Clinical Utility of Pre-Therapeutic [18F]FDG PET/CT Imaging for Predicting Outcomes in Breast Cancer
Abstract
1. Introduction
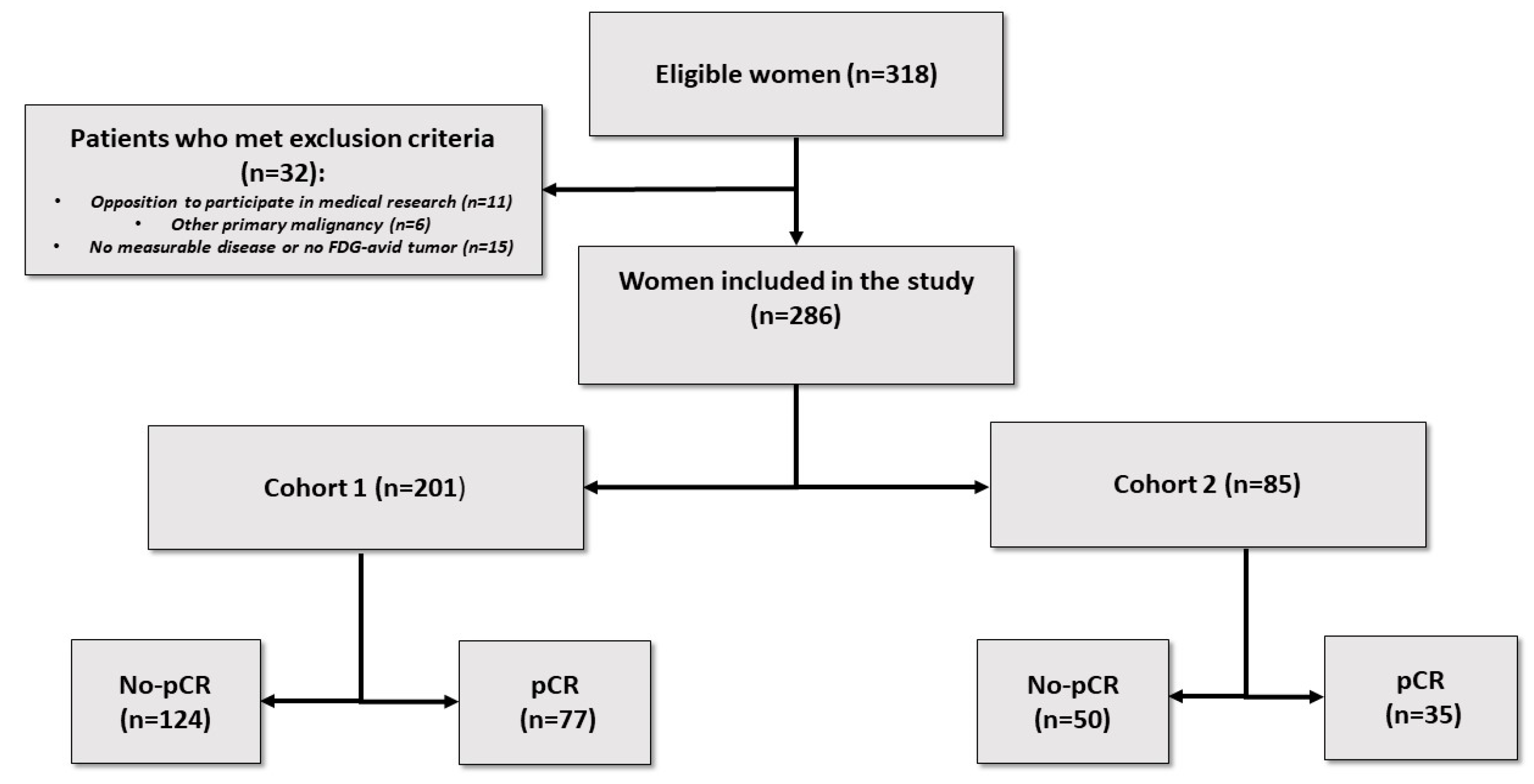
2. Materials and Methods
2.1. Patients
2.2. Clinicopathological Data
2.3. Treatment, Pathological Complete Response, and Surveillance
2.4. PET/CT Imaging
2.5. Outcomes Measures
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
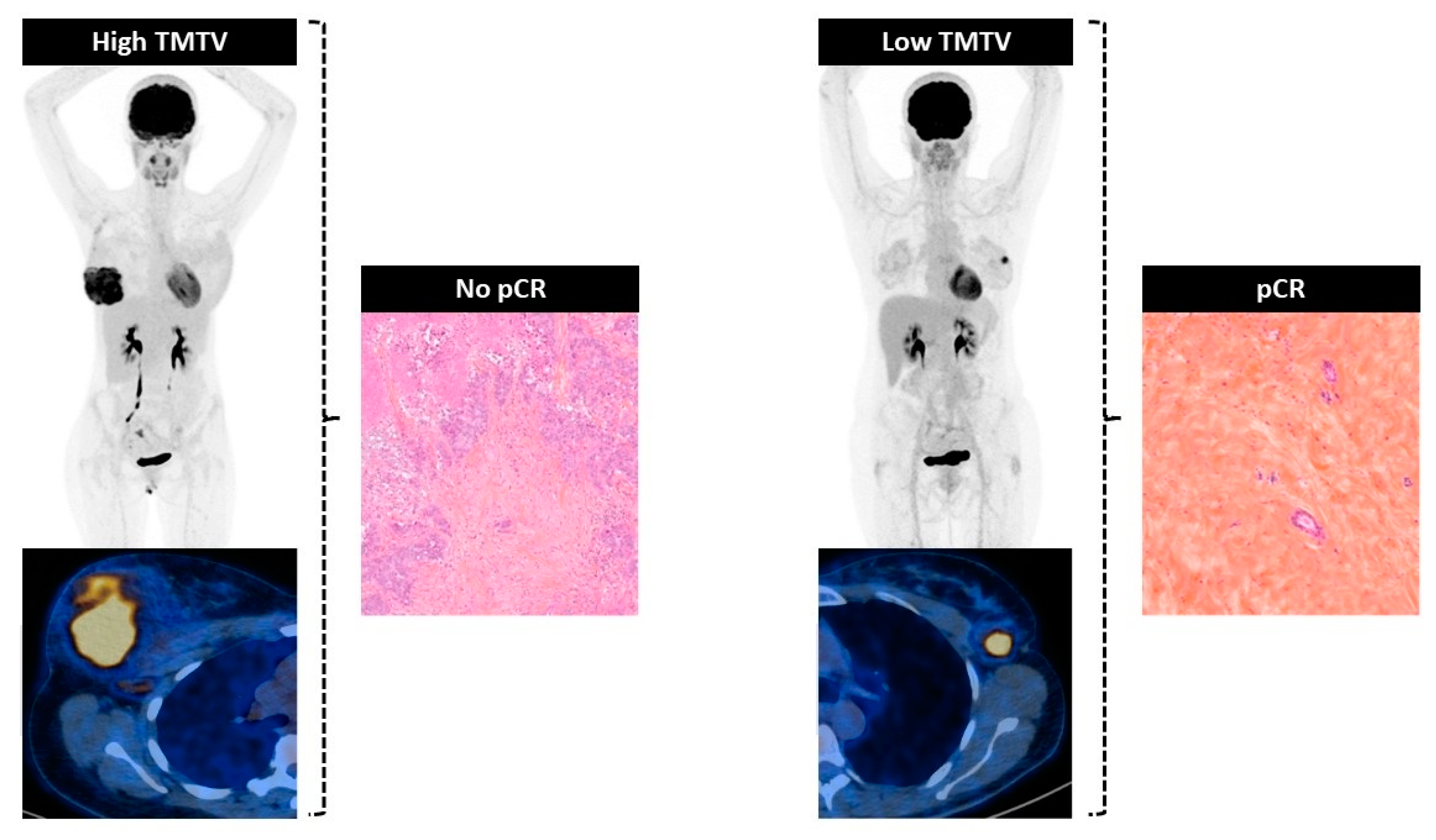
3.2. Association with Pathological Complete Response
3.2.1. Relationship between Biomarkers and pCR
3.2.2. Determination of Cut-Off Value of TMTV to Predict pCR
3.2.3. Univariate and Multivariate Analyses of pCR Including TMTV (High versus Low)
3.3. Association with Recurrence-Free Survival
3.3.1. Determination of the Best Cut-Off Value of TMTV to Predict 3-Year RFS
3.3.2. Survival Analysis
3.4. Subgroup Analysis: Triple Negative Breast Cancer
3.4.1. Association with Pathological Complete Response
3.4.2. Association with Recurrence-Free Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rubens, R.D.; Sexton, S.; Tong, D.; Winter, P.J.; Knight, R.K.; Hayward, J.L. Combined chemotherapy and radiotherapy for locally advanced breast cancer. Eur. J. Cancer 1980, 16, 351–356. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Baghdadi, Y.; Love, C.; Sparano, J.A. Clinical Utility of 18F-FDG PET/CT in Staging Localized Breast Cancer Before Initiating Preoperative Systemic Therapy. J. Natl. Compr. Cancer Netw. 2020, 18, 1240–1246. [Google Scholar] [CrossRef]
- Hyland, C.J.; Varghese, F.; Yau, C.; Beckwith, H.; Khoury, K.; Varnado, W.; Hirst, G.L.; Flavell, R.R.; Chien, A.J.; Yee, D.; et al. Use of 18F-FDG PET/CT as an Initial Staging Procedure for Stage II-III Breast Cancer: A Multicenter Value Analysis. J. Natl. Compr. Cancer Netw. 2020, 18, 1510–1517. [Google Scholar] [CrossRef]
- Groheux, D.; Mankoff, D.; Espié, M.; Hindié, E. ¹⁸F-FDG PET/CT in the early prediction of pathological response in aggressive subtypes of breast cancer: Review of the literature and recommendations for use in clinical trials. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 983–993. [Google Scholar] [CrossRef]
