Use of the Free Endometriosis Risk Advisor App as a Non-Invasive Screening Test for Endometriosis in Patients with Chronic Pelvic Pain and/or Unexplained Infertility
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Surgical Evaluation
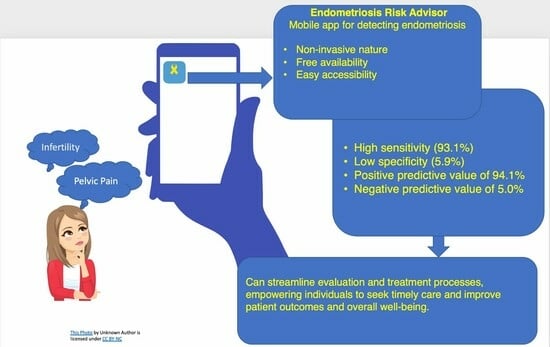
2.3. The Endometriosis Risk Advisor
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nezhat, C.; Nezhat, F.; Nezhat, C. Endometriosis: Ancient Disease, Ancient Treatments. Fertil. Steril. 2012, 98, S1–S62. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.R.; Nezhat, F.; Nezhat, C. Nezhat’s Operative Gynecologic Laparoscopy and Hysteroscopy, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Nisolle, M.; Donnez, J. Peritoneal Endometriosis, Ovarian Endometriosis, and Adenomyotic Nodules of the Rectovaginal Septum Are Three Different Entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Alimi, Y.; Iwanaga, J.; Loukas, M.; Tubbs, R.S. The Clinical Anatomy of Endometriosis: A Review. Cureus 2018, 10, e3361. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Farina, R.; Palmucci, S.; Vizzini, I.A.A.; Libertini, N.; Coronella, M.; Spadola, S.; Caltabiano, R.; Iraci, M.; Basile, A.; et al. Endometriosis: Clinical Features, MR Imaging Findings and Pathologic Correlation. Insights Imaging 2018, 9, 149–172. [Google Scholar] [CrossRef]
- Leonardi, M.; Espada, M.; Kho, R.M.; Magrina, J.F.; Millischer, A.-E.; Savelli, L.; Condous, G. Endometriosis and the Urinary Tract: From Diagnosis to Surgical Treatment. Diagnostics 2020, 10, 771. [Google Scholar] [CrossRef]
- Rousset, P.; Rousset-Jablonski, C.; Alifano, M.; Mansuet-Lupo, A.; Buy, J.-N.; Revel, M.-P. Thoracic Endometriosis Syndrome: CT and MRI Features. Clin. Radiol. 2014, 69, 323–330. [Google Scholar] [CrossRef]
- Ellis, K.; Munro, D.; Clarke, J. Endometriosis Is Undervalued: A Call to Action. Front. Glob. Womens Health 2022, 3. [Google Scholar] [CrossRef]
- Meuleman, C.; Vandenabeele, B.; Fieuws, S.; Spiessens, C.; Timmerman, D.; D’Hooghe, T. High Prevalence of Endometriosis in Infertile Women with Normal Ovulation and Normospermic Partners. Fertil. Steril. 2009, 92, 68–74. [Google Scholar] [CrossRef]
- Carter, J.E. Combined Hysteroscopic and Laparoscopic Findings in Patients with Chronic Pelvic Pain. J. Am. Assoc. Gynecol. Laparosc. 1994, 2, 43–47. [Google Scholar] [CrossRef]
- Nezhat, C.; Li, A.; Abed, S.; Balassiano, E.; Soliemannjad, R.; Nezhat, A.; Nezhat, C.H.; Nezhat, F. Strong Association between Endometriosis and Symptomatic Leiomyomas. JSLS J. Soc. Laparoendosc. Surg. 2016, 20, e2016.00053. [Google Scholar] [CrossRef]
- Lin, K.Y.-H.; Yang, C.-Y.; Lam, A.; Chang, C.Y.-Y.; Lin, W.-C. Uterine Leiomyoma Is Associated with the Risk of Developing Endometriosis: A Nationwide Cohort Study Involving 156,195 Women. PLoS ONE 2021, 16, e0256772. [Google Scholar] [CrossRef]
