Immunohistochemical Expression of Upregulated Gene 4 Protein Expression (URG4/URGCP) and Its Association with 5-Year Survival in Patients with Colon Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tumour Samples
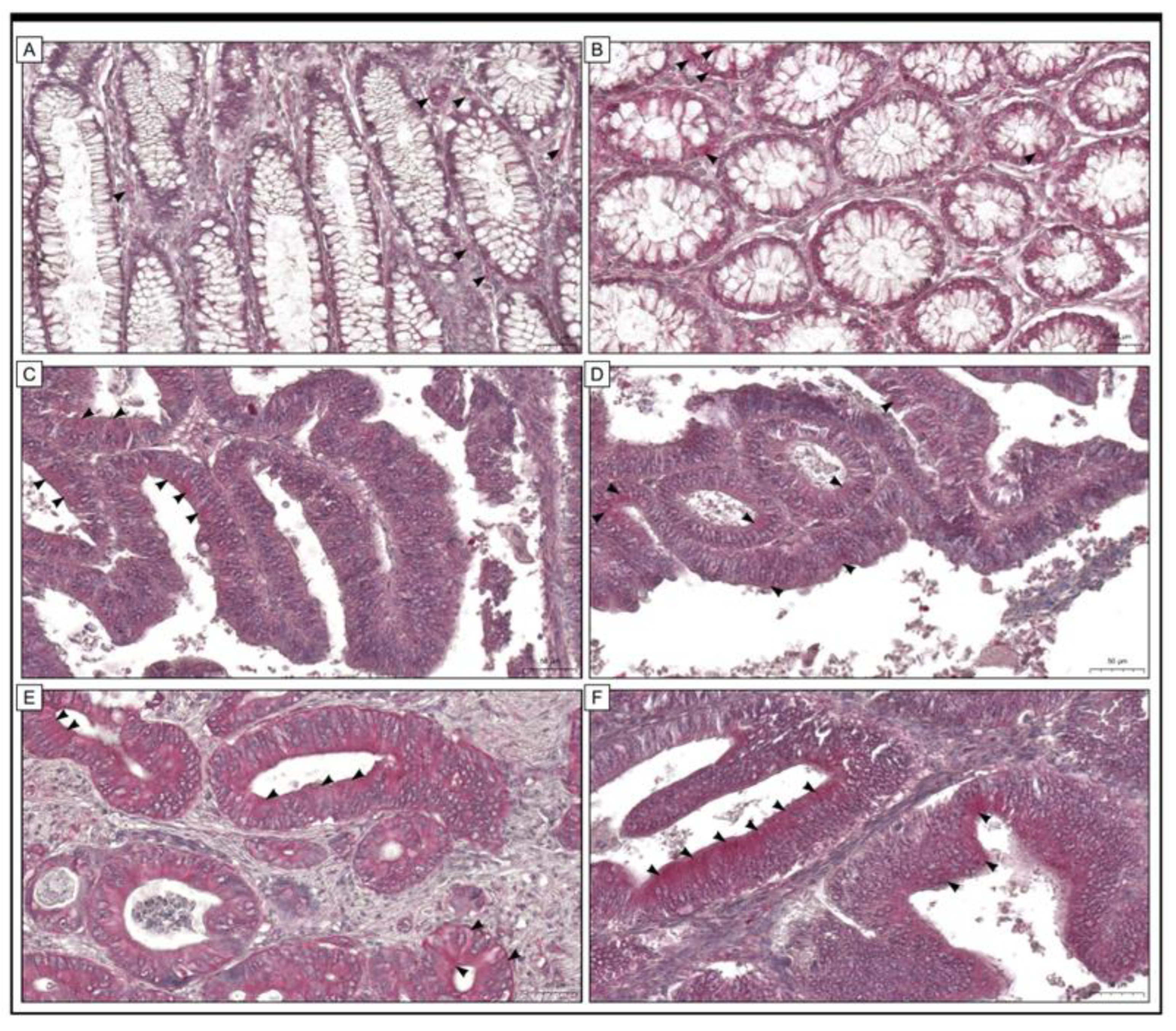
2.2. Immunohistochemical Staining
2.3. Statistical Analysis
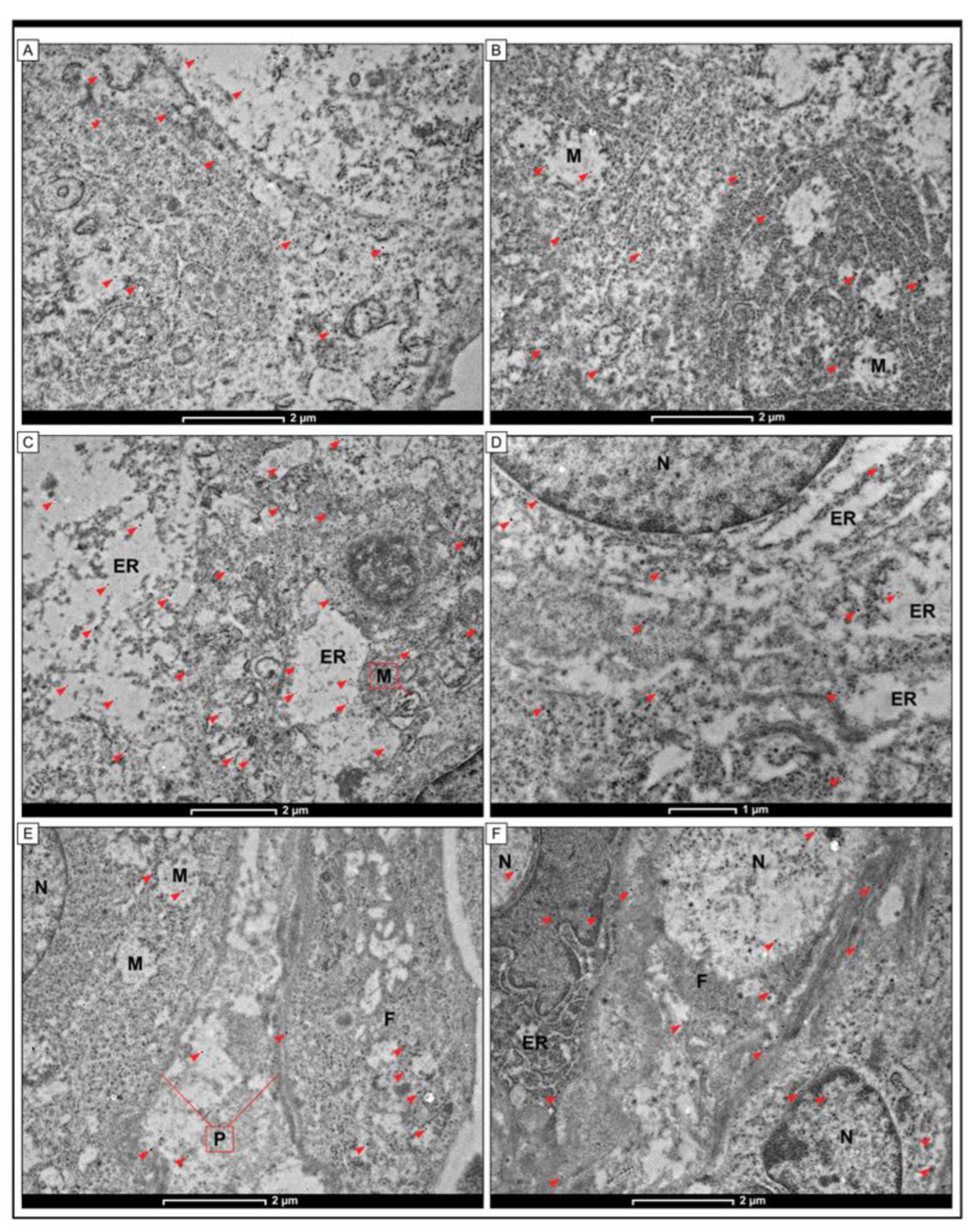
2.4. Immunogold Electron Microscopy
3. Results
3.1. Characteristics of the Patients
3.2. The Relationship between the Immunohistochemical Expression of URG4 and the Most Important Clinical Features of the Patients
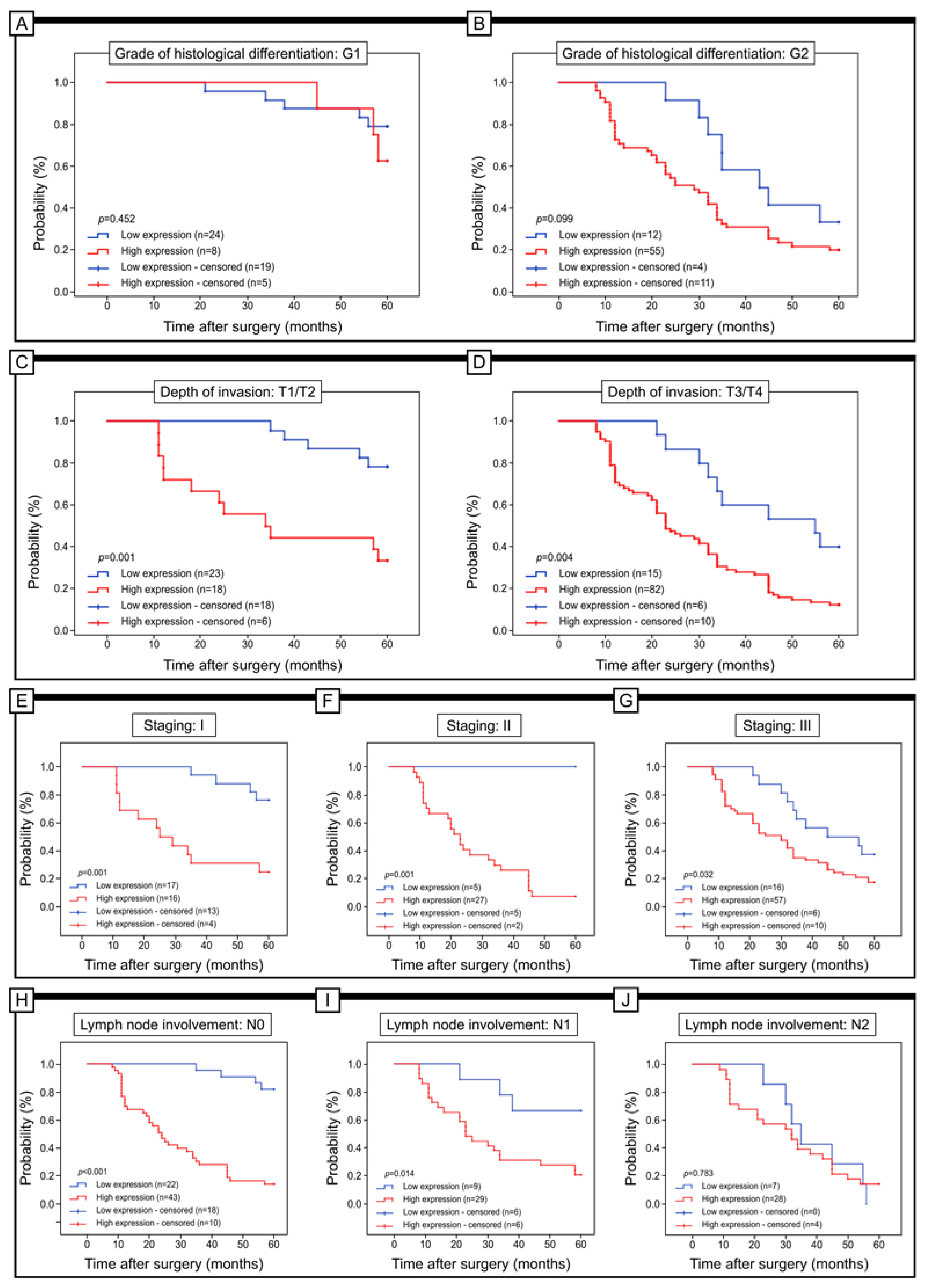
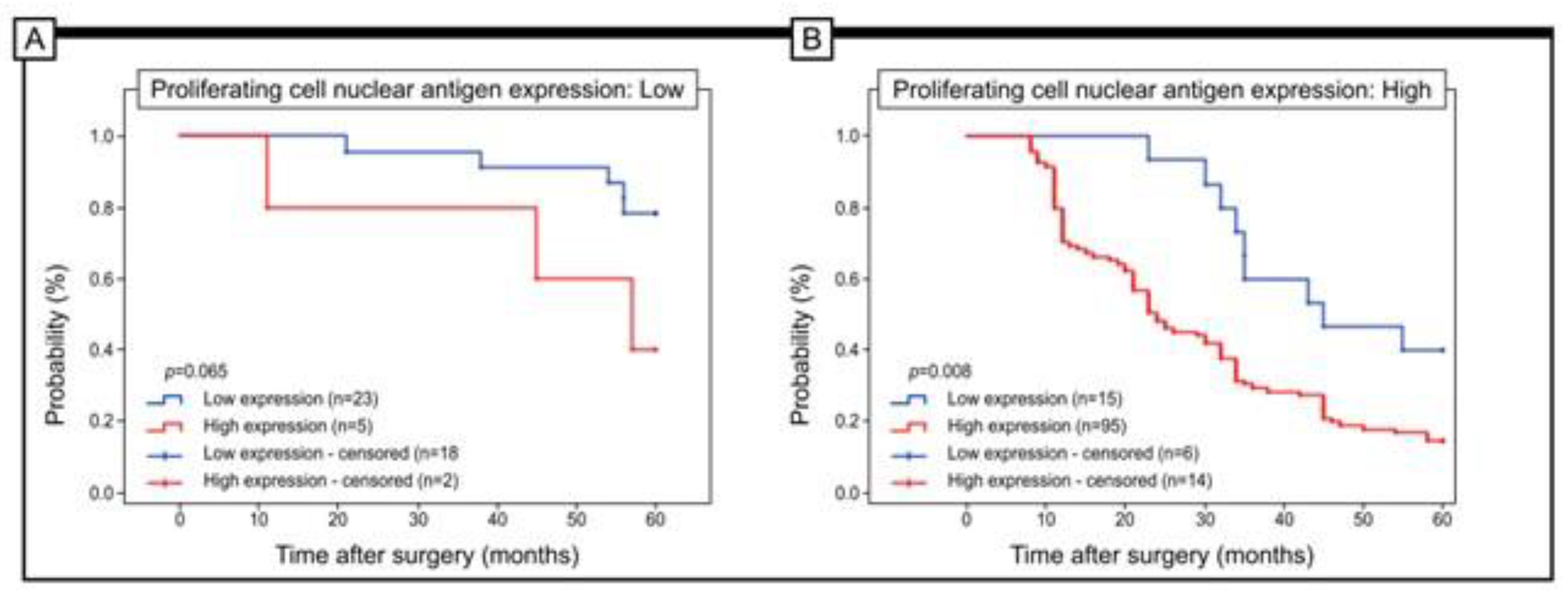
3.3. The Prognostic Role of URG4 Expression in Association with 5-Year Survival
3.4. Detection of URG4 at the Cellular Level by the Use of TEM
4. Discussion
5. Conclusions and Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Douaiher, J.; Ravipati, A.; Grams, B.; Chowdhury, S.; Alatise, O.; Are, C. Colorectal cancer-global burden, trends, and geographical variations. J. Surg. Oncol. 2017, 115, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer. J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.; Lok, V.; Wang, J.; Fung, F.; Ding, H.; Zheng, Z.J. Differences in Incidence and Mortality Trends of Colorectal Cancer Worldwide Based on Sex, Age, and Anatomic Location. Clin. Gastroenterol. Hepatol. 2021, 19, 955–966.e61. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal carcinoma: Pathologic aspects. J. Gastrointest. Oncol. 