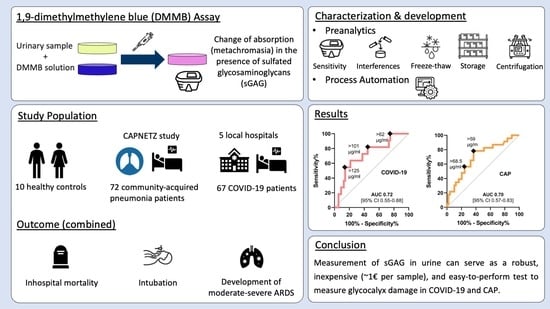
Analysis of Urinary Glycosaminoglycans to Predict Outcome in COVID-19 and Community-Acquired Pneumonia—A Proof-of-Concept Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. COVID-19 Cohort and Healthy Controls
2.1.2. Community-Acquired Pneumonia (CAP) Cohort
2.2. Reagents and Consumables
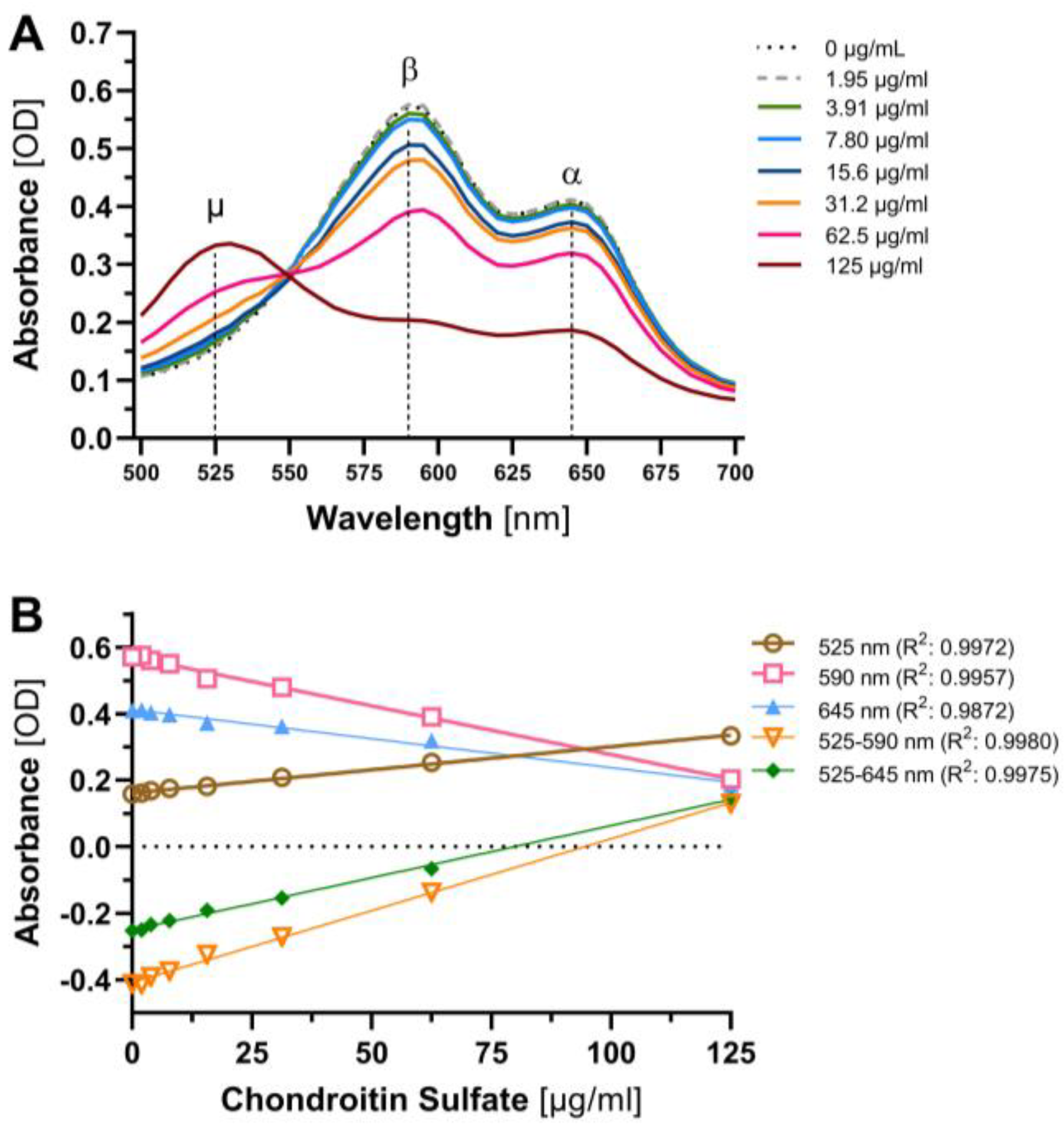
2.3. Evaluation and Further Development of DMMB Assay
- Analytical assay sensitivity, hereafter assay sensitivity, defined as the slope of the calibration curve.
