Evolution of the Chronic Venous Leg Ulcer Microenvironment and Its Impact on Medical Devices and Wound Care Therapies
Abstract
:1. Introduction
2. Pathophysiology of Venous Leg Ulcers
2.1. Etiologic and Pathophysiologic Classification
2.2. Pathophysiological Mechanisms in CVD-VLU
3. Overview of the VLU Microenvironment
3.1. Structural Components of a Chronic VLU Wound Bed
3.1.1. Exudate
Importance and Origin of Wound Exudate
Exudate Types
3.1.2. Granulation and Epithelial Tissues
3.1.3. Devitalized Tissue (Slough, Fibrinous Tissue)
- Surgical/sharp debridement;
- Mechanical debridement (washing solutions, whirlpool therapy, wet-to-dry dressings, ultrasound-assisted debridement, and lavage);
- Enzymatic debridement (topical application of enzymes breaks down the tissue, attaching necrotic tissue to the wound bed);
- Autolytic debridement (application of dressings facilitates the development of the body’s own enzymes to rid a wound of necrotic tissue);
- Biosurgical debridement (sterile larvae).
3.2. Wound Bed Temperature
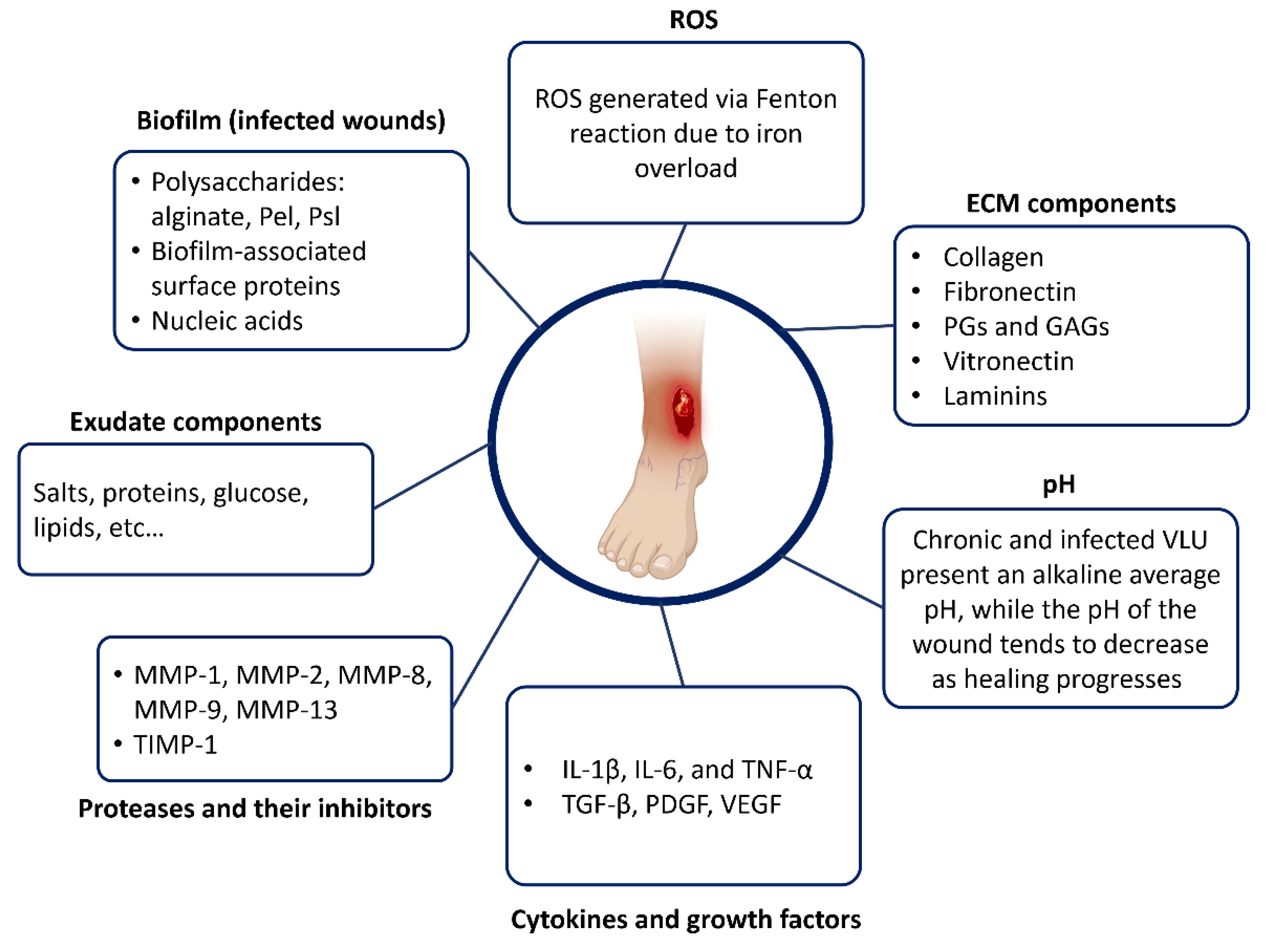
3.3. Biochemical Composition of the VLU Microenvironment
3.3.1. Wound Bed pH
3.3.2. Extracellular Matrix Components
Collagens
- -
- Type I collagen: It is a fibrillar collagen composed of two alpha-1 chains and one alpha-2 chain. It forms a triple helix structure with a one-residue stagger between adjacent chains. Type I collagen is the most abundant type and provides tensile strength to tissues such as skin, bones, tendons, and ligaments.