2012, 3, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.; Chung, L.; Wilkinson, K.; Lea, V.; Lim, S.H.; Abubakar, A.; Ng, W.; Lee, M.; Roberts, T.L.; Chua, W.; et al. Prognostic Significance of MRE11 Overexpression in Colorectal Cancer Patients. Cancers 2023, 15, 2438. [Google Scholar] [CrossRef]
- Dayde, D.; Tanaka, I.; Jain, R.; Tai, M.C.; Taguchi, A. Predictive and Prognostic Molecular Biomarkers for Response to Neoadjuvant Chemoradiation in Rectal Cancer. Int. J. Mol. Sci. 2017, 18, 573. [Google Scholar] [CrossRef]
- Carpinetti, P.; Donnard, E.; Bettoni, F.; Asprino, P.; Koyama, F.; Rozanski, A.; Sabbaga, J.; Habr-Gama, A.; Parmigiani, R.B.; Galante, P.A.; et al. The use of personalized biomarkers and liquid biopsies to monitor treatment response and disease recurrence in locally advanced rectal cancer after neoadjuvant chemoradiation. Oncotarget 2015, 6, 38360–38371. [Google Scholar] [CrossRef]
- Tufan, N.L.; Lian, Z.; Liu, J.; Pan, J.; Arbuthnot, P.; Kew, M.; Clayton, M.M.; Zhu, M.; Feitelson, M.A. Hepatitis Bx antigen stimulates expression of a novel cellular gene, URG4, that promotes hepatocellular growth and survival. Neoplasia 2002, 4, 355–368. [Google Scholar] [CrossRef]
- Li, W.; Zhou, N. URG4 upregulation is associated with tumor growth and poor survival in epithelial ovarian cancer. Arch. Gynecol. Obstet. 2012, 286, 209–215. [Google Scholar] [CrossRef]
