The Prognostic Nutritional Index (PNI): A New Biomarker for Determining Prognosis in Metastatic Castration-Sensitive Prostate Carcinoma
Abstract
:1. Introduction
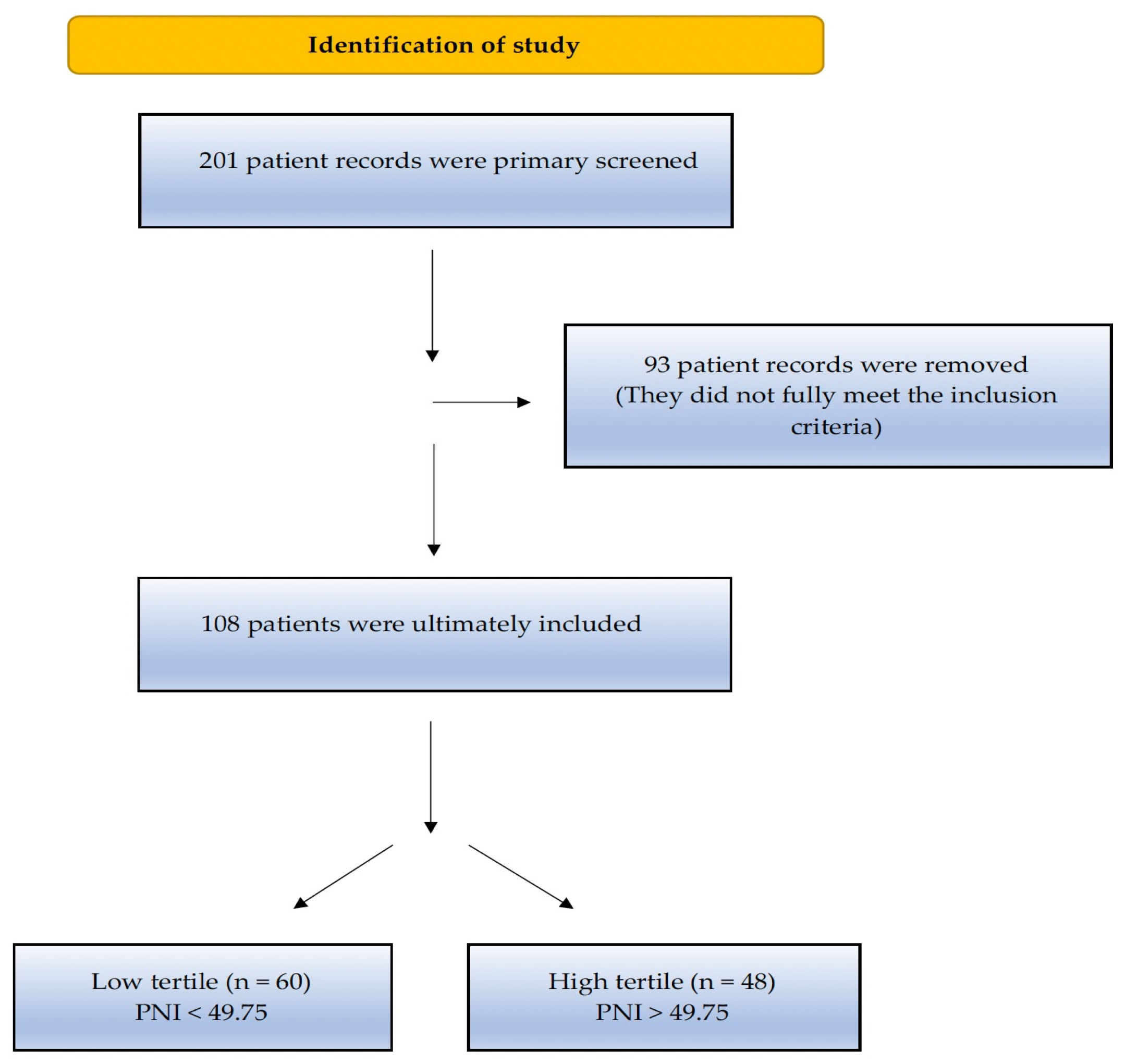
2. Material and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Shore, N.D.; Antonarakis, E.S.; Cookson, M.S.; Crawford, E.D.; Morgans, A.K.; Albala, D.M.; Hafron, J.; Harris, R.G.; Saltzstein, D.; Brown, G.A.; et al. Optimizing the role of androgen deprivation therapy in advanced prostate cancer: Challenges beyond the guidelines. Prostate 2020, 80, 527–544. [Google Scholar] [CrossRef]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Aly, M.; Leval, A.; Schain, F.; Liwing, J.; Lawson, J.; Vágó, E.; Nordström, T.; Andersson, T.M.-L.; Sjöland, E.; Wang, C.; et al. Survival in patients diagnosed with castration-resistant prostate cancer: A population-based observational study in Sweden. Scand. J. Urol. 2020, 54, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Francini, E.; Gray, K.P.; Xie, W.; Shaw, G.K.; Valença, L.; Bernard, B.; Albiges, L.; Harshman, L.C.; Kantoff, P.W.; Taplin, M.-E.; et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC). Prostate 2018, 78, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Lucarelli, G.; Crocetto, F.; Dolce, P.; Verde, A.; La Civita, E.; Zappavigna, S.; de Cobelli, O.; Di Lorenzo, G.; Facchini, B.A.; et al. First-line systemic therapy for metastatic castration-sensitive prostate cancer: An updated systematic review with novel findings. Crit. Rev. Oncol. Hematol. 2021, 157, 103198. [Google Scholar] [CrossRef] [PubMed]
- Semiz, H.S.; Keskinkılıç, M.; Ellez, H.I.; Arayıcı, M.E.; Karaoglu, A. Approach to the Therapy of Metastatic Castration-Sensitive Prostate Carcinoma: A Single Center Experience. J. Basic Clin. Health Sci. 2022, 6, 296–304. [Google Scholar] [CrossRef]
- Tan, C.S.; Read, J.A.; Phan, V.H.; Beale, P.J.; Peat, J.K.; Clarke, S.J. The relationship between nutritional status, inflammatory markers and survival in patients with advanced cancer: A prospective cohort study. Support. Care Cancer 2015, 23, 385–391. [Google Scholar] [CrossRef]
- Yapar Taskoylu, B.; Avci, E.; Degirmencioglu, S.; Yaren, A.; Ergin, A.; Kilic, D.; Karan, C.; Cakan, B.; Doğan, T.; Ozdemir, M. Relationship between neutrophil/lymphocyte, platelet/lymphocyte, CRP/Albumin ratio and survival in ovarian cancer. Pam. Med. J. 2021, 14, 666–674. [Google Scholar]