- Groheux, D. Predicting pathological complete response in breast cancer early. Lancet Oncol. 2014, 15, 1415–1416. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.; Xu, C.; Liu, C.; Zheng, C.; Fulham, M.J.; Feng, D.; Wang, L.; Song, S.; Huang, G. 18F-FDG PET/CT radiomic predictors of pathologic complete response (pCR) to neoadjuvant chemotherapy in breast cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Lemarignier, C.; Martineau, A.; Teixeira, L.; Vercellino, L.; Espié, M.; Merlet, P.; Groheux, D. Correlation between tumour characteristics, SUV measurements, metabolic tumour volume, TLG and textural features assessed with 18F-FDG PET in a large cohort of oestrogen receptor-positive breast cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Giacchetti, S.; Moretti, J.-L.; Porcher, R.; Espié, M.; Lehmann-Che, J.; de Roquancourt, A.; Hamy, A.-S.; Cuvier, C.; Vercellino, L.; et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Nishimukai, A.; Inoue, N.; Kira, A.; Takeda, M.; Morimoto, K.; Araki, K.; Kitajima, K.; Watanabe, T.; Hirota, S.; Katagiri, T.; et al. Tumor size and proliferative marker geminin rather than Ki67 expression levels significantly associated with maximum uptake of 18F-deoxyglucose levels on positron emission tomography for breast cancers. PLoS ONE 2017, 12, e0184508. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diao, W.; Tian, F.; Jia, Z. The prognostic value of SUVmax measuring on primary lesion and ALN by 18F-FDG PET or PET/CT in patients with breast cancer. Eur. J. Radiol. 2018, 105, 1–7. [Google Scholar] [CrossRef]
- Soret, M.; Bacharach, S.L.; Buvat, I. Partial-volume effect in PET tumor imaging. J. Nucl. Med. 2007, 48, 932–945. [Google Scholar] [CrossRef]
- Seban, R.D.; Rouzier, R.; Latouche, A.; Deleval, N.; Guinebretiere, J.M.; Buvat, I.; Bidard, F.C.; Champion, L. Total metabolic tumor volume and spleen metabolism on baseline [18F]-FDG PET/CT as independent prognostic biomarkers of recurrence in resected breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3560–3570. [Google Scholar] [CrossRef]
