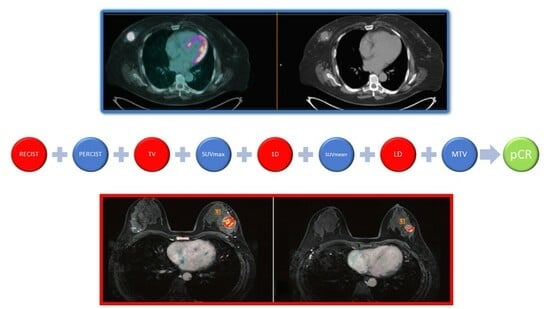
Comparison of MRI vs. [18F]FDG PET/CT for Treatment Response Evaluation of Primary Breast Cancer after Neoadjuvant Chemotherapy: Literature Review and Future Perspectives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy and Selection of the Studies
2.2. Data Collection and Extraction
2.3. Quality Assessment
3. Results
3.1. Literature Search
3.2. Basic Characteristics
3.3. Imaging and Technical Aspects
3.4. Main Findings
3.5. Risk of Bias Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Spring, L.M.; Bar, Y.; Isakoff, S.J. The Evolving Role of Neoadjuvant Therapy for Operable Breast Cancer. J. Natl. Compr. Cancer Netw. 2022, 20, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Laas, E.; Labrosse, J.; Hamy, A.S.; Benchimol, G.; de Croze, D.; Feron, J.G.; Coussy, F.; Balezeau, T.; Guerin, J.; Lae, M.; et al. Determination of breast cancer prognosis after neoadjuvant chemotherapy: Comparison of Residual Cancer Burden (RCB) and Neo-Bioscore. Br. J. Cancer 2021, 124, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Volders, J.H.; Negenborn, V.L.; Spronk, P.E.; Krekel, N.M.A.; Schoonmade, L.J.; Meijer, S.; Rubio, I.T.; van den Tol, M.P. Breast-conserving surgery following neoadjuvant therapy-a systematic review on surgical outcomes. Breast Cancer Res. Treat. 2018, 168, 1–12. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Brackstone, M.; Palma, D.; Tuck, A.B.; Scott, L.; Potvin, K.; Vandenberg, T.; Perera, F.; D’Souza, D.; Taves, D.; Kornecki, A.; et al. Concurrent Neoadjuvant Chemotherapy and Radiation Therapy in Locally Advanced Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 769–776. [Google Scholar] [CrossRef]
- Dialani, V.; Chadashvili, T.; Slanetz, P.J. Role of Imaging in Neoadjuvant Therapy for Breast Cancer. Ann. Surg. Oncol. 2015, 22, 1416–1424. [Google Scholar] [CrossRef]
- Reig, B.; Lewin, A.A.; Du, L.; Heacock, L.; Toth, H.K.; Heller, S.L.; Gao, Y.; Moy, L. Breast mri for evaluation of response to neoadjuvant therapy. Radiographics 2021, 41, 665–679. [Google Scholar] [CrossRef]
- Negrão, E.M.S.; Bitencourt, A.G.V.; de Souza, J.A.; Marques, E.F. Accuracy of breast magnetic resonance imaging in evaluating the response to neoadjuvant chemotherapy: A study of 310 cases at a cancer center. Radiol. Bras. 2019, 52, 299–304. [Google Scholar] [CrossRef]
- Loo, C.E.; Straver, M.E.; Rodenhuis, S.; Muller, S.H.; Wesseling, J.; Vrancken Peeters, M.J.; Gilhuijs, K.G. Magnetic resonance imaging response monitoring of breast cancer during neoadjuvant chemotherapy: Relevance of Breast Cancer Subtype. J. Clin. Oncol. 2011, 29, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Zasadny, K.; Helvie, M.; Hutchins, G.D.; Weber, B.; Cody, R. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: Initial evaluation. J. Clin. Oncol. 1993, 11, 2101–2111. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; The PRISMA-DTA Group. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ 2020, 370, m2632. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, U.; Miyake, M.; Nagaoka, T.; Terauchi, T.; Kubota, K.; Kinoshita, T.; Daisaki, H.; Macapinlac, H.A. Neoadjuvant chemotherapy in breast cancer: Prediction of pathologic response with PET/CT and dynamic contrast-enhanced MR imaging—Prospective assessment. Radiology 2012, 263, 53–63. [Google Scholar] [CrossRef]