- Shu, W.; Tao, W.; Chunyan, H.; Jie, F.; Yuan, L.; Yan, X.; Huan, Z.; Liang, X. Preoperative nutritional evaluation of prostate cancer patients undergoing laparoscopic radical prostatectomy. PLoS ONE 2022, 17, e0262630. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkaishi Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Yan, L.; Nakamura, T.; Casadei-Gardini, A.; Bruixola, G.; Huang, Y.L.; Hu, Z.D. Long-term and short-term prognostic value of the prognostic nutritional index in cancer: A narrative review. Ann. Transl. Med. 2021, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, X.; Chi, C.; Wang, Y.; Cai, W.; Shao, X.; Xu, F.; Pan, J.; Zhu, Y.; Shangguan, X.; et al. Prognostic nutritional index predicts initial response to treatment and prognosis in metastatic castration-resistant prostate cancer patients treated with abiraterone. Prostate 2017, 77, 1233–1241. [Google Scholar] [CrossRef]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, M.D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Velho, P.I.; Bastos, D.A.; Antonarakis, E.S. New approaches to targeting the androgen receptor pathway in prostate cancer. Clin. Adv. Hematol. Oncol. 2021, 19, 228–240. [Google Scholar] [PubMed]
- Li, B.; Lu, Z.; Wang, S.; Hou, J.; Xia, G.; Li, H.; Yin, B.; Lu, W. Pretreatment elevated prognostic nutritional index predicts a favorable prognosis in patients with prostate cancer. BMC Cancer 2020, 20, 361. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Mohri, Y.; Inoue, Y.; Tanaka, K.; Hiro, J.; Uchida, K.; Kusunoki, M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J. Surg. 2013, 37, 2688–2692. [Google Scholar] [CrossRef]
- Kobayashi, H.; Shiota, M.; Sato, N.; Kobayashi, S.; Matsumoto, T.; Monji, K.; Kashiwagi, E.; Takeuchi, A.; Inokuchi, J.; Shiga, K.-I.; et al. Differential prognostic impact of complete blood count-related parameters by prior use of novel androgen receptor pathway inhibitors in docetaxel-treated castration-resistant prostate cancer patients. Anticancer Drugs 2022, 33, e541–e547. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [PubMed]
- Baron, A.; Mancini, M.; Caldwell, E.; Cabrelle, A.; Bernardi, P.; Pagano, F. Serenoa repens extract targets mitochondria and activates the intrinsic apoptotic pathway in human prostate cancer cells. BJU Int. 2009, 103, 1275–1283. [Google Scholar] [CrossRef]
- Kawahara, T.; Yoneyama, S.; Ohno, Y.; Hashimoto, J.I.Y.; Tsumura, H.; Tabata, K.-I.; Nakagami, Y.; Tanabe, K.; Iwamura, M.; Uemura, H.; et al. Prognostic Value of the LATITUDE and CHAARTED Risk Criteria for Predicting the Survival of Men with Bone Metastatic Hormone-Naïve Prostate Cancer Treated with Combined Androgen Blockade Therapy: Real-World Data from a Japanese Multi-Institutional Study. BioMed Res. Int. 2020, 2020, 7804932. [Google Scholar] [CrossRef]