- Urso, L.; Evangelista, L.; Alongi, P.; Quartuccio, N.; Cittanti, C.; Rambaldi, I.; Ortolan, N.; Borgia, F.; Nieri, A.; Uccelli, L.; et al. The Value of Semiquantitative Parameters Derived from 18F-FDG PET/CT for Predicting Response to Neoadjuvant Chemotherapy in a Cohort of Patients with Different Molecular Subtypes of Breast Cancer. Cancers 2022, 14, 5869. [Google Scholar] [CrossRef]
- Jiménez-Ballvé, A.; García García-Esquinas, M.; Salsidua-Arroyo, O.; Serrano-Palacio, A.; García-Sáenz, J.A.; Ortega Candil, A.; Fuentes Ferrer, M.E.; Rodríguez Rey, C.; Román-Santamaría, J.M.; Moreno, F.; et al. Prognostic value of metabolic tumour volume and total lesion glycolysis in 18F-FDG PET/CT scans in locally advanced breast cancer staging. Rev. Esp. Med. Nucl. Imagen Mol. 2016, 35, 365–372. [Google Scholar]
- Farrugia, M.K.; Wen, S.; Jacobson, G.M.; Salkeni, M.A. Prognostic factors in breast cancer patients evaluated by positron-emission tomography/computed tomography before neoadjuvant chemotherapy. World J. Nucl. Med. 2018, 17, 275–280. [Google Scholar] [CrossRef]
- Higuchi, T.; Fujimoto, Y.; Ozawa, H.; Bun, A.; Fukui, R.; Miyagawa, Y.; Imamura, M.; Kitajima, K.; Yamakado, K.; Miyoshi, Y. Significance of metabolic tumor volume at baseline and reduction of mean standardized uptake value in 18F-FDG-PET/CT imaging for predicting pathological complete response in breast cancers treated with preoperative chemotherapy. Ann. Surg. Oncol. 2019, 26, 2175–8324. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G.; Chmielik, E.; Cserni, B.; Tot, T. The new TNM-based staging of breast cancer. Virchows Arch. 2018, 472, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J.; Panel members. Strategies for subtypes–dealing with the diversity of breast cancer: Highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Provenzano, E.; Bossuyt, V.; Viale, G.; Cameron, D.; Badve, S.; Denkert, C.; MacGrogan, G.; Penault-Llorca, F.; Boughey, J.; Curigliano, G.; et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: Recommendations from an international working group. Mod. Pathol. 2015, 28, 1185–1201. [Google Scholar] [CrossRef]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Haque, W.; Verma, V.; Hatch, S.; Suzanne Klimberg, V.; Brian Butler, E.; Teh, B.S. Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2018, 170, 559–567. [Google Scholar] [CrossRef]