- Park, S.H.; Moon, W.K.; Cho, N.; Chang, J.M.; Im, S.A.; Park, I.A.; Kang, K.W.; Han, W.; Noh, D.Y. Comparison of diffusion-weighted MR imaging and FDG PET/CT to predict pathological complete response to neoadjuvant chemotherapy in patients with breast cancer. Eur. Radiol. 2012, 22, 18–25. [Google Scholar] [CrossRef]
- Schmitz, A.M.T.; Teixeira, S.C.; Pengel, K.E.; Loo, C.E.; Vogel, W.V.; Wesseling, J.; Rutgers, E.J.T.; Valdés Olmos, R.A.; Sonke, G.S.; Rodenhuis, S.; et al. Monitoring tumor response to neoadjuvant chemotherapy using MRI & 18F-FDG PET/CT in breast cancer subtypes. PLoS ONE 2017, 12, e0176782. [Google Scholar] [CrossRef]
- Kitajima, K.; Miyoshi, Y.; Yamano, T.; Odawara, S.; Higuchi, T.; Yamakado, K. Assessment of tumor response to neoadjuvant chemotherapy in patients with breast cancer using MRI and FDG-PET/CT-RECIST 1.1 vs. PERCIST 1.0. Med. Sci. 2018, 80, 183–197. [Google Scholar] [CrossRef]
- Tokuda, Y.; Yanagawa, M.; Fujita, Y.; Honma, K.; Tanei, T.; Shimoda, M.; Miyake, T.; Naoi, Y.; Kim, S.J.; Shimazu, K.; et al. Prediction of pathological complete response after neoadjuvant chemotherapy in breast cancer: Comparison of diagnostic performances of dedicated breast PET, whole-body PET, and dynamic contrast-enhanced MRI. Breast Cancer Res. Treat. 2021, 188, 107–115. [Google Scholar] [CrossRef]
- Baysal, H.; Serdaroglu, A.Y.; Ozemir, I.A.; Baysal, B.; Gungor, S.; Erol, C.I.; Ozsoy, M.S.; Ekinci, O.; Alimoglu, O. Comparison of Magnetic Resonance Imaging with Positron Emission Tomography/Computed Tomography in the Evaluation of Response to Neoadjuvant Therapy of Breast Cancer. J. Surg. Res. 2022, 278, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Simo, M.; Gonzales Cao, M.; Ubeda, B.; Treserras, F.; Ara, C.; Brown, J.; Fabregas, R.; Baules, S.; Martinez, A.; Cubido, M. Tumor response evaluation to neoadjuvant chemotherapy by functional imaging technologies. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, S275. [Google Scholar]
- Kim, T.; Kang, D.K.; An, Y.S.; Yim, H.; Jung, Y.S.; Kim, K.S.; Kang, S.Y.; Kim, T.H. Utility of MRI and PET/CT after neoadjuvant chemotherapy in breast cancer patients: Correlation with pathological response grading system based on tumor cellularity. Acta Radiol. 2014, 55, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Pengel, K.E.; Koolen, B.B.; Loo, C.E.; Vogel, W.V.; Wesseling, J.; Lips, E.H.; Rutgers, E.J.; Valdés Olmos, R.A.; Vrancken Peeters, M.J.; Rodenhuis, S.; et al. Combined use of 18F-FDG PET/CT and MRI for response monitoring of breast cancer during neoadjuvant chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1515–1524. [Google Scholar] [CrossRef]