- -
- Type II collagen: It is also a fibrillar collagen but is composed of three identical alpha-1 chains. It forms a triple helix structure with a different stagger than type I collagen. Type II collagen is found in cartilage and provides structural support for this tissue.
- -
- Type III collagen: It is a fibrillar collagen composed of three identical alpha-1 chains. It forms a triple helix structure with a different stagger than type I or II collagens. Type III collagen is found in blood vessels and internal organs and contributes to their mechanical properties.
- -
- Type IV collagen: It is a non-fibrillar collagen that forms networks rather than fibers. It consists of three alpha-1 chains and three alpha-2 chains that form a triple helix structure with interruptions in the repeating sequence of amino acids. Type IV collagen provides structural support for basement membranes [38].
Fibronectin
Proteoglycans and Glycosaminoglycans
Vitronectin
Laminins
3.3.3. Proteases and Their Inhibitors
3.3.4. Cytokines and Growth Factors
- -
- Transforming Growth Factor-beta (TGF-β): TGF-β is a growth factor involved in inflammation, cell migration, angiogenesis, proliferation, collagen production, and differentiation. It is a key regulator of epithelial–mesenchymal transition (EMT), a process by which epithelial cells lose their cell–cell adhesion and apical–basal polarity and acquire a mesenchymal phenotype [52]. It is a key regulator of epithelial–mesenchymal transition (EMT), a process by which epithelial cells lose their cell–cell adhesion and apical–basal polarity and acquire a mesenchymal phenotype. Among the three isoforms of TGF-b (TGF-β1, TGF-β2, and TGF-β3), TGF-β2 has been shown to play a specific role in EMT in various tissues [53].
- -
- Platelet-Derived Growth Factor (PDGF): this GF triggers the formation of granulation tissue at the wound site and tissue regeneration processes including chemotaxis, fibroblast proliferation, and collagenase production [52].
- -
- Vascular Endothelial Growth Factor (VEGF): VEGF is a potent inducer of angiogenesis, promoting the formation of new blood vessels in the wound bed [54].
- -
- Interleukin-1β (IL-1 β): IL-1β is a pro-inflammatory cytokine that regulates inflammation and immune responses. IL-1β is primarily released by certain immune cells like monocytes and macrophages, as well as nonimmune cells including fibroblasts and endothelial cells, in response to cellular injury, infection, invasion, and inflammation [51].
- -
- Interleukin-4 (IL-4): IL-4 programs macrophages to down-regulate pro-inflammatory mediators and to promote wound healing processes by contributing to the production of extracellular matrix and by activating fibroblasts [55].
- -
- Interleukin-6 (IL-6): IL-6, a pro-inflammatory cytokine, is an important mediator of protein catabolism in infectious diseases such as infected VLUs [36]. IL-6 is involved in the acute phase of the response to injury or infection. It has been shown to convert naïve T-cells into CD4þ/CD25þ T Reg or IL-10-secreting CD4þ T-cells (Tr1) [55].
- -
- Interleukin-10 (IL-10): IL-10 is an immunoregulatory cytokine that plays a role in modulating the immune response to support wound healing. IL-10 is discussed in this article as an important immunoregulatory cytokine that exhibits both suppressive and stimulatory effects on the immune system. It acts in an anti-inflammatory manner on macrophages and DCs by reducing the production of pro-inflammatory cytokines and presenting antigens to T-cells [55].