- Viallon, V.; Latouche, A. Discrimination measures for survival outcomes: Connection between the AUC and the predictiveness curve. Biom. J. 2011, 53, 217–236. [Google Scholar] [CrossRef]
- Empereur-Mot, C.; Guillemain, H.; Latouche, A.; Zagury, J.-F.; Viallon, V.; Montes, M. Predictiveness curves in virtual screening. J. Cheminform. 2015, 7, 52. [Google Scholar] [CrossRef]

| All Patients N = 286 | Overall N = 286 | Cohort 1 N = 201 | Cohort 2 N = 85 | |
|---|---|---|---|---|
| Mean (±SD), n (%) | p Value | |||
| Clinicopathological characteristics | ||||
| Age (years) | 49.1 (±12.4) | 48.7 (±12.65) | 50.0 (±11.8) | 0.39 |
| pCR | 112 (39.2) | 77 (38.3) | 35 (41.2) | 0.65 |
| TNM | ||||
| T stage | 0.14 | |||
| 1 | 26 (9.1) | 15 (7.5) | 11 (12.9) | |
| 2 | 162 (56.6) | 113 (56.2) | 49 (57.6) | |
| 3 | 75 (26.2) | 59 (29.4) | 16 (18.8) | |
| 4 | 23 (8.0) | 14 (7.0) | 9 (10.6) | |
| N+ | 166 (58.0) | 124 (61.7) | 42 (49.4) | 0.07 |
| Subtype | 0.43 | |||
| Luminal A | 28 (9.8) | 43 (21.4) | 25 (29.4) | |
| Luminal B HER2- | 71 (24.8) | 20 (10.0) | 8 (9.4) | |
| HER2+ * | 68 (23.8) | 54 (26.9) | 17 (20.0) | |
| TNBC | 119 (41.6) | 84 (41.8) | 35 (41.2) | |
| Histologic parameters | ||||
| Ki67 | 49.8 (±24.2) | 49.7 (±24.1) | 50.1 (±24.6) | 0.21 |
| Vascular invasion | 25 (8.7) | 20 (10.0) | 8 (9.4) | 0.27 |
| Grade | 0.79 | |||
| I/II | 95 (33.2) | 68 (33.8) | 27 (31.8) | |
| III | 191 (66.8) | 133 (66.2) | 58 (68.2) | |
| Mitotic index | 0.64 | |||
| 1 | 62 (22.2) | 44 (22.7) | 18 (21.2) | |
| 2 | 97 (34.8) | 64 (33.0) | 33 (38.8) | |
| 3 | 120 (43.0) | 86 (44.3) | 34 (40.0) | |
| Tumor markers (ng/mL) | ||||
| CEA | 2.76 (±6.0) | 2.89 (±6.6) | 2.1 (±1.6) | 0.53 |
| CA 15-3 | 24.4 (±43.2) | 26.2 (±42.8) | 22.8 (±22.5) | 0.35 |
| PET imaging characteristics | ||||
| SUVmax | 11.7 (±6.4) | 11.1 (±6.2) | 13.14 (±6.7) | 0.01 |
| TMTV | 24.4 (±43.2) | 27.4 (±42.8) | 17.3 (±43.6) | 0.07 |
| Treatment | ||||
| Neoadjuvant | ||||
| HER2-targeted therapy | 68 (23.8) | 43 (21.4) | 25 (29.4) | 0.19 |
| Adjuvant | ||||
| Radiotherapy | 276 (96.5) | 193 (96) | 83 (97.6) | 0.74 |