- Song, J.; Xie, H.; Lian, Z.; Yang, G.; Du, R.; Du, Y.; Zou, X.; Jin, H.; Gao, J.; Liu, J.; et al. Enhanced cell survival of gastric cancer cells by a novel gene URG4. Neoplasia 2006, 8, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, J.; Wang, Y.; Hu, J.; Liu, C.; Feng, C.; Zeng, X. URGCP/URG4 promotes apoptotic resistance in bladder cancer cells by activating NF-κB signaling. Oncotarget 2015, 6, 30887–30901. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, L.C.; Zhang, H.Y.; Qin, Z.Y.; Wang, Y.; Mao, Y.; Yao, Y.; Zhou, L.F. Serological identification of URGCP as a potential biomarker for glioma. CNS Neurosci. Ther. 2014, 20, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, R.; Xu, X.; Zhang, L.; Wu, S.; Yang, T.; Fang, L.; Wu, J.; Zhu, X.; Li, M.; et al. URGCP promotes non-small cell lung cancer invasiveness by activating the NF-κB-MMP-9 pathway. Oncotarget 2015, 6, 36489–36504. [Google Scholar] [CrossRef][Green Version]
- Dodurga, Y.; Eroğlu, C.; Seçme, M.; Elmas, L.; Avcı, Ç.B.; Şatıroğlu-Tufan, N.L. Anti-proliferative and anti-invasive effects of ferulic acid in TT medullary thyroid cancer cells interacting with URG4/URGCP. Tumour Biol. 2016, 37, 1933–1940. [Google Scholar] [CrossRef]
- Dodurga, Y.; Avcı, C.B.; Susluer, S.Y.; Satıroğlu Tufan, N.L.; Gündüz, C. The expression of URGCP gene in prostate cancer cell lines: Correlation with rapamycin. Mol. Biol. Rep. 2012, 39, 10173–10177. [Google Scholar] [CrossRef]