- Limit of Blank (LoB) is the highest apparent analyte concentration expected to be found when replicates of a blank sample containing no analyte are tested. LoB = meanblank + 1.645 × (SDblank)
- Limit of Detection (LoD) is defined as the lowest analyte concentration that can be reliably distinguished from the LoB and at which detection is feasible. The LoD is determined by using both the measured LoB and test replicates of a sample known to contain a low concentration of analyte. LoD = LoB + 1.645 × (SD low-concentration sample)
- Intra-assay precision, defined as the within-run precision of the assay and assessed as the coefficient of variation of 8 parallel measurements of four samples (SD × 100/mean) and inter-assay precision.
- Inter-assay precision, assessed by measurement of four samples in different runs by different operators.
- Stability of the urine samples was assessed by evaluating the effect of storage, exposure to light as well as freeze–thaw cycles and centrifugation.
2.4. Statistical Analysis
3. Results
3.1. Sensitivity, Precision and Freeze–Thaw Studies
3.2. Interference, Specificity and Centrifugation Studies
3.3. Baseline Characteristics
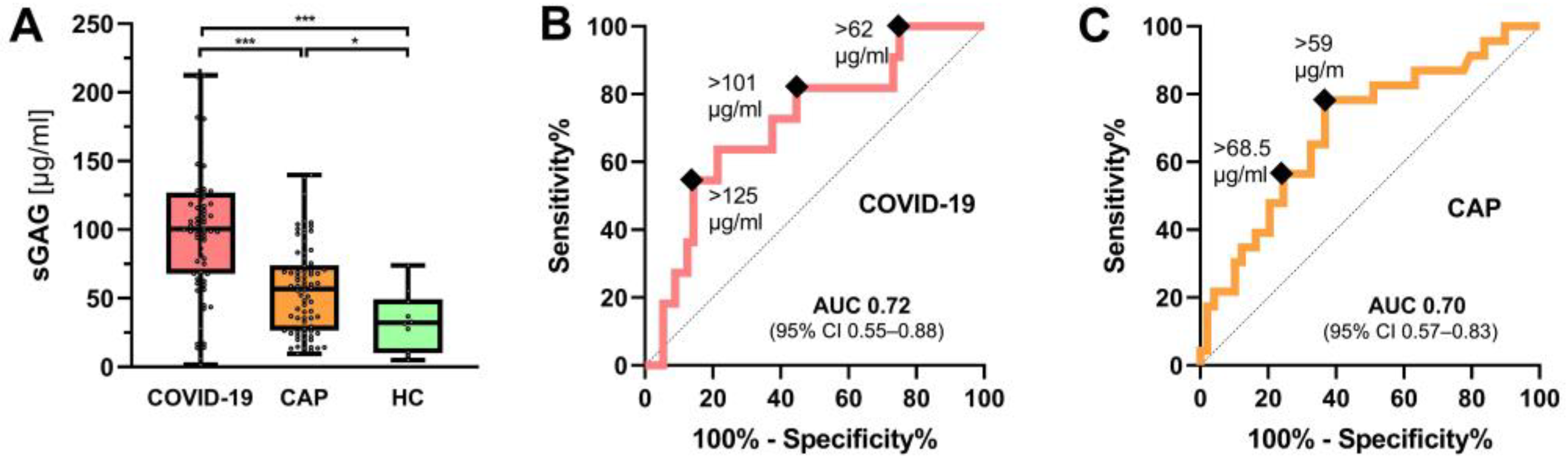
3.4. Urinary sGAG Concentrations in COVID-19 and CAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smadja, D.M.; Mentzer, S.J.; Fontenay, M.; Laffan, M.A.; Ackermann, M.; Helms, J.; Jonigk, D.; Chocron, R.; Pier, G.B.; Gendron, N.; et al. COVID-19 is a systemic vascular hemopathy: Insight for mechanistic and clinical aspects. Angiogenesis 2021, 24, 755–788. [Google Scholar] [CrossRef]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care 2020, 24, 353. [Google Scholar] [CrossRef]
- Rovas, A.; Seidel, L.M.; Vink, H.; Pohlkotter, T.; Pavenstadt, H.; Ertmer, C.; Hessler, M.; Kumpers, P. Association of sublingual MICROCIRCULATION parameters and endothelial glycocalyx dimensions in resuscitated sepsis. Crit. Care 2019, 23, 260. [Google Scholar] [CrossRef] [Green Version]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef] [Green Version]
- Rovas, A.; Osiaevi, I.; Buscher, K.; Sackarnd, J.; Tepasse, P.R.; Fobker, M.; Kuhn, J.; Braune, S.; Gobel, U.; Tholking, G.; et al. Microvascular dysfunction in COVID-19: The MYSTIC study. Angiogenesis 2021, 24, 145–157. [Google Scholar] [CrossRef]