| Chemotherapy | 68 (23.8) | 30 (14.19) | 38 (45.2) | <0.01 |
| HER2-targeted therapy | 32 (11.2) | 16 (7.69) | 16 (18.82) | 0.01 |
| Endocrine therapy | 153 (53.5) | 104 (51.7) | 49 (57.6) | 0.43 |
| Factor Associated with No-pCR after NACT | ||||
|---|---|---|---|---|
| N = 286 | Univariate | Multivariate | ||
| Events = 112 | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Age < 40 years (vs. ≥40) | 0.6 (0.4–1.1) | 0.09 | 0.7 (0.4–1.2) | 0.16 |
| T stage 3–4 (vs. 1–2) | 2.1 (1.2–3.5) | <0.01 | 1.6 (0.9–3.1) | 0.13 |
| N+ (vs. N−) | 2.19 (1.3–3.6) | <0.01 | - | - |
| Subtype | - | <0.01 | ||
| Luminal | 1.0 (Reference) | - | - | - |
| HER2+ | 0.3 (0.1–0.5) | <0.01 | 0.3 (0.1–0.6) | - |
| TNBC | 0.3 (0.2–-0.5) | <0.01 | 0.4 (0.2–0.7) | - |
| Vascular invasion (yes vs. no) | 1.7 (0.7–4.6) | 0.20 | - | - |
| Histologic grade 3 (vs. 1–2) | 0.4 (0.2–0.7) | <0.01 | - | - |
| Ki67 ≥ 20% (vs. <20%) | 0.3 (0.1–0.6) | <0.01 | 0.3 (0.1–0.7) | <0.01 |
| TMTV > 9.0 cm3 (vs. ≤9.0 cm3) | 2.9 (1.8–4.9) | <0.01 | 2.4 (1.3–4.2) | <0.01 |
| 3-Year Recurrence-Free Survival | ||||
|---|---|---|---|---|
| n = 286 | Univariate | Multivariate | ||
| Events = 65 | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age < 40 years (vs. ≥40) | 1.1 (0.5–2.3) | 0.78 | - | - |
| pCR | 0.5 (0.2–0.9) | 0.04 | 0.6 (0.3–1.2) | 0.14 |
| T stage 3–4 (vs. 1–2) | 1.51(0.8–2.9) | 0.21 | - | - |
| N+ (vs. N−) | 2.4 (1.1–5.1) | 0.02 | - | - |
| Molecular subtype | ||||
| Luminal | 1.0 (Reference) | - | - | - |
| HER2+ | 0.3 (0.1–1.2) | 0.09 | - | - |
| TNBC | 1.6 (0.8–3.2) | 0.20 | - | - |
| Vascular invasion (yes vs. no) | 1.7 (0.6–4.4) | 0.26 | - | - |
| Histologic grade 3 (vs. 1–2) | 0.8 (0.4–1.6) | 0.55 | - | - |
| Ki67 ≥ 20% (vs. <20%) | 2.4 (0.7–7.7) | 0.15 | 2.8 (0.8–9.0) | 0.09 |
| TMTV > 13.5 cm3 (vs. ≤13.5 cm3) | 4.4 (2.1–9.1) | <0.01 | 4.0 (1.9–8.4) | <0.01 |
| Factor Associated with no-pCR after NACT | ||||
|---|---|---|---|---|
| n = 119 | Univariate | Multivariate | ||
| Events = 57 | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Age < 40 years (vs. ≥40) | 1.0 (0.5–2.3) | 0.99 | - | - |
| T stage 3–4 (vs. 1–2) | 4.1 (1.8–10.3) | <0.01 | 2.2 (0.8–6.0) | 0.12 |
| N+ (vs. N−) | 1.9 (0.9–4.1) | 0.07 | - | - |