- Dodurga, Y.; Oymak, Y.; Gündüz, C.; Satıroglu-Tufan, N.L.; Vergin, C.; Cetingül, N.; Biray Avci, C.; Topçuoğlu, N. Leukemogenesis as a new approach to investigate the correlation between up regulated gene 4/upregulator of cell proliferation (URG4/URGCP) and signal transduction genes in leukemia. Mol. Biol. Rep. 2013, 40, 3043–3048. [Google Scholar] [CrossRef]
- Aslan, F.; Avcıkurt, A.S. URG4 expression in invasive breast carcinoma and its relation to clinicopathological characteristics. Breast Cancer 2019, 26, 485–491. [Google Scholar] [CrossRef]
- Stoimenov, I.; Helleday, T. PCNA on the crossroad of cancer. Biochem. Soc. Trans. 2009, 37, 605–613. [Google Scholar] [CrossRef]
- Guzińska-Ustymowicz, K.; Pryczynicz, A.; Kemona, A.; Czyzewska, J. Correlation between proliferation markers: PCNA, Ki-67, MCM-2 and antiapoptotic protein Bcl-2 in colorectal cancer. Anticancer Res. 2009, 29, 3049–3052. [Google Scholar] [PubMed]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Wang, Y.; Ye, L.H. Hepatitis B virus X protein accelerates the development of hepatoma. Cancer Biol. Med. 2014, 11, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xi, Y.; Chen, G.; Wu, X.; He, M. URG4 mediates cell proliferation and cell cycle in osteosarcoma via GSK3β/β-catenin/cyclin D1 signaling pathway. J. Orthop. Surg. Res. 2020, 15, 226. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Meng, Q.; Zhang, T.; Zeng, C.; He, B.; Zhang, S. URG4 expression is a novel prognostic factor for the progression of nasopharyngeal carcinoma and overall survival of patient. Onco. Targets. Ther. 2016, 9, 3059–3065. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, H.; Zhang, L.; Hou, T.; Wu, S.; Huang, Q.; Song, L.; Liu, J. URG4 overexpression is correlated with cervical cancer progression and poor prognosis in patients with early-stage cervical cancer. BMC Cancer 2014, 14, 885. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, B.; Hua, R.; Tai, W.C.; Zeng, Z.; Xie, B.; Huang, C.; Xue, J.; Xiong, S.; Yang, J.; et al. URG4/URGCP enhances the angiogenic capacity of human hepatocellular carcinoma cells in vitro via activation of the NF-κB signaling pathway. BMC Cancer 2015, 15, 368. [Google Scholar] [CrossRef]

| n (Number of Cases) | % | ||
|---|---|---|---|
| Gender | Females | 68 | 49.28 |
| Males | 70 | 50.72 | |
| Age [years] | ≤60 years | 50 | 36.23 |
| 61–75 years | 50 | 36.23 | |
| >75 years | 38 | 27.54 | |
| M ± SD | 65.12 ± 12.66 | ||
| Me (Q1–Q3) | 65 (56–77) | ||
| Min—Max | 33–89 | ||
| The degree of the histological differentiation | G1 | 32 | 23.19 |
| G2 | 67 | 48.55 | |
| G3 | 39 | 28.26 | |
| Depth of invasion | T1 | 22 | 15.94 |
| T2 | 19 | 13.77 | |
| T3 | 73 | 52.90 | |
| T4 | 24 | 17.39 | |
| Regional Lymph Node involvement | N0 | 65 | 47.10 |
| N1 | 38 | 27.54 | |
| N2 | 35 | 25.36 | |
| Location of tumour | Right part of the colon | 72 | 52.17 |
| Left part of the colon | 66 | 47.83 | |
| URG4 expression in non-cancerous tissue | Low | 124 | 89.86 |
| High | 14 | 10.14 | |
| Angioinvasion | No | 38 | 27.54 |