- Nicolaus, K.; Bräuer, D.; Sczesny, R.; Lehmann, T.; Diebolder, H.; Runnebaum, I.B. Unexpected Coexistent Endometriosis in Women with Symptomatic Uterine Leiomyomas Is Independently Associated with Infertility, Nulliparity and Minor Myoma Size. Arch. Gynecol. Obstet. 2019, 300, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Culley, L.; Law, C.; Hudson, N.; Denny, E.; Mitchell, H.; Baumgarten, M.; Raine-Fenning, N. The Social and Psychological Impact of Endometriosis on Women’s Lives: A Critical Narrative Review. Hum. Reprod. Update 2013, 19, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Parker, M.; Sneddon, A.; Lopez, V.; Ellwood, D. Impact of Endometriosis on Women’s Lives: A Qualitative Study. BMC Womens Health 2014, 14, 123. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Mu, F.; Terry, K.L.; Harris, H.R.; Poole, E.M.; Farland, L.; Missmer, S.A. Endometriosis: A High-Risk Population for Major Chronic Diseases? Hum. Reprod. Update 2015, 21, 500–516. [Google Scholar] [CrossRef]
- Soliman, A.M.; Coyne, K.S.; Gries, K.S.; Castelli-Haley, J.; Snabes, M.C.; Surrey, E.S. The Effect of Endometriosis Symptoms on Absenteeism and Presenteeism in the Workplace and at Home. J. Manag. Care Spec. Pharm. 2017, 23, 745–754. [Google Scholar] [CrossRef]
- Soliman, A.M.; Surrey, E.; Bonafede, M.; Nelson, J.K.; Castelli-Haley, J. Real-World Evaluation of Direct and Indirect Economic Burden among Endometriosis Patients in the United States. Adv. Ther. 2018, 35, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, V.H.; Decter, D.H.; Chodick, G.; Shalev, V.; Weil, C. Burden of Endometriosis: Infertility, Comorbidities, and Healthcare Resource Utilization. J. Clin. Med. 2022, 11, 1133. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Chapron, C.; Giudice, L.C.; Laufer, M.R.; Leyland, N.; Missmer, S.A.; Singh, S.S.; Taylor, H.S. Clinical Diagnosis of Endometriosis: A Call to Action. Am. J. Obstet. Gynecol. 2019, 220, 354.e1–354.e12. [Google Scholar] [CrossRef]
- Ghai, V.; Jan, H.; Shakir, F.; Haines, P.; Kent, A. Diagnostic Delay for Superficial and Deep Endometriosis in the United Kingdom. J. Obstet. Gynaecol. 2020, 40, 83–89. [Google Scholar] [CrossRef]
- Husby, G.K.; Haugen, R.S.; Moen, M.H. Diagnostic Delay in Women with Pain and Endometriosis: Diagnostic Delay of Endometriosis. Acta Obstet. Gynecol. Scand. 2003, 82, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Prentice, L.; Bossuyt, P.M.M.; Farquhar, C.; Hull, M.L.; Johnson, N. Combination of the Non-Invasive Tests for the Diagnosis of Endometriosis. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T. Clinical Aspects of Adolescent Endometriosis. Endocrines 2021, 2, 301–310. [Google Scholar] [CrossRef]
- Surrey, E.; Soliman, A.M.; Trenz, H.; Blauer-Peterson, C.; Sluis, A. Impact of Endometriosis Diagnostic Delays on Healthcare Resource Utilization and Costs. Adv. Ther. 2020, 37, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Fuldeore, M.; Snabes, M.C. Factors Associated with Time to Endometriosis Diagnosis in the United States. J. Womens. Health 2017, 26, 788–797. [Google Scholar] [CrossRef]