- de Bono, J.S.; Smith, M.R.; Saad, F.; Rathkopf, D.E.; Mulders, P.F.A.; Small, E.J.; Shore, N.D.; Fizazi, K.; De Porre, P.; Kheoh, T.; et al. Subsequent Chemotherapy and Treatment Patterns After Abiraterone Acetate in Patients with Metastatic Castration-resistant Prostate Cancer: Post Hoc Analysis of COU-AA-302. Eur. Urol. 2017, 71, 656–664. [Google Scholar] [CrossRef]









| Variables | Total (n = 108) |
|---|---|
| Age group, n (%) | |
| <70 | 63 (58.3%) |
| ≥70 | 45 (41.7%) |
| Survival Status, n (%) | |
| Alive | 45 (41.7%) |
| Ex | 63 (58.3%) |
| Received chemotherapy in the castration-sensitive stage, n (%) | |
| Yes | 48 (44.4%) |
| No | 60 (55.6%) |
| Disease severity grade (ISUP) groups, n (%) | |
| 1 | 1 (0.9%) |
| 2 | 12 (11.1%) |
| 3 | 16 (14.8%) |
| 4 | 24 (22.2%) |
| 5 | 49 (45.4%) |
| History of primary surgery, n (%) | |
| None | 93 (86.1%) |
| Radical | 15 (13.9%) |
| CHAARTED criteria groups, n (%) | |
| Low-volume disease | 31 (28.7%) |
| High-volume disease | 77 (71.3%) |
| LATITUDE criteria groups, n (%) | |
| Low risk | 51 (47.2%) |
| High risk | 57 (52.8%) |
| PNI groups, n (%) | |
| <49.75 | 60 (55.6%) |
| >49.75 | 48 (44.4%) |
| PNI Groups | p Value * | ||||
|---|---|---|---|---|---|
| ≥49.75 | <49.75 | 1.000 | |||
| Age groups | n | % | n | % | |
| <70 | 28 | 44.4 | 35 | 55.6 | |
| ≥70 | 20 | 44.4 | 25 | 55.6 | |
| PNI Groups | p value ** | ||||
| ≥49.75 | <49.75 | 0.127 | |||
| PSA (median, percentiles (25, 75), μg/L) | 99.42 (20.49–156.25) | 126.19 (56.70–154.52) | |||
| PNI Groups | p value * | ||||
| ≥49.75 | <49.75 | 0.002 | |||
| CHAARTED criteria | n | % | n | % | |
| Low volume | 21 | 67.7 | 10 | 32.3 | |
| High volume | 27 | 35.1 | 50 | 64.9 | |
| PNI Groups | p value * | ||||
| ≥49.75 | <49.75 | 0.196 | |||
| LATITUDE criteria | n | % | n | % | |
| Low risk | 26 | 51.0 | 25 | 49.0 | |
| High risk | 22 | 38.6 | 35 | 61.4 | |
| PNI Groups | p value * | ||||
| ≥49.75 | <49.75 | 0.017 | |||
| Metastasis group | n | % | n | % | |
| M1a | 11 | 73.3 | 4 | 26.7 | |
| M1b | 32 | 43.8 | 41 | 56.2 | |
| M1c | 5 | 25.0 | 15 | 75.0 | |
| LATITUDE risk | p value * | ||||
| Low risk | High risk | <0.001 | |||
| CHAARTED volume | n | % | n | % | |
| Low volume | 30 | 96.8 | 1 | 3.2 | |
| High volume | 21 | 27.3 | 56 | 72.7 | |