- Pahk, K.; Kim, S.; Choe, J.G. Early prediction of pathological complete response in luminal B type neoadjuvant chemotherapy-treated breast cancer patients: Comparison between interim 18 F-FDG PET/CT and MRI. Nucl. Med. Commun. 2015, 36, 887–891. [Google Scholar] [CrossRef]
- An, Y.Y.; Kim, S.H.; Kang, B.J.; Lee, A.W. Treatment response evaluation of breast cancer after neoadjuvant chemotherapy and usefulness of the imaging parameters of MRI and PET/CT. J. Korean Med. Sci. 2015, 30, 808–815. [Google Scholar] [CrossRef]
- Amioka, A.; Masumoto, N.; Gouda, N.; Kajitani, K.; Shigematsu, H.; Emi, A.; Kadoya, T.; Okada, M. Ability of contrast-enhanced ultrasonography to determine clinical responses of breast cancer to neoadjuvant chemotherapy. Jpn. J. Clin. Oncol. 2016, 46, 303–309. [Google Scholar] [CrossRef]
- Cho, N.; Im, S.A.; Kang, K.W.; Park, I.A.; Song, I.C.; Lee, K.H.; Kim, T.Y.; Lee, H.; Chun, I.K.; Yoon, H.J. Early prediction of response to neoadjuvant chemotherapy in breast cancer patients: Comparison of single-voxel 1H-magnetic resonance spectroscopy and 18F-fluorodeoxyglucose positron emission tomography. Eur. Radiol. 2016, 26, 2279–2290. [Google Scholar] [CrossRef]
- Choi, E.K.; Yoo, I.R.; Kim, S.H.; Park, S.Y.; Hyun, O.J.; Kang, B.J. The value of pre- and post-neoadjuvant chemotherapy F-18 FDG PET/CT scans in breast cancer: Comparison with MRI. Acta Radiol. 2018, 59, 41–49. [Google Scholar] [CrossRef]
- O’Connor, M.K.; Tran, T.D.; Swanson, T.N.; Ellingson, L.R.; Hunt, K.N.; Whaley, D.H. Improved visualization of breast tissue on a dedicated breast PET system through ergonomic redesign of the imaging table. EJNMMI Res. 2017, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, E.P. Impact of Neoadjuvant Chemotherapy on Locoregional Surgical Treatment of Breast Cancer. Ann. Surg. Oncol. 2015, 22, 1425–1433. [Google Scholar] [CrossRef]
- Gu, Y.L.; Pan, S.M.; Ren, J.; Yang, Z.X.; Jiang, G.Q. Role of Magnetic Resonance Imaging in Detection of Pathologic Complete Remission in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: A Meta-analysis. Clin. Breast Cancer 2017, 17, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Rauch, G.M.; Adrada, B.E.; Kuerer, H.M.; van la Parra, R.F.; Leung, J.W.; Yang, W.T. Multimodality imaging for evaluating response to neoadjuvant chemotherapy in breast cancer. Am. J. Roentgenol. 2017, 208, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Scheel, J.R.; Kim, E.; Partridge, S.C.; Lehman, C.D.; Rosen, M.A.; Bernreuter, W.K.; Pisano, E.D.; Marques, H.S.; Morris, E.A.; Weatherall, P.T.; et al. MRI, Clinical Examination, and Mammography for Preoperative Assessment of Residual Disease and Pathologic Complete Response after Neoadjuvant Chemotherapy for Breast Cancer: ACRIN 6657 Trial. AJR Am. J. Roentgenol. 2018, 210, 1376–1385. [Google Scholar] [CrossRef]
- Gampenrieder, S.P.; Peer, A.; Weismann, C.; Meissnitzer, M.; Rinnerthaler, G.; Webhofer, J.; Westphal, T.; Riedmann, M.; Meissnitzer, T.; Egger, H.; et al. Radiologic complete response (rCR) in contrast-enhanced magnetic resonance imaging (CE-MRI) after neoadjuvant chemotherapy for early breast cancer predicts recurrence-free survival but not pathologic complete response (pCR). Breast Cancer Res. 2019, 21, 19. [Google Scholar] [CrossRef]