- Ballweg, M.L. Impact of Endometriosis on Women’s Health: Comparative Historical Data Show That the Earlier the Onset, the More Severe the Disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 201–218. [Google Scholar] [CrossRef]
- de Ziegler, D.; Toner, J.P. Fertility Workups: The Times They Are a-Changin’. Fertil. Steril. 2022, 118, 5–7. [Google Scholar] [CrossRef]
- Pirtea, P.; Vulliemoz, N.; de Ziegler, D.; Ayoubi, J.M. Infertility Workup: Identifying Endometriosis. Fertil. Steril. 2022, 118, 29–33. [Google Scholar] [CrossRef]
- Chapron, C.; Lafay-Pillet, M.-C.; Santulli, P.; Bourdon, M.; Maignien, C.; Gaudet-Chardonnet, A.; Maitrot-Mantelet, L.; Borghese, B.; Marcellin, L. A New Validated Screening Method for Endometriosis Diagnosis Based on Patient Questionnaires. EClinicalMedicine 2022, 44, 101263. [Google Scholar] [CrossRef]
- Anastasiu, C.V.; Moga, M.A.; Elena Neculau, A.; Bălan, A.; Scârneciu, I.; Dragomir, R.M.; Dull, A.-M.; Chicea, L.-M. Biomarkers for the Noninvasive Diagnosis of Endometriosis: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 1750. [Google Scholar] [CrossRef]
- Kimber-Trojnar, Ż.; Pilszyk, A.; Niebrzydowska, M.; Pilszyk, Z.; Ruszała, M.; Leszczyńska-Gorzelak, B. The Potential of Non-Invasive Biomarkers for Early Diagnosis of Asymptomatic Patients with Endometriosis. J. Clin. Med. 2021, 10, 2762. [Google Scholar] [CrossRef] [PubMed]
- Bendifallah, S.; Puchar, A.; Suisse, S.; Delbos, L.; Poilblanc, M.; Descamps, P.; Golfier, F.; Touboul, C.; Dabi, Y.; Daraï, E. Machine Learning Algorithms as New Screening Approach for Patients with Endometriosis. Sci. Rep. 2022, 12, 639. [Google Scholar] [CrossRef]
- Evans-Hoeker, E.; Lessey, B.A.; Jeong, J.W.; Savaris, R.F.; Palomino, W.A.; Yuan, L.; Schammel, D.P.; Young, S.L. Endometrial BCL6 Overexpression in Eutopic Endometrium of Women with Endometriosis. Reprod. Sci. 2016, 23, 1234–1241. [Google Scholar] [CrossRef]
- Somigliana, E.; Vercellini, P.; Vigano’, P.; Benaglia, L.; Crosignani, P.G.; Fedele, L. Non-Invasive Diagnosis of Endometriosis: The Goal or Own Goal? Hum. Reprod. 2010, 25, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, L.; Nisolle, M.; Noël, J.-C.; Fastrez, M. Three Types of Endometriosis: Pathogenesis, Diagnosis and Treatment. State of the Art. J. Clin. Med. 2023, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Endomarchnews.org. Available online: https://www.endomarchnews.org/about-us-i (accessed on 30 June 2023).
- Jackman, J.M.; Vaid, A.; Agarwal, S.; Nezhat, A.; Nezhat, C. Can We Diagnosis Endometriosis with a Phone App? Nezhat Endometriosis Advisor Mobile Application as a Predictor for Endometriosis in Patients Experiencing Pelvic Pain, Infertility or Unexplained Infertility. Fertil. Steril. 2020, 114, e539. [Google Scholar] [CrossRef]
- Sadeghi, M.R. Unexplained Infertility, the Controversial Matter in Management of Infertile Couples. J. Reprod. Infertil. 2015, 16, 1–2. [Google Scholar]
- Infertility. Cdc.gov. Available online: https://www.cdc.gov/reproductivehealth/infertility/index.htm (accessed on 30 June 2023).