- Kubaski, F.; Osago, H.; Mason, R.W.; Yamaguchi, S.; Kobayashi, H.; Tsuchiya, M.; Orii, T.; Tomatsu, S. Glycosaminoglycans detection methods: Applications of mass spectrometry. Mol. Genet. Metab. 2017, 120, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, E.P.; Overdier, K.H.; Sun, X.; Lin, L.; Liu, X.; Yang, Y.; Ammons, L.A.; Hiller, T.D.; Suflita, M.A.; Yu, Y.; et al. Urinary Glycosaminoglycans Predict Outcomes in Septic Shock and Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2016, 194, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Yeo, T.W.; Bush, P.A.; Chen, Y.; Young, S.P.; Zhang, H.; Millington, D.S.; Granger, D.L.; Mwaikambo, E.D.; Anstey, N.M.; Weinberg, J.B. Glycocalyx breakdown is increased in African children with cerebral and uncomplicated falciparum malaria. FASEB J. 2019, 33, 14185–14193. [Google Scholar] [CrossRef] [Green Version]
- Yeo, T.W.; Weinberg, J.B.; Lampah, D.A.; Kenangalem, E.; Bush, P.; Chen, Y.; Price, R.N.; Young, S.; Zhang, H.Y.; Millington, D.; et al. Glycocalyx Breakdown Is Associated With Severe Disease and Fatal Outcome in Plasmodium falciparum Malaria. Clin. Infect. Dis. 2019, 69, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Welte, T.; Suttorp, N.; Marre, R. CAPNETZ-community-acquired pneumonia competence network. Infection 2004, 32, 234–238. [Google Scholar] [CrossRef]
- Sun, X.; Li, L.; Overdier, K.H.; Ammons, L.A.; Douglas, I.S.; Burlew, C.C.; Zhang, F.; Schmidt, E.P.; Chi, L.; Linhardt, R.J. Analysis of Total Human Urinary Glycosaminoglycan Disaccharides by Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2015, 87, 6220–6227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladner, Y.D.; Alini, M.; Armiento, A.R. The Dimethylmethylene Blue Assay (DMMB) for the Quantification of Sulfated Glycosaminoglycans. Methods Mol. Biol. 2023, 2598, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29, S49-52. [Google Scholar]
- Zheng, C.H.; Levenston, M.E. Fact versus artifact: Avoiding erroneous estimates of sulfated glycosaminoglycan content using the dimethylmethylene blue colorimetric assay for tissue-engineered constructs. Eur. Cell Mater. 2015, 29, 224–236, discussion 236. [Google Scholar] [CrossRef]
- Nouailles, G.; Wyler, E.; Pennitz, P.; Postmus, D.; Vladimirova, D.; Kazmierski, J.; Pott, F.; Dietert, K.; Muelleder, M.; Farztdinov, V.; et al. Temporal omics analysis in Syrian hamsters unravel cellular effector responses to moderate COVID-19. Nat. Commun. 2021, 12, 4869. [Google Scholar] [CrossRef]