- Schrading, S.; Kuhl, C.K. Breast Cancer: Influence of Taxanes on Response Assessment with Dynamic Contrast-enhanced MR Imaging. Radiology 2015, 277, 687–696. [Google Scholar] [CrossRef]
- You, S.; Kang, D.K.; Jung, Y.S.; An, Y.S.; Jeon, G.S.; Kim, T.H. Evaluation of lymph node status after neoadjuvant chemotherapy in breast cancer patients: Comparison of diagnostic performance of ultrasound, MRI and ¹⁸F-FDG PET/CT. Br. J. Radiol. 2015, 88, 20150143. [Google Scholar] [CrossRef]
- Hayashi, N.; Tsunoda, H.; Namura, M.; Ochi, T.; Suzuki, K.; Yamauchi, H.; Nakamura, S. Magnetic Resonance Imaging Combined With Second-look Ultrasonography in Predicting Pathologic Complete Response After Neoadjuvant Chemotherapy in Primary Breast Cancer Patients. Clin. Breast Cancer 2019, 19, 71–77. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liu, J.; Huang, G. Is 18F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis. Breast Cancer Res. Treat. 2012, 131, 357–369. [Google Scholar] [CrossRef]
- Goktas Aydin, S.; Bilici, A.; Olmez, O.F.; Oven, B.B.; Acikgoz, O.; Cakir, T.; Basim, P.; Cakir, A.; Kutlu, Y.; Hamdard, J. The Role of 18F-FDG PET/CT in Predicting the Neoadjuvant Treatment Response in Patients with Locally Advanced Breast Cancer. Breast Care 2022, 17, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, L.; Jin, P.; Hu, L.; Li, X.; Guo, T.; Yang, K. MRI and PET/CT for evaluation of the pathological response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. Breast 2018, 40, 106–115. [Google Scholar] [CrossRef]
- Fowler, A.M.; Strigel, R.M. Clinical advances in PET–MRI for breast cancer. Lancet Oncol. 2022, 23, e32–e43. [Google Scholar] [CrossRef]
- Roy, S.; Whitehead, T.D.; Li, S.; Ademuyiwa, F.O.; Wahl, R.L.; Dehdashti, F.; Shoghi, K.I. Co-clinical FDG-PET radiomic signature in predicting response to neoadjuvant chemotherapy in triple-negative breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Sekine, C.; Uchiyama, N.; Watase, C.; Murata, T.; Shiino, S.; Jimbo, K.; Iwamoto, E.; Takayama, S.; Kurihara, H.; Satomi, K.; et al. Preliminary experiences of PET/MRI in predicting complete response in patients with breast cancer treated with neoadjuvant chemotherapy. Mol. Clin. Oncol. 2022, 16, 1–8. [Google Scholar] [CrossRef]
- de Mooij, C.M.; van Nijnatten, T.J.A.; Goorts, B.; Kooreman, L.F.; Raymakers, I.W.M.; van Meijl, S.P.L.; de Boer, M.; Keymeule, K.B.M.I.; Wildberger, J.E.; Mottaghy, F.M.; et al. Prediction of Primary tumor and Axillary Lymph Node Response to Neoadjuvant Chemo (Targeted) Therapy with with Dedicated Breast [18F]FDG PET/MRI in Breast Cancer. Cancers 2023, 15, 401. [Google Scholar] [CrossRef]
- Ming, Y.; Wu, N.; Qian, T.; Li, X.; Wan, D.Q.; Li, C.; Li, Y.; Wu, Z.; Wang, X.; Liu, J.; et al. Progress and Future Trends in PET/CT and PET/MRI Molecular Imaging Approaches for Breast Cancer. Front. Oncol. 2020, 10, 1301. [Google Scholar] [CrossRef]