- Lee, S.-Y.; Koo, Y.-J.; Lee, D.-H. Classification of Endometriosis. Yeungnam Univ. J. Med. 2021, 38, 10–18. [Google Scholar] [CrossRef]
- Brilhante, A.V.M.; Augusto, K.L.; Portela, M.C.; Sucupira, L.C.G.; Oliveira, L.A.F.; Pouchaim, A.J.M.V.; Nóbrega, L.R.M.; de Magalhães, T.F.; Sobreira, L.R.P. Endometriosis and Ovarian Cancer: An Integrative Review (Endometriosis and Ovarian Cancer). Asian Pac. J. Cancer Prev. 2017, 18, 11–16. [Google Scholar] [CrossRef]
- Yu, X.; Cai, H.; Guan, J.; Zheng, X.; Han, H. Laparoscopic Surgery: Any Role in Patients with Unexplained Infertility and Failed in Vitro Fertilization Cycles? Medicine 2019, 98, e14957. [Google Scholar] [CrossRef]

| Variable | Number (%)/Mean ± SD |
|---|---|
| Age (year) | 35.79 ± 0.4 |
| BMI (kg/cm2) | 24.16 ± 0.3 |
| Nulligravida | 169 (59.1) |
| Smoking (current or past) | 18 (6.2) |
| Family History of Endometriosis | 41 (14.0) |
| Pelvic Pain | 233 (81.5) |
| Unexplained Infertility | 212 (75) |
| Pelvic Pain and Unexplained Infertility | 148 (50.5) |
| Endometriosis Stage (rASRM) | |
| I | 35 (12.7) |
| II | 95 (34.4) |
| III | 46 (16.7) |
| IV | 100 (36.2) |
| Total | Infertility Group | Pain Group | Infertility and Pain Group | Stage I/II | Stage III/IV | |
|---|---|---|---|---|---|---|
| Sensitivity (%) | 93.1 | 94.0 | 93.5 | 95.1 | 88.3 | 96.8 |
| Specificity (%) | 5.9 | 9.1 | N/A | N/A | N/A | 100.0 |
| PPV (%) | 94.1 | 95.0 | 94.5 | 95.8 | 86.9 | 100.0 |
| NPV (%) | 5.0 | 7.7 | N/A | N/A | N/A | 16.7 |
| PLR | 0.98 | 1.03 | 0.93 | 0.95 | 0.88 | N/A |
| NLR | 1.17 | 0.65 | N/A | N/A | N/A | 0.03 |
| DOR | 0.84 | 1.57 | N/A | N/A | N/A | N/A |
| Accuracy % | 88.1 | 89.6 | 88.8 | 91.2 | 77.9 | 89.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nezhat, C.; Armani, E.; Chen, H.-C.C.; Najmi, Z.; Lindheim, S.R.; Nezhat, C. Use of the Free Endometriosis Risk Advisor App as a Non-Invasive Screening Test for Endometriosis in Patients with Chronic Pelvic Pain and/or Unexplained Infertility. J. Clin. Med. 2023, 12, 5234. https://doi.org/10.3390/jcm12165234
Nezhat C, Armani E, Chen H-CC, Najmi Z, Lindheim SR, Nezhat C. Use of the Free Endometriosis Risk Advisor App as a Non-Invasive Screening Test for Endometriosis in Patients with Chronic Pelvic Pain and/or Unexplained Infertility. Journal of Clinical Medicine. 2023; 12(16):5234. https://doi.org/10.3390/jcm12165234
Chicago/Turabian StyleNezhat, Camran, Ellie Armani, Hsuan-Chih Carolina Chen, Zahra Najmi, Steven R. Lindheim, and Ceana Nezhat. 2023. "Use of the Free Endometriosis Risk Advisor App as a Non-Invasive Screening Test for Endometriosis in Patients with Chronic Pelvic Pain and/or Unexplained Infertility" Journal of Clinical Medicine 12, no. 16: 5234. https://doi.org/10.3390/jcm12165234
APA StyleNezhat, C., Armani, E., Chen, H.-C. C., Najmi, Z., Lindheim, S. R., & Nezhat, C. (2023). Use of the Free Endometriosis Risk Advisor App as a Non-Invasive Screening Test for Endometriosis in Patients with Chronic Pelvic Pain and/or Unexplained Infertility. Journal of Clinical Medicine, 12(16), 5234. https://doi.org/10.3390/jcm12165234