- Schmidt, E.P.; Yang, Y.; Janssen, W.J.; Gandjeva, A.; Perez, M.J.; Barthel, L.; Zemans, R.L.; Bowman, J.C.; Koyanagi, D.E.; Yunt, Z.X.; et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012, 18, 1217–1223. [Google Scholar] [CrossRef] [Green Version]
- Rovas, A.; Buscher, K.; Osiaevi, I.; Drost, C.C.; Sackarnd, J.; Tepasse, P.R.; Fobker, M.; Kuhn, J.; Braune, S.; Gobel, U.; et al. Microvascular and proteomic signatures overlap in COVID-19 and bacterial sepsis: The MICROCODE study. Angiogenesis 2022, 25, 503–515. [Google Scholar] [CrossRef]
- Collignon, F.; Frydman, A.; Caplain, H.; Ozoux, M.L.; Le Roux, Y.; Bouthier, J.; Thebault, J.J. Comparison of the pharmacokinetic profiles of three low molecular mass heparins--dalteparin, enoxaparin and nadroparin--administered subcutaneously in healthy volunteers (doses for prevention of thromboembolism). Thromb. Haemost. 1995, 73, 630–640. [Google Scholar] [CrossRef]
- Maroclo, M.V.; Pereira, S.D.; Sampaio, F.J.; Cardoso, L.E. Urinary glycosaminoglycan excretion during the menstrual cycle in normal young women. J. Urol. 2005, 173, 1789–1792. [Google Scholar] [CrossRef] [Green Version]
- Zamboni, M.J.; Cabral, C.A.; Sampaio, F.J.; Cardoso, L.E. Oral hormonal contraceptives affect the concentration and composition of urinary glycosaminoglycans in young women. Int. Urogynecol. J. Pelvic Floor. Dysfunct. 2009, 20, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, L.; Chen, Y.; Ma, J.; Yang, Y.; Aodeng, S.; Cui, Q.; Wen, K.; Xiao, M.; Xie, J.; et al. Syndecan-1, an indicator of endothelial glycocalyx degradation, predicts outcome of patients admitted to an ICU with COVID-19. Mol. Med. 2021, 27, 151. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, F.; Morales, D.; Diaz-Papapietro, C.; Valdes, C.; Fernandez, C.; Valls, N.; Lazo, M.; Espinoza, C.; Gonzalez, R.; Gutierrez, R.; et al. Relationship Between Endothelial and Angiogenesis Biomarkers Envisage Mortality in a Prospective Cohort of COVID-19 Patients Requiring Respiratory Support. Front. Med. 2022, 9, 826218. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.; Moreno-Castano, A.B.; Palomo, M.; Martinez-Sanchez, J.; Torramade-Moix, S.; Tellez, A.; Ventosa, H.; Segui, F.; Escolar, G.; Carreras, E.; et al. Distinctive Biomarker Features in the Endotheliopathy of COVID-19 and Septic Syndromes. Shock 2022, 57, 95–105. [Google Scholar] [CrossRef] [PubMed]

| Variable | COVID-19 Cohort | CAP Cohort * |
|---|---|---|