- Aertgeerts, K.; Levin, I.; Shi, L.; Snell, G.P.; Jennings, A.; Prasad, G.S.; Zhang, Y.; Kraus, M.L.; Salakian, S.; Sridhar, V.; et al. Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein α. J. Biol. Chem. 2005, 280, 19441–19444. [Google Scholar] [CrossRef]
- Backhaus, P.; Burg, M.C.; Asmus, I.; Pixberg, M.; Büther, F.; Breyholz, H.J.; Yeh, R.; Weigel, S.B.; Stichling, P.; Heindel, W.; et al. Initial Results of 68Ga-FAPI-46 PET/MRI to Assess Response to Neoadjuvant Chemotherapy in Breast Cancer. J. Nucl. Med. 2023, 64, 717–723. [Google Scholar] [CrossRef]
- Evangelista, L.; Urso, L.; Caracciolo, M.; Stracuzzi, F.; Panareo, S.; Cistaro, A.; Catalano, O. FDG PET/CT Volume-Based Quantitative Data and Survival Analysis in Breast Cancer Patients: A Systematic Review of the Literature. Curr. Med. Imaging 2023, 19, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Cervino, A.R.; Ghiotto, C.; Saibene, T.; Michieletto, S.; Fernando, B.; Orvieto, E.; Guarneri, V.; Conte, P. Could semiquantitative FDG analysis add information to the prognosis in patients with stage II/III breast cancer undergoing neoadjuvant treatment? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1648–1655. [Google Scholar] [CrossRef]
- Urso, L.; Evangelista, L.; Alongi, P.; Quartuccio, N.; Cittanti, C.; Rambaldi, I.; Ortolan, N.; Borgia, F.; Nieri, A.; Uccelli, L. The Value of Semiquantitative Parameters Derived from 18F-FDG PET/CT for Predicting Response to Neoadjuvant Chemotherapy in a Cohort of Patients with Different Molecular Subtypes of Breast Cancer. Cancers 2022, 14, 5869. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Cochet, A.; Humbert, O.; Alberini, J.L.; Hindié, E.; Mankoff, D. 18F-FDG PET/CT for staging and restaging of breast cancer. J. Nucl. Med. 2016, 57, 17S–26S. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Quartuccio, N.; Caracciolo, M.; Evangelista, L.; Schirone, A.; Frassoldati, A.; Arnone, G.; Panareo, S.; Bartolomei, M. Impact on the long-term prognosis of FDG PET/CT in luminal-A and luminal-B breast cancer. Nucl. Med. Commun. 2022, 43, 212–219. [Google Scholar] [CrossRef]
- Son, S.H.; Lee, S.W.; Jeong, S.Y.; Song, B.I.; Chae, Y.S.; Ahn, B.C.; Lee, J. Whole-Body Metabolic Tumor Volume, as Determined by 18F-FDG PET/CT, as a Prognostic Factor of Outcome for Patients With Breast Cancer Who Have Distant Metastasis. Am. J. Roentgenol. 2015, 205, 878–885. [Google Scholar] [CrossRef]
- Kitajima, K.; Miyoshi, Y.; Sekine, T.; Takei, H.; Ito, K.; Suto, A.; Kaida, H.; Ishii, K.; Daisaki, H.; Yamakado, K. Harmonized pretreatment quantitative volume-based FDG-PET/CT parameters for prognosis of stage I-III breast cancer: Multicenter study. Oncotarget 2021, 12, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Manco, L.; Castello, A.; Evangelista, L.; Guidi, G.; Castellani, M.; Florimonte, L.; Cittanti, C.; Turra, A.; Panareo, S. PET-Derived Radiomics and Artificial Intelligence in Breast Cancer: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 13409. [Google Scholar] [CrossRef]