| Parameter | Overall Survival (OS) | Cancer Specific Survival (CSS) | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (years) | 1.020 (0.988–1.053) | 0.223 | 1.019 (0.988–1.050) | 0.226 |
| PSA (μg/L) | 1.000 (1.000–1.001) | 0.555 | 1.001 (1.00–1.001) | 0.847 |
| PNI | 0.018 | 0.001 | ||
| >49.75 | 1 | 1 | ||
| <49.75 | 1.893 (1.117–3.208) | 2.460 (1.440–4.202) | ||
| ISUP grade group | 0.006 | 0.102 | ||
| 1–3 | 1 | 1 | ||
| 4–5 | 2.467 (1.304–4.668) | 1.648 (0.906–2.998) | ||
| CHAARTED | <0.001 | <0.001 | ||
| Low volume | 1 | 1 | ||
| High volume | 4.249 (2.013–8.965) | 3.980 (1.831–8.348) | ||
| LATITUDE | <0.001 | <0.001 | ||
| Low risk | 1 | 1 | ||
| High risk | 3.322 (1.926–5.731) | 2.921 (1.718–4.966) | ||
| Parameter | Overall Survival (OS) | Cancer Specific Survival (CSS) | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (years) | 1.040 (1.001–1.080) | 0.042 | 1.040 (1.003–1.078) | 0.034 |
| PSA (μg/L) | 1.000 (1.000–1.001) | 0.781 | 1.001 (1.00–1.001) | 0.835 |
| PNI | 0.005 | <0.001 | ||
| >48.9 | 1 | 1 | ||
| <48.9 | 2.280 (1.285–4.046) | 3.011 (1.664–5.447) | ||
| ISUP grade group | 0.001 | 0.025 | ||
| 1–3 | 1 | 1 | ||
| 4–5 | 2.863 (1.501–5.459) | 2.014 (1.093–3.711) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellez, H.I.; Keskinkilic, M.; Semiz, H.S.; Arayici, M.E.; Kısa, E.; Oztop, I. The Prognostic Nutritional Index (PNI): A New Biomarker for Determining Prognosis in Metastatic Castration-Sensitive Prostate Carcinoma. J. Clin. Med. 2023, 12, 5434. https://doi.org/10.3390/jcm12175434
Ellez HI, Keskinkilic M, Semiz HS, Arayici ME, Kısa E, Oztop I. The Prognostic Nutritional Index (PNI): A New Biomarker for Determining Prognosis in Metastatic Castration-Sensitive Prostate Carcinoma. Journal of Clinical Medicine. 2023; 12(17):5434. https://doi.org/10.3390/jcm12175434
Chicago/Turabian StyleEllez, Halil Ibrahim, Merve Keskinkilic, Hüseyin Salih Semiz, Mehmet Emin Arayici, Erdem Kısa, and Ilhan Oztop. 2023. "The Prognostic Nutritional Index (PNI): A New Biomarker for Determining Prognosis in Metastatic Castration-Sensitive Prostate Carcinoma" Journal of Clinical Medicine 12, no. 17: 5434. https://doi.org/10.3390/jcm12175434
APA StyleEllez, H. I., Keskinkilic, M., Semiz, H. S., Arayici, M. E., Kısa, E., & Oztop, I. (2023). The Prognostic Nutritional Index (PNI): A New Biomarker for Determining Prognosis in Metastatic Castration-Sensitive Prostate Carcinoma. Journal of Clinical Medicine, 12(17), 5434. https://doi.org/10.3390/jcm12175434