- -
- Tumour Necrosis Factor-alpha (TNF-α): TNF-α is a pro-inflammatory cytokine involved in different cellular processes, including inflammation, cell proliferation, and apoptosis. It influences the early stages of wound healing and can contribute to chronic inflammation when dysregulated, which is associated with pain [51].
3.3.5. Reactive Oxygen Species (ROS) and Antioxidants
3.3.6. Wound Exudate Composition as a Function of the Physiological Condition
4. Chronic VLU Progression: Healing and Infected Stages
4.1. Overview of the Wound Healing Steps
4.1.1. Haemostasis
4.1.2. Inflammation
4.1.3. Proliferation
4.1.4. Remodelling
4.2. Infection and Its Impact on the Wound Microenvironment
4.2.1. The Pathogenesis of Wound Infection
- Contamination: this stage refers to the presence of non-replicating microorganisms on the surface of a wound. These microorganisms may be introduced during dressing changes or other procedures, but they do not necessarily cause an infection.
- Colonization: this stage refers to the presence of replicating microorganisms on the surface of a wound. These microorganisms are able to multiply and form biofilms, but they do not necessarily cause an infection.
- Local infection (covert and overt stages): this stage refers to the presence of replicating microorganisms that have caused damage to host tissues and are causing an inflammatory response. The covert stage is characterized by subtle clinical indicators that suggest an infection is present, while the overt stage is characterized by classic signs and symptoms of local wound infection, such as erythema, warmth, pain, and purulent discharge.
- Spreading infection: this stage refers to the spread of microorganisms beyond the local area of tissue damage into adjacent tissues or structures.
- Systemic infection: this stage refers to the spread of microorganisms beyond the local area of tissue damage into the bloodstream or other body systems, leading to sepsis or other systemic complications.
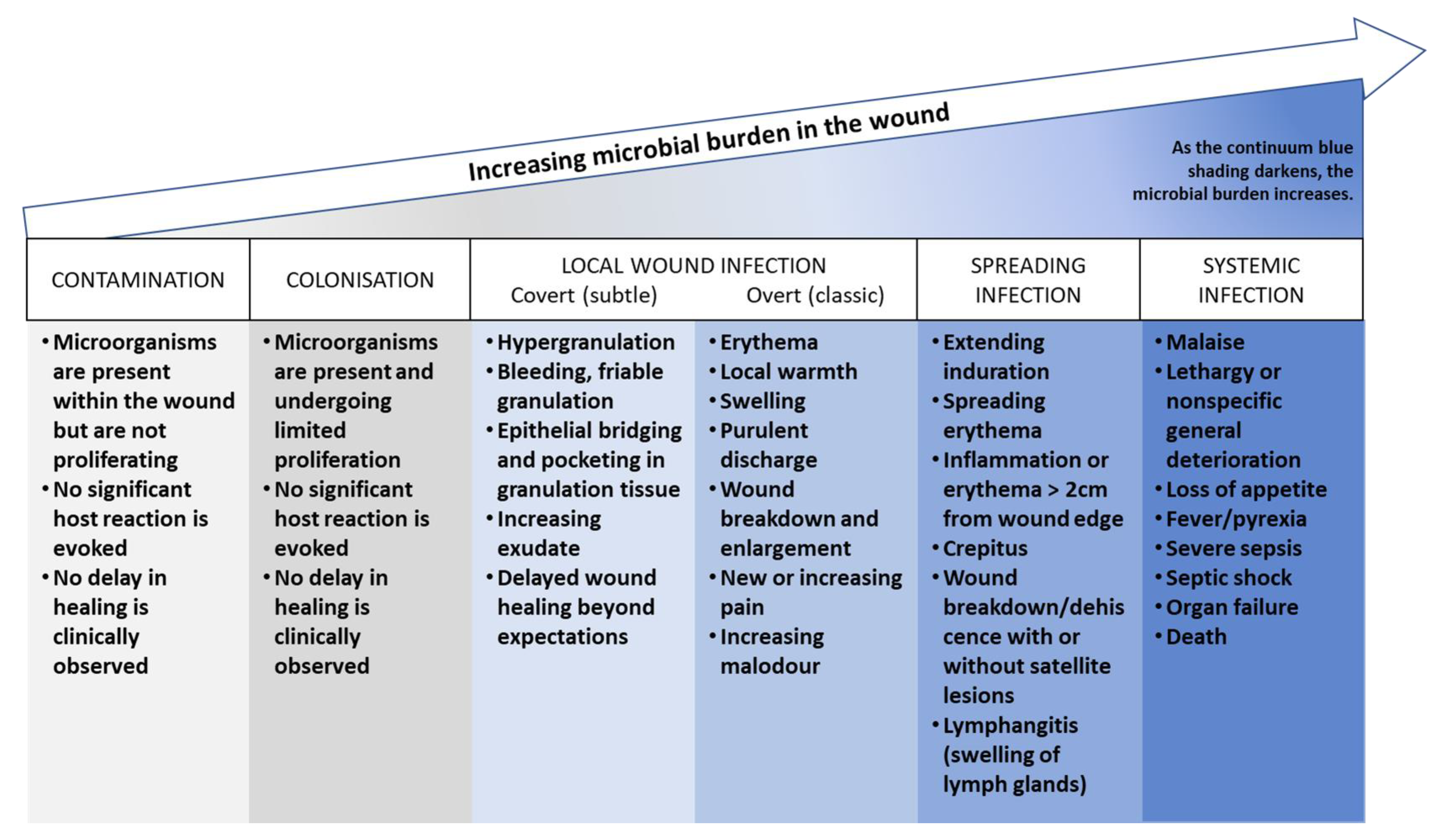
4.2.2. Biofilm Formation
4.2.3. Biofilm Biochemistry
Pseudomonas aeruginosa Biofilm
Staphylococcus aureus Biofilm
4.2.4. Impact of Infection on Exudate Characteristics
5. Impact of the Wound Microenvironment on Wound Care Products
5.1. Simulated Conditions
5.1.1. Saline SWF