| Vascular invasion (yes vs. no) | 1.7 (0.5–6.7) | 0.40 | - | - |
| Histologic grade 3 (vs. 1–2) | 0.5 (0.2–1.5) | 0.30 | - | - |
| Ki67 ≥ 25% (vs. <25%) | 0.3 (0.02–2.8) | 0.40 | - | - |
| TMTV > 9.0 cm3 (vs. ≤9.0 cm3) | 4.9 (2.3–11.0) | <0.01 | 3.6 (1.5–8.6) | <0.01 |
| 3-Year Recurrence-Free Survival | ||||
|---|---|---|---|---|
| n = 119 | Univariate | Multivariate | ||
| Events = 30 | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age < 40 years (vs. ≥40) | 1.5 (0.5–4.1) | 0.42 | - | - |
| pCR | 0.3 (0.1–0.8) | 0.01 | 0.4 (0.1–1.1) | 0.07 |
| T stage 3–4 (vs. 1–2) | 1.4 (0.6–3.4) | 0.41 | - | - |
| N+ (vs. N−) | 2.9 (1.2–7.3) | 0.02 | - | - |
| Vascular invasion (yes vs. no) | 1.8 (0.5–6.0) | 0.36 | - | - |
| Histologic grade 3 (vs. 1–2) | 0.5 (0.2–1.5) | 0.22 | - | - |
| Ki67 ≥ 25% (vs. <25%) | 0.7 (0.1–5.5) | 0.77 | - | - |
| TMTV > 13.5 cm3 (vs. ≤13.5 cm3) | 4.0 (1.6–9.8) | < 0.01 | 3.1 (1.2–7.9) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najid, S.; Seban, R.-D.; Champion, L.; De Moura, A.; Sebbag, C.; Salaün, H.; Cabel, L.; Bonneau, C. Clinical Utility of Pre-Therapeutic [18F]FDG PET/CT Imaging for Predicting Outcomes in Breast Cancer. J. Clin. Med. 2023, 12, 5487. https://doi.org/10.3390/jcm12175487
Najid S, Seban R-D, Champion L, De Moura A, Sebbag C, Salaün H, Cabel L, Bonneau C. Clinical Utility of Pre-Therapeutic [18F]FDG PET/CT Imaging for Predicting Outcomes in Breast Cancer. Journal of Clinical Medicine. 2023; 12(17):5487. https://doi.org/10.3390/jcm12175487
Chicago/Turabian StyleNajid, Sophia, Romain-David Seban, Laurence Champion, Alexandre De Moura, Clara Sebbag, Hélène Salaün, Luc Cabel, and Claire Bonneau. 2023. "Clinical Utility of Pre-Therapeutic [18F]FDG PET/CT Imaging for Predicting Outcomes in Breast Cancer" Journal of Clinical Medicine 12, no. 17: 5487. https://doi.org/10.3390/jcm12175487
APA StyleNajid, S., Seban, R.-D., Champion, L., De Moura, A., Sebbag, C., Salaün, H., Cabel, L., & Bonneau, C. (2023). Clinical Utility of Pre-Therapeutic [18F]FDG PET/CT Imaging for Predicting Outcomes in Breast Cancer. Journal of Clinical Medicine, 12(17), 5487. https://doi.org/10.3390/jcm12175487