| Yes | 100 | 72.46 | |
| Immunohistochemical expression of proliferating cell nuclear antigen (PCNA) | Low | 28 | 20.29 |
| High | 110 | 79.71 | |
| Staging | I | 33 | 23.91 |
| II | 32 | 23.19 | |
| III | 73 | 52.90 | |
| The Immunoexpression Level of URG4 | p-Value | |||||
|---|---|---|---|---|---|---|
| Low | High | |||||
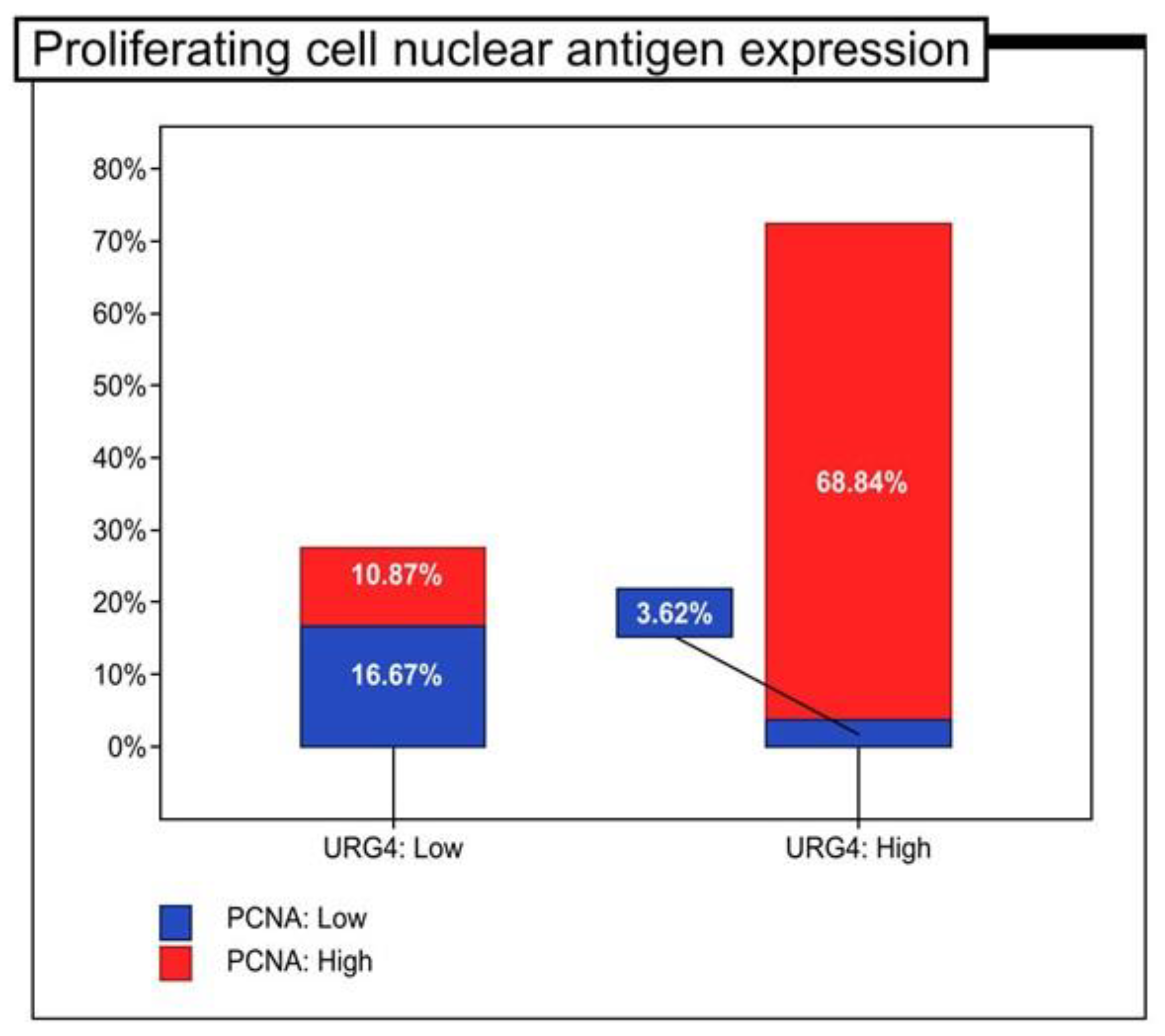
| Proliferating cell nuclear antigen expression | Low | 23 | (16.67%) | 5 | (3.62%) | p < 0.001 |
| High | 15 | (10.87%) | 95 | (68.84%) | p = 0.044 | |
| The Immunoexpression Level of URG4 | p-Value | |||||
|---|---|---|---|---|---|---|
| Low | High | |||||
| Age [Years] | ≤60 years | 15 | (30.00%) | 35 | (70.00%) | p = 0.777 |
| 61–75 years | 12 | (24.00%) | 38 | (76.00%) | ||
| >75 years | 11 | (28.95%) | 27 | (71.05%) | ||
| Gender | Females | 23 | (33.82%) | 45 | (66.18%) | p = 0.103 |
| Males | 15 | (21.43%) | 55 | (78.57%) | ||
| Grade of histological differentiation (G) | G1 | 24 | (75.00%) | 8 | (25.00%) | p < 0.001 |
| G2 | 12 | (17.91%) | 55 | (82.09%) | ||
| G3 | 2 | (5.13%) | 37 | (94.87%) | ||
| Depth of invasion (T) | T1/T2 | 23 | (56.10%) | 18 | (43.90%) | p < 0.001 |
| T3/T4 | 15 | (15.46%) | 82 | (84.54%) | ||
| Regional Lymph Node involvement | N0 | 22 | (33.85%) | 43 | (66.15%) | p = 0.276 |
| N1 | 9 | (23.68%) | 29 | (76.32%) | ||
| N2 | 7 | (20.00%) | 28 | (80.00%) | ||
| Localisation | Left part of the colon | 19 | (26.39%) | 53 | (73.61%) | p = 0.753 |
| Right part of the colon | 19 | (28.79%) | 47 | (71.21%) | ||
| Angioinvasion | Yes | 25 | (65.79%) | 13 | (34.21%) | p < 0.001 |
| No | 13 | (13.00%) | 87 | (87.00%) | ||
| Expression of proliferating cell nuclear antigen | Low | 23 | (82.14%) | 5 | (17.86%) | p < 0.001 |
| High | 15 | (13.64%) | 95 | (86.36%) | ||
| Staging of colon cancer (I, II, III) | I | 17 | (51.52%) | 16 | (48.48%) | p = 0.002 |
| II | 5 | (15.63%) | 27 | (84.38%) | ||
| III | 16 | (21.92%) | 57 | (78.08%) | ||
| Prognostic Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Gender Reference kategory: Female | 1.017 | 0.684–1.511 | 0.935 | – | – | – |
| Age | 1.004 | 0.989–1.019 | 0.633 | – | – | – |
| Staging | 1.365 | 1.066–1.749 | 0.014 | 0.756 | 0.433–1.318 | 0.324 |
| Grade of histological differentiation | 2.758 | 2.051–3.708 | <0.001 | 1.958 | 1.295–2.962 | 0.001 |
| Depth of invasion | 1.836 | 1.450–2.324 | <0.001 | 1.278 | 0.880–1.856 | 0.198 |
| Regional Lymph Node involvement | 1.270 | 1.003–1.609 | 0.047 | 1.011 | 0.673–1.520 | 0.957 |
| Localisation Reference category: Left | 1.100 | 0.740–1.636 | 0.637 | – | – | – |
| Immunohistochemical expression of URG4 in cancer tissue | 4.296 | 2.427–7.605 | <0.001 | 2.217 | 1.159–4.241 | 0.016 |
| Angioinvasion Reference category: No | 3.957 | 2.233–7.013 | <0.001 | 1.003 | 0.446–2.256 | 0.994 |
| Expression of proliferating cell nuclear antigen (PCNA) Reference category: Low | 5.392 | 2.602–11.174 | <0.001 | 1.592 | 0.534–4.743 | 0.404 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzozowa-Zasada, M.; Piecuch, A.; Michalski, M.; Stęplewska, K.; Matysiak, N.; Kucharzewski, M. Immunohistochemical Expression of Upregulated Gene 4 Protein Expression (URG4/URGCP) and Its Association with 5-Year Survival in Patients with Colon Adenocarcinoma. J. Clin. Med. 2023, 12, 5477. https://doi.org/10.3390/jcm12175477
Brzozowa-Zasada M, Piecuch A, Michalski M, Stęplewska K, Matysiak N, Kucharzewski M. Immunohistochemical Expression of Upregulated Gene 4 Protein Expression (URG4/URGCP) and Its Association with 5-Year Survival in Patients with Colon Adenocarcinoma. Journal of Clinical Medicine. 2023; 12(17):5477. https://doi.org/10.3390/jcm12175477
Chicago/Turabian StyleBrzozowa-Zasada, Marlena, Adam Piecuch, Marek Michalski, Katarzyna Stęplewska, Natalia Matysiak, and Marek Kucharzewski. 2023. "Immunohistochemical Expression of Upregulated Gene 4 Protein Expression (URG4/URGCP) and Its Association with 5-Year Survival in Patients with Colon Adenocarcinoma" Journal of Clinical Medicine 12, no. 17: 5477. https://doi.org/10.3390/jcm12175477
APA StyleBrzozowa-Zasada, M., Piecuch, A., Michalski, M., Stęplewska, K., Matysiak, N., & Kucharzewski, M. (2023). Immunohistochemical Expression of Upregulated Gene 4 Protein Expression (URG4/URGCP) and Its Association with 5-Year Survival in Patients with Colon Adenocarcinoma. Journal of Clinical Medicine, 12(17), 5477. https://doi.org/10.3390/jcm12175477