5.1.2. SWF Containing 2% Bovine Serum Albumin
5.1.3. FBS-Based SWF
5.1.4. Other SWF Approaches
| SWF Composition | Applications in Wound Dressing Assessment | References |
|---|---|---|
| 2% BSA 0.02 M 0.02 M calcium chloride 0.4 M sodium chloride 0.08 M tris-aminomethane Dissolved in deionized water Adjusted pH 7.5 | Swelling studies, water absorption and equilibrium water content, evaporative water loss, and adhesion studies of a newly formulated wound dressing. Release of active molecules/drug dissolution. | [83,84,85,86,87] |
| 50% foetal calf serum and 50% maximum recovery diluent (0.1% w/v peptone [beef protein extract] and 0.9% w/v sodium chloride) | Investigation of the rate of silver release and antibacterial activity. | [67] |
| Foetal bovine serum mixed with an equal amount of peptone water | Development of a test method for measuring silver release from silver-containing dressings that are not fully saturated with test fluid. | [89] |
| Filtered foetal bovine serum | Interaction of biological fluids with materials used for wound healing applications. Changes in osmolality and total protein content were evaluated after contact with wound dressing material. | [5] |
| British Pharmacopoeia Solution A (142 mM NaCl, 2.5 mM CaCl.2H2O in distilled water) | Development of a new measurement system for real-time monitoring of moisture levels. | [90] |
| 8298 g of NaCl (142 mM) 0.368 g of CaCl2 (3.32 mM) in deionized water making up to 1 L | Evaluation of certain aspects relating to the absorbency of dressings in contact with the wound. | [81,91] |
| Foetal calf serum (70%) Lactic acid (11–12 mM) Lactoferrin (20–30 μg/mL) Fibrinogen (200–400 μg/mL) Fibronectin (30–60 μg/mL) Collagen (10–12 μg/mL) | The study of planktonic growth, biofilm features, and interspecies interactions of Staphylococcus aureus and Pseudomonas aeruginosa. | [37] |
| Sodium (3.24 g/L) Potassium (0.56 g/L) Calcium (0.05 g/L) Magnesium (0.01 g/L) Chloride (3.40 g/L) Whey protein isolate (40.06 g/L) Vegetable oil (2.32 g/L) Sugars (1.05 g/L) Simethicone (0.04 g/L) Dissolved in PBS | Development of a new in vivo test method to compare wound dressing fluid handling characteristics and wear time. | [78] |
| 0.2% w/v fatty acids, 4.0% w/v albumin, 2.5% w/v globulins 0.05% w/v triglycerides Dissolved in PBS