| Baseline characteristics | ||
| Number of participants (n; %) | 67 (100) | 72 (100) |
| Female sex (n; %) | 36 (53.7) | 22 (30.6) |
| Age (years, median (IQR)) | 62 (50–74) | 78 (70–85) |
| BMI (kg/m2, median (IQR)) | 27 (24–31) | na |
| Positive SARS-CoV-2 swap * (n; %) | 67 (100) | na |
| Quick SOFA score (pts, median (IQR) | 0 (0–2) | 0 (0–2) |
| Oxygen supply (n; %) | 33 (49.3) | 50 (69.4) |
| C-reactive protein (mg/dl median (IQR)) | 4.6 (1.5–4.2) | 7.4 (3.1–15.2) |
| Comorbidities (n; %) | ||
| Cardiovascular disease | 40 (59.7) | 20 (27.8) |
| Kidney disease | 11 (16.4) | 9 (12.5) |
| Lung disease | 14 (20.9) | 30 (41.7) |
| Diabetes | 19 (28.4) | na |
| Malignancy | 1 (1.5) | 10 (13.9) |
| Outcomes (n; %) | ||
| ARDS | 15 (22.4) | na |
| Intubation | 8 (11.9) | 2 (2.8) |
| NIV or HFNC | 7 (10.4) | 2 (2.8) |
| In-hospital mortality | 8 (11.9) | 23 (31.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovas, A.; Neumann, J.K.; Drost, C.C.; Vollenberg, R.; Thölking, G.; Fobker, M.; Witzenrath, M.; Kümpers, P.; AGAMOTTO Study Group; CAPNETZ Study Group. Analysis of Urinary Glycosaminoglycans to Predict Outcome in COVID-19 and Community-Acquired Pneumonia—A Proof-of-Concept Study. J. Clin. Med. 2023, 12, 5269. https://doi.org/10.3390/jcm12165269
Rovas A, Neumann JK, Drost CC, Vollenberg R, Thölking G, Fobker M, Witzenrath M, Kümpers P, AGAMOTTO Study Group, CAPNETZ Study Group. Analysis of Urinary Glycosaminoglycans to Predict Outcome in COVID-19 and Community-Acquired Pneumonia—A Proof-of-Concept Study. Journal of Clinical Medicine. 2023; 12(16):5269. https://doi.org/10.3390/jcm12165269
Chicago/Turabian StyleRovas, Alexandros, Julia Katharina Neumann, Carolin Christina Drost, Richard Vollenberg, Gerold Thölking, Manfred Fobker, Martin Witzenrath, Philipp Kümpers, AGAMOTTO Study Group, and CAPNETZ Study Group. 2023. "Analysis of Urinary Glycosaminoglycans to Predict Outcome in COVID-19 and Community-Acquired Pneumonia—A Proof-of-Concept Study" Journal of Clinical Medicine 12, no. 16: 5269. https://doi.org/10.3390/jcm12165269
APA StyleRovas, A., Neumann, J. K., Drost, C. C., Vollenberg, R., Thölking, G., Fobker, M., Witzenrath, M., Kümpers, P., AGAMOTTO Study Group, & CAPNETZ Study Group. (2023). Analysis of Urinary Glycosaminoglycans to Predict Outcome in COVID-19 and Community-Acquired Pneumonia—A Proof-of-Concept Study. Journal of Clinical Medicine, 12(16), 5269. https://doi.org/10.3390/jcm12165269