- Molina-García, D.; García-Vicente, A.M.; Pérez-Beteta, J.; Amo-Salas, M.; Martínez-González, A.; Tello-Galán, M.J.; Soriano-Castrejón, Á.; Pérez-García, V.M. Intratumoral heterogeneity in 18F-FDG PET/CT by textural analysis in breast cancer as a predictive and prognostic subrogate. Ann. Nucl. Med. 2018, 32, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Umutlu, L.; Kirchner, J.; Bruckmann, N.M.; Morawitz, J.; Antoch, G.; Ting, S.; Bittner, A.K.; Hoffmann, O.; Häberle, L.; Ruckhäberle, E. Multiparametric18F-FDG PET/MRI-Based Radiomics for Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancers 2022, 14, 1727. [Google Scholar] [CrossRef]
- Oliveira, C.; Oliveira, F.; Vaz, S.C.; Marques, H.P.; Cardoso, F. Prediction of pathological response after neoadjuvant chemotherapy using baseline FDG PET heterogeneity features in breast cancer. Br. J. Radiol. 2023, 96, 20220655. [Google Scholar] [CrossRef]
- Hustinx, R.; Pruim, J.; Lassmann, M.; Visvikis, D. An EANM position paper on the application of artificial intelligence in nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 61–66. [Google Scholar] [CrossRef] [PubMed]



| Authors [Ref.] | Year | Country | Study Design/N° of Involved Centers | Funding Sources |
|---|---|---|---|---|
| Amioka et al. [28] | 2016 | Japan | Prospective/monocentric | None |
| An et al. [27] | 2015 | South Korea | Retrospective/monocentric | National Research Foundation of Korea |
| Baysal et al. [22] | 2022 | Turkey | Retrospective/monocentric | None |
| Choi et al. [30] | 2018 | South Korea | Prospective/monocentric | None |
| Kim et al. [24] | 2014 | South Korea | Retrospective/monocentric | None |
| Kitajima et al. [20] | 2018 | Japan | Retrospective/monocentric | None |
| Cho et al. [29] | 2016 | South Korea | Prospective/monocentric | National Research Foundation of Korea |
| Pahk et al. [26] | 2015 | South Korea | Retrospective/monocentric | Korea Health Industry Development Institute |
| Park et al. [18] | 2012 | South Korea | Retrospective/monocentric | Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Innovative Research Institute for Cell Therapy |
| Pengel et al. [25] | 2014 | Netherlands | Prospective/monocentric | Project Breast CARE |
| Schmitz et al. [19] | 2017 | Netherlands | Prospective/monocentric | Project Breast CARE |
| Simo et al. [23] | 2013 | Spain | Prospective/monocentric | Not reported |
| Tateishi et al. [17] | 2012 | Japan, USA | Prospective/bicentric | None |
| Tokuda et al. [21] | 2021 | Japan | Prospective/monocentric | None |
| Authors [Ref.] | Sample Size | Mean/Median Age (Years) | Histology | PET Scanner | Response Assessment | pCR |
|---|---|---|---|---|---|---|
| Amioka et al. [28] | 63 | 53.0 (31–69) | LU (5A, 18B, 11HER2), HER2 (8), TP (21) | whole-body | RECIST 1.1 | YES |
| An et al. [27] | 16 | 51.6 (29–69) | DC (19), LC (1) | whole-body | ∆SUVmax, ∆LD | NR |
| Baysal et al. [22] | 88 | 53.09 ± 12.57 | LU (26A, 39B, 9HER2), TP (14) | whole-body | RECIST 1.1, PERCIST 1.0 | YES |
| Choi et al. [30] | 33 | 50.0 ± 10 | IDC (28), micropapillary (2), ILC (2), metaplastic (1) | whole-body | ∆SULpeak, ∆MTV, ∆1D, ∆TV | YES |
| Kim et al. [24] | 38 | 47.0 (27–70) | DC (54), LC (1), MUC (1) | whole-body | ∆SUVmax | NR |
| Kitajima et al. [20] | 32 | 52.4 (29–74) | DC (29), LC (1), MUC (2) | whole-body | RECIST 1.1, PERCIST 1.0 | YES |
| Cho et al. [29] | 35 | 49.6 (35–65) | DC (33), LC (2) | whole-body | ∆SUVmax, ∆LD | YES |
| Pahk et al. [26] | 21 | 51 (NR) | DC (21) | whole-body | ∆SUVmax | NR |
| Park et al. [18] | 34 | 44 (27–60) | DC (32), MUC (1), other (1) | whole-body | ∆SUVmax | NR |
| Pengel et al. [25] | 93 | 48 (26–68) | DC (85), LC (7) | whole-body | ∆SUVmax | YES |
| Schmitz et al. [19] | 188 | 47 (25–73) | IDC (167), ILC (18), others (3) | whole-body | ∆SUVmax, ∆LD | NR |
| Simo et al. [23] | 30 | 47 (31–70) | LU (12A, 9B), TN (10), HER2 (10) | whole-body | RECIST 1.1, PERCIST 1.0 | NR |
| Tateishi et al. [17] | 142 | 57 (43–72) | DC (131), LC (11) | whole-body | ∆SUVmax, ∆LD | NR |
| Tokuda et al. [21] | 29 | 55 (35–78) | LU (7A, 13B, 3HER2), TP (6) | dedicated for breast | RECIST 1.1, PERCIST 1.0 | YES |
| Authors [Ref.] | Performance Measure | MRI | PET/CT | MRI + PET |
|---|---|---|---|---|
| SE | 69.6 | SUVmax 100 | NR | |
| Amioka et al. [28] | SP | 85.0 | SUVmax 52.5 | NR |
| Acc | 79.4 | SUVmax 69.8 | NR | |
| SE | ΔLD 66.67 ΔTV 66.67 ΔPE 66.67 ΔLD + ΔTV + ΔPE 66.67 ΔADC 66.67 | ΔSUV 66.67 | LD + SUV 33.33 TV + SUV 33.33 PE + SUV 33.33 ADC + SUV 33.33 | |
| An et al. [27] | SP | ΔLD 94.12 ΔTV 94.12 ΔPE 70.59 ΔLD + ΔTV + ΔPE 94.12 ΔADC 70.59 | ΔSUV 92.31 | LD + SUV 100 TV + SUV 100 PE + SUV 92.32 ADC + SUV 100 |
| Acc | ΔLD 90.00 ΔTV 90.00 ΔPE 70.00 ΔLD + ΔTV + ΔPE 90.00 ΔADC 70.00 | ΔSUV 87.50 | LD + SUV 87.50 TV + SUV 87.50 PE + SUV 81.25 ADC + SUV 87.50 | |
| Baysal et al. [22] | SE | 86.96 | PERCIST 100 | NR |
| SP | 30.7 | PERCIST 75.6 | NR | |
| Acc | 57.1 | PERCIST 81.8 | NR | |
| Choi et al. [30] | SE | 1D 88.2 | SULpeak 100 | NR |
| SP | 1D 62.5 | SULpeak 25 | NR | |
| Acc | 1D 75.7 | SULpeak 63.6 | NR | |
| Kim et al. [24] | SE | Δ diameter 64.7 Δ volume 91.2 | ΔSUV 91.3 | NR |
| SP | Δ diameter 95.5 Δ volume 77.3 | ΔSUV 73.3 | NR | |
| Acc | Δ diameter 76.8 Δ volume 85.7 | ΔSUV 81.6 | NR | |
| Kitajima et al. [20] | SE | RECIST1.1 28.6 | PERCIST 100 | NR |
| SP | RECIST1.1 94.4 | PERCIST 22.2 | NR | |
| Acc | RECIST1.1 65.6 | PERCIST 56.3 | NR | |
| Cho et al. [29] | SE | MRS 75.9 | SUVmax 100 SUVpeak 100 TLG 79.3 | NR |
| SP | MRS 100 | SUVmax 66.7 SUVpeak 66.7 TLG 100 | NR | |
| Acc | MRS 91.1 | SUVmax 82.2 SUVpeak 86.2 TLG 87.9 | NR | |
| Pahk et al. [26] | SE | Δ size 64.3 | ΔSUV 85.7 | NR |
| SP | Δ size 71.4 | ΔSUV 100 | NR | |
| Acc | Δ size 65 | ΔSUV 90 | NR | |
| Park et al. [18] | SE | DWI 100 | SUV 100 | DWI + SUV 100 |
| SP | DWI 70.4 | SUV 77.8 | DWI + SUV 88.9 | |
| Acc | DWI 76.5 | SUV 82.4 | DWI + SUV 91.2 | |
| Pengel et al. [25] | SE | NR | NR | NR |
| SP | NR | NR | NR | |
| Acc | NR | NR | NR | |
| Schmitz et al. [19] | SE | ER+ 36.2 TP 45.5 | NR | HER2+ 55.8 |
| SP | NR | NR | NR | |
| Acc | NR | NR | NR | |
| Simo et al. [23] | SE | NR | NR | NR |
| SP | NR | NR | NR | |
| Acc | NR | NR | NR | |
| Tateishi et al. [17] | SE | Δ rate costant 51.7 | ΔSUVmax 66.7 | NR |
| SP | Δ rate costant 92 | ΔSUVmax 96.4 | NR | |
| Acc | Δ rate costant 83.8 | ΔSUVmax 90.1 | NR | |
| Tokuda et al. [21] | SE | 100 | dbPET 85.7 WB-PET 71.4 | NR |
| SP | 50 | dbPET 72.7 WB-PET 77.3 | NR | |
| Acc | 77.3 | dbPET 82 WB-PET 73 | NR |
| Study | Riks of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Text | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Tateishi; 2012 [17] | ? | ? | + | ? | − | − | + |
| Park; 2012 [18] | ? | ? | + | ? | + | + | + |
| Simo; 2013 [23] | + | ? | + | ? | + | − | + |
| Kim; 2014 [24] | + | ? | + | ? | + | + | + |
| Pengel; 2014 [25] | ? | ? | + | + | + | + | + |
| Pahk; 2015 [26] | − | ? | + | + | − | + | + |
| An; 2015 [27] | ? | + | + | + | + | + | + |
| Cho; 2016 [29] | ? | ? | + | + | + | + | + |
| Amioka; 2016 [28] | ? | ? | + | ? | − | − | + |
| Choi; 2017 [30] | − | + | + | − | + | + | + |
| Schmitz; 2017 [19] | + | + | + | ? | − | + | + |
| Kitajima; 2018 [20] | + | ? | + | ? | + | + | + |
| Tokuda; 2021 [21] | + | ? | + | ? | + | − | + |
| Baysal; 2022 [22] | ? | ? | + | ? | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caracciolo, M.; Castello, A.; Urso, L.; Borgia, F.; Marzola, M.C.; Uccelli, L.; Cittanti, C.; Bartolomei, M.; Castellani, M.; Lopci, E. Comparison of MRI vs. [18F]FDG PET/CT for Treatment Response Evaluation of Primary Breast Cancer after Neoadjuvant Chemotherapy: Literature Review and Future Perspectives. J. Clin. Med. 2023, 12, 5355. https://doi.org/10.3390/jcm12165355
Caracciolo M, Castello A, Urso L, Borgia F, Marzola MC, Uccelli L, Cittanti C, Bartolomei M, Castellani M, Lopci E. Comparison of MRI vs. [18F]FDG PET/CT for Treatment Response Evaluation of Primary Breast Cancer after Neoadjuvant Chemotherapy: Literature Review and Future Perspectives. Journal of Clinical Medicine. 2023; 12(16):5355. https://doi.org/10.3390/jcm12165355
Chicago/Turabian StyleCaracciolo, Matteo, Angelo Castello, Luca Urso, Francesca Borgia, Maria Cristina Marzola, Licia Uccelli, Corrado Cittanti, Mirco Bartolomei, Massimo Castellani, and Egesta Lopci. 2023. "Comparison of MRI vs. [18F]FDG PET/CT for Treatment Response Evaluation of Primary Breast Cancer after Neoadjuvant Chemotherapy: Literature Review and Future Perspectives" Journal of Clinical Medicine 12, no. 16: 5355. https://doi.org/10.3390/jcm12165355
APA StyleCaracciolo, M., Castello, A., Urso, L., Borgia, F., Marzola, M. C., Uccelli, L., Cittanti, C., Bartolomei, M., Castellani, M., & Lopci, E. (2023). Comparison of MRI vs. [18F]FDG PET/CT for Treatment Response Evaluation of Primary Breast Cancer after Neoadjuvant Chemotherapy: Literature Review and Future Perspectives. Journal of Clinical Medicine, 12(16), 5355. https://doi.org/10.3390/jcm12165355