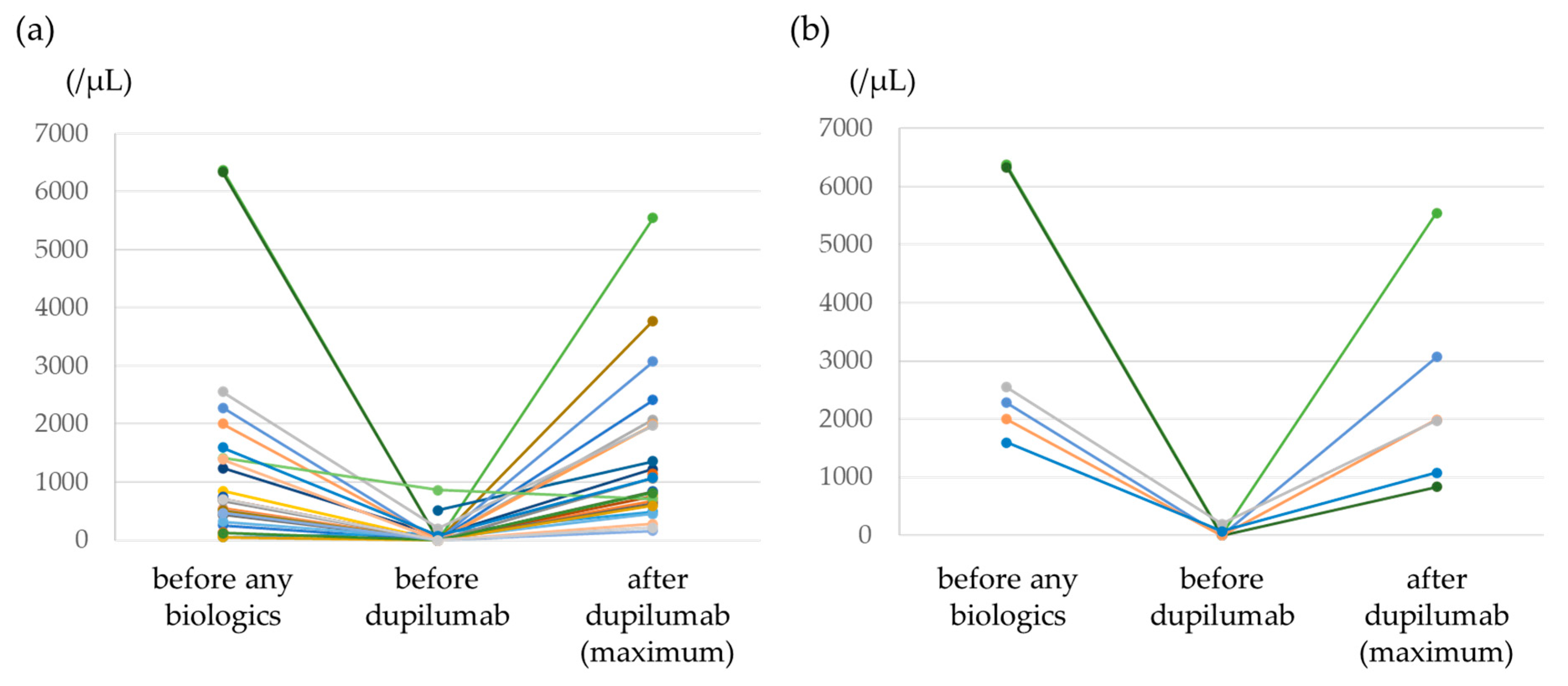
3.2. Efficacy of Dupilumab
3.3. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brusselle, G.G.; Koppelman, G.H. Biologic therapies for severe asthma. N. Engl. J. Med. 2022, 386, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Hekking, P.W.; Wener, R.R.; Amelink, M.; Zwinderman, A.H.; Bouvy, M.L.; Bel, E.H. The prevalence of severe refractory asthma. J. Allergy Clin. Immunol. 2015, 135, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, G.; Gallelli, L.; Renda, T.; Romeo, P.; Busceti, M.T.; Grembiale, R.D.; Maselli, R.; Marsico, S.A.; Vatrella, A. Update on optimal use of omalizumab in management of asthma. J. Asthma Allergy 2011, 4, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Humbert, M.; Beasley, R.; Ayres, J.; Slavin, R.; Hébert, J.; Bousquet, J.; Beeh, K.M.; Ramos, S.; Canonica, G.W.; Hedgecock, S.; et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005, 60, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Farne, H.A.; Wilson, A.; Powell, C.; Bax, L.; Milan, S.J. Anti-IL5 therapies for asthma. Cochrane Database Syst. Rev. 2017, 9, CD010834. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Pirozzi, G.; Graham, N.M.H. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert. Rev. Clin. Immunol. 2017, 13, 425–437. [Google Scholar] [CrossRef]
- Maspero, J.; Adir, Y.; Al-Ahmad, M.; Celis-Preciado, C.A.; Colodenco, F.D.; Giavina-Bianchi, P.; Lababidi, H.; Ledanois, O.; Mahoub, B.; Perng, D.-W.; et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022, 8, 00576–2021. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; Fitzgerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Dupin, C.; Belhadi, D.; Guilleminault, L.; Gamez, A.; Berger, P.; De Blay, F.; Bonniaud, P.; Leroyer, C.; Mahay, G.; Girodet, P.O.; et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort. Clin. Exp. Allergy 2020, 50, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Araya, J.; Miyagawa, H.; Okuda, K.; Takekoshi, D.; Hashimoto, M.; Minagawa, S.; Ishikawa, T.; Hara, H.; Kuwano, K. Real-World Effectiveness of Dupilumab for Patients with Severe Asthma: A Retrospective Study. J. Asthma Allergy 2022, 15, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Mümmler, C.; Munker, D.; Barnikel, M.; Veit, T.; Kayser, M.Z.; Welte, T.; Behr, J.; Kneidinger, N.; Suhling, H.; Milger, K. Dupilumab improves asthma control and lung function in patients with insufficient outcome during previous antibody therapy. J. Allergy Clin. Immunol. Pract. 2021, 9, 1177–1185.e4. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, M.; Sugiura, H.; Nagase, H.; Yamaguchi, M.; Inoue, H.; Sagara, H.; Tamaoki, J.; Tohda, Y.; Munakata, M.; Yamauchi, K.; et al. Japanese guidelines for adult asthma 2017. Allergol. Int. 2017, 66, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Asthma Management and Prevention; Global Initiative for Asthma: Fontana, WI, USA, 2019; Volume 49. [Google Scholar]
- Tokunaga, T.; Sakashita, M.; Haruna, T.; Asaka, D.; Takeno, S.; Ikeda, H.; Nakayama, T.; Seki, N.; Ito, S.; Murata, J.; et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: The JESREC Study. Allergy 2015, 70, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Humbert, M.; Taille, C.; Mala, L.; Le Gros, V.; Just, J.; Molimard, M. Investigators S: Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: The STELLAIR study. Eur. Respir. J. 2018, 51, 1702523. [Google Scholar] [CrossRef]
- Lloyd, A.; Turk, F.; Leighton, T.; Walter Canonica, G. Psychometric evaluation of Global Evaluation of Treatment Effectiveness: A tool to assess patients with moderate-to-severe allergic asthma. J. Med. Econ. 2007, 10, 285–296. [Google Scholar] [CrossRef]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- Schatz, M.; Kosinski, M.; Yarlas, A.S.; Hanlon, J.; Watson, M.E.; Jhingran, P. The minimally important difference of the Asthma Control Test. J. Allergy Clin. Immunol. 2009, 124, 719–723.e711. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Kayser, M.Z.; Drick, N.; Milger, K.; Fuge, J.; Kneidinger, N.; Korn, S.; Buhl, R.; Behr, J.; Welte, T.; Suhling, H. Real-world multicenter experience with mepolizumab and benralizumab in the treatment of uncontrolled severe eosinophilic asthma over 12 months. J. Asthma Allergy 2021, 14, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Crimi, C.; Benfante, A.; Caiaffa, M.F.; Calabrese, C.; Carpagnano, G.E.; Ciotta, D.; D’Amato, M.; Macchia, L.; Nolasco, S.; et al. Therapeutic effects of benralizumab assessed in patients with severe eosinophilic asthma: Real-life evaluation correlated with allergic and non-allergic phenotype expression. J. Asthma Allergy 2021, 14, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Pianigiani, T.; Alderighi, L.; Meocci, M.; Messina, M.; Perea, B.; Luzzi, S.; Bergantini, L.; D’Alessandro, M.; Refini, R.M.; Bargagli, E.; et al. Exploring the interaction between fractional exhaled nitric oxide and biologic treatment in severe asthma: A systematic review. Antioxidants 2023, 12, 400. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Menzies-Gow, A.; Zangrilli, J.G.; Hirsch, I.; Metcalfe, P.; Newbold, P.; Goldman, M. Predictors of enhanced response with benralizumab for patients with severe asthma: Pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir. Med. 2018, 6, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, J.E.; Hearn, A.P.; Dhariwal, J.; d’Ancona, G.; Douiri, A.; Roxas, C.; Fernandes, M.; Green, L.; Thomson, L.; Nanzer, A.M.; et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest 2020, 159, 496–506. [Google Scholar] [CrossRef]
- Sandhu, Y.; Harada, N.; Sasano, H.; Harada, S.; Ueda, S.; Takeshige, T.; Tanabe, Y.; Ishimori, A.; Matsuno, K.; Abe, S.; et al. Pretreatment frequency of circulating th17 cells and feno levels predicted the real-world response after 1 year of benralizumab treatment in patients with severe asthma. Biomolecules 2023, 13, 538. [Google Scholar] [CrossRef]
- Watanabe, H.; Shirai, T.; Hirai, K.; Akamatsu, T.; Nakayasu, H.; Tamura, K.; Masuda, T.; Takahashi, S.; Tanaka, Y.; Kishimoto, Y.; et al. Blood eosinophil count and FeNO to predict benralizumab effectiveness in real-life severe asthma patients. J. Asthma 2021, 59, 1796–1804. [Google Scholar] [CrossRef]
- Menigoz, C.; Dirou, S.; Chambellan, A.; Hassoun, D.; Moui, A.; Magnan, A.; Blanc, F.X. Use of FeNO to predict anti-IL-5 and IL-5R biologics efficacy in a real-world cohort of adults with severe eosinophilic asthma. J. Asthma 2023, 60, 1162–1170. [Google Scholar] [CrossRef]
- Hanania, N.A.; Wenzel, S.; Rosen, K.; Hsieh, H.J.; Mosesova, S.; Choy, D.F.; Lal, P.; Arron, J.R.; Harris, J.M.; Busse, W. Exploring the effects of omalizumab in allergic asthma: An analysis of biomarkers in the EXTRA study. Am. J. Respir. Crit. Care Med. 2013, 187, 804–811. [Google Scholar] [CrossRef]
- Takeno, S.; Taruya, T.; Ueda, T.; Noda, N.; Hirakawa, K. Increased exhaled nitric oxide and its oxidation metabolism in eosinophilic chronic rhinosinusitis. Auris Nasus Larynx 2013, 40, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Maspero, J.F.; Katelaris, C.H.; Busse, W.W.; Castro, M.; Corren, J.; Chipps, B.E.; Peters, A.T.; Pavord, I.D.; Ford, L.B.; Sher, L.; et al. Dupilumab efficacy in uncontrolled, moderate-to-severe asthma with self-reported chronic rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 527–539.e9. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Han, J.K.; Bachert, C.; Fokkens, W.; Desrosiers, M.; Wagenmann, M.; Lee, S.E.; Smith, S.G.; Martin, N.; Mayer, B.; Yancey, S.W.; et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 1141–1153. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.Y.; Gevaert, P.; Heffler, E.; Hopkins, C.; Tversky, J.R.; Barker, P.; Cohen, D.; Emson, C.; et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2022, 149, 1309–1317.e1312. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef] [Green Version]
- Wechsler, M.E.; Klion, A.D.; Paggiaro, P.; Nair, P.; Staumont-Salle, D.; Radwan, A.; Johnson, R.R.; Kapoor, U.; Khokhar, F.A.; Daizadeh, N.; et al. Effect of dupilumab on blood eosinophil counts in patients with asthma, chronic rhinosinusitis with nasal polyps, atopic dermatitis, or eosinophilic esophagitis. J. Allergy Clin. Immunol. Pract. 2022, 10, 2695–2709. [Google Scholar] [CrossRef]
| Characteristics | n = 27 |
|---|---|
| Age, years—median (IQR) | 57 (45–68) |
| Male sex—n (%) | 15 (55.6) |
| Disease duration, years—median (IQR) | 12 (8–26) |
| Body mass index—median (IQR) | 24.3 (20.0–27.4) |
| Smoking history | |
| Never—n (%) | 16 (59.3) |
| Former or current—n (%) | 11 (40.7) |
| ACT—median (IQR) | 20.5 (16–23) * |
| Allergies—n (%) | 16 (59.3) |
| Comorbidity | |
| ABPA—n (%) | 3 (11.1) |
| ECRS—n (%) | 23 (85.2) |
| EOM—n (%) | 11 (40.7) |
| AD—n (%) | 2 (7.4) |
| AR—n (%) | 10 (37.0) |
| Treatment | |
| High dose ICS—n (%) | 18 (66.7) |
| Medium dose ICS—n (%) | 9 (33.3) |
| LABA—n (%) | 26 (96.3) |
| LAMA—n (%) | 14 (51.9) |
| LTRA—n (%) | 18 (66.7) |
| Xanthine—n (%) | 8 (29.6) |
| Maintenance OCS—n (%) | 9 (33.3) |
| Previous biologics | |
| Omalizumab—n (%) | 3 (11.1) |
| Mepolizumab—n (%) | 3 (11.1) |
| Benralizumab—n (%) | 21 (77.8) |
| Treatment period—median (IQR) | 421 (301–673) |
| Biomarkers (before the use of any biologics) | |
| Blood eosinophil count (/μL)—median (IQR) | 690 (352–1407) * |
| Serum IgE (IU/mL)—median (IQR) | 436 (188–881) † |
| FeNO (ppb)—median (IQR) | 82 (56–116) ‡ |
| FeNO before dupilumab use (ppb)—median (IQR) | 60 (37–77) ‡ |
| Pulmonary function | |
| FEV1 (L)—median (IQR) | 1.84 (1.50–2.39) ‡ |
| %FEV1 (%)—median (IQR) | 80.0% (60.1–84.9) ‡ |
| FEV1/FVC (%)—median (IQR) | 63.5 (54.0–75.3) ‡ |
| Reasons (n = 27) | n (%) |
|---|---|
| Asthmatic symptoms | 5 (18.5) |
| Asthmatic and ECRS symptoms | 7 (25.9) |
| ECRS symptoms | 10 (37.0) |
| EOM symptoms | 3 (11.1) |
| ECRS and EOM symptoms | 1 (3.7) |
| Self-administration | 1 (3.7) |
| GETE Score | Previous Biologics: n (%) | Dupilumab: n (%) |
|---|---|---|
| Excellent | 3 (11.1) | 6 (22.2) |
| Good | 8 (29.6) | 15 (55.6) |
| Moderate | 12 (44.4) | 4 (14.8) |
| Poor | 4 (14.8) | 1 (3.7) |
| Worsening | 0 (0) | 1 (3.7) |
| Excellent/Good | 11 (40.7) | 21 (77.8) |
| GETE-Improved (n = 14) | Non-GETE-Improved (n = 13) | p-Value | |
|---|---|---|---|
| Age, years—median (IQR) | 62 (52–72) | 55 (41–60) | 0.14 |
| Male sex—n (%) | 10 (71.4) | 5 (38.5) | 0.13 |
| Body mass index—median (IQR) | 24.6 (22.8–27.8) | 20.8 (18.5–26.4) | 0.26 |
| Never smoker—n (%) | 6 (42.9) | 10 (76.9) | 0.12 |
| ABPA—n (%) | 3 (21.4) | 0 (0) | 0.22 |
| ECRS—n (%) | 11 (78.6) | 12 (92.3) | 0.60 |
| EOM—n (%) | 7 (50.0) | 4 (30.8) | 0.44 |
| Maintenance OCS—n (%) | 4 (28.6) | 5 (38.5) | 0.70 |
| Biomarkers (before the use of any biologics) | |||
| Blood eosinophil count (/μL)—median (IQR) | 601 (270–1144) | 703 (498–1695) | 0.53 |
| Serum IgE (IU/mL)—median (IQR) | 395 (296–710) | 272 (135–584) | 0.43 |
| FeNO (ppb)—median (IQR) | 82 (50–124) | 87 (57–103) | 0.97 |
| FeNO before dupilumab use (ppb)—median (IQR) | 71 (49–125) | 51 (36–68) | 0.39 |
| ACT-Improved (n = 8) | Non-ACT-Improved (n = 12) | p-Value | |
|---|---|---|---|
| Age, years—median (IQR) | 56 (43–64) | 60 (46–73) | 0.33 |
| Male sex—n (%) | 4 (50.0) | 8 (66.7) | 0.65 |
| Body mass index—median (IQR) | 25.5 (23.0–27.7) | 21.2 (19.4–25.0) | 0.22 |
| Never smoker—n (%) | 5 (62.5) | 7 (58.3) | 1.00 |
| ABPA—n (%) | 2 (25.0) | 0 (0) | 0.15 |
| ECRS—n (%) | 7 (87.5) | 9 (75.0) | 0.62 |
| EOM—n (%) | 3 (37.5) | 5 (41.7) | 1.00 |
| Maintenance OCS—n (%) | 3 (37.5) | 2 (16.7) | 0.35 |
| Biomarkers (before the use of any biologics) | |||
| Blood eosinophil count (/μL)—median (IQR) | 1045 (699–1682) | 439 (95–516) * | <0.01 |
| Serum IgE (IU/mL)—median (IQR) | 388 (183–710) † | 272 (150–433) ‡ | 0.54 |
| FeNO (ppb)—median (IQR) | 87 (40–126) | 67 (57–101) § | 0.89 |
| FeNO before dupilumab use (ppb)—median (IQR) | 68 (58–88) | 36 (33–47) ‡ | 0.04 |
| FEV1-Improved (n = 14) | Non-FEV1-Improved (n = 6) | p-Value | |
|---|---|---|---|
| Age, years—median (IQR) | 62 (57–69) | 46 (44–67) | 0.54 |
| Male sex—n (%) | 10 (71.4) | 3 (50.0) | 0.61 |
| Body mass index—median (IQR) | 23.2 (20.5–25.8) | 27.2 (22.6–32.0) | 0.19 |
| Never smoker—n (%) | 6 (42.9) | 5 (83.3) | 0.16 |
| ABPA—n (%) | 1 (7.1) | 1 (16.7) | 0.52 |
| ECRS—n (%) | 12 (85.7) | 5 (83.3) | 1.00 |
| EOM—n (%) | 6 (42.9) | 2 (33.3) | 1.00 |
| Maintenance OCS—n (%) | 4 (28.6) | 1 (16.7) | 1.00 |
| Biomarkers (before the use of any biologics) | |||
| Blood eosinophil count (/μL)—median (IQR) | 692 (460–1848) | 488 (359–1059) | 0.44 |
| Serum IgE (IU/mL)—median (IQR) | 395 (157–710) * | 188 (150–206) † | 0.28 |
| FeNO (ppb)—median (IQR) | 73 (55–100) ‡ | 101 (56–124) § | 0.57 |
| FeNO before dupilumab use (ppb)—median (IQR) | 68 (57–77) ¶ | 39 (36–41) § | 0.27 |
| ECRS Symptoms (n = 23) | no. (%) |
|---|---|
| Improved | 20 (87.0) |
| No change | 3 (13.0) |
| Worsening | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higo, H.; Ichikawa, H.; Arakawa, Y.; Mori, Y.; Itano, J.; Taniguchi, A.; Senoo, S.; Kimura, G.; Tanimoto, Y.; Miyake, K.; et al. Switching to Dupilumab from Other Biologics without a Treatment Interval in Patients with Severe Asthma: A Multi-Center Retrospective Study. J. Clin. Med. 2023, 12, 5174. https://doi.org/10.3390/jcm12165174
Higo H, Ichikawa H, Arakawa Y, Mori Y, Itano J, Taniguchi A, Senoo S, Kimura G, Tanimoto Y, Miyake K, et al. Switching to Dupilumab from Other Biologics without a Treatment Interval in Patients with Severe Asthma: A Multi-Center Retrospective Study. Journal of Clinical Medicine. 2023; 12(16):5174. https://doi.org/10.3390/jcm12165174
Chicago/Turabian StyleHigo, Hisao, Hirohisa Ichikawa, Yukako Arakawa, Yoshihiro Mori, Junko Itano, Akihiko Taniguchi, Satoru Senoo, Goro Kimura, Yasushi Tanimoto, Kohei Miyake, and et al. 2023. "Switching to Dupilumab from Other Biologics without a Treatment Interval in Patients with Severe Asthma: A Multi-Center Retrospective Study" Journal of Clinical Medicine 12, no. 16: 5174. https://doi.org/10.3390/jcm12165174
APA StyleHigo, H., Ichikawa, H., Arakawa, Y., Mori, Y., Itano, J., Taniguchi, A., Senoo, S., Kimura, G., Tanimoto, Y., Miyake, K., Katsuta, T., Kataoka, M., Maeda, Y., Kiura, K., Miyahara, N., & Okayama Respiratory Disease Study Group (ORDSG). (2023). Switching to Dupilumab from Other Biologics without a Treatment Interval in Patients with Severe Asthma: A Multi-Center Retrospective Study. Journal of Clinical Medicine, 12(16), 5174. https://doi.org/10.3390/jcm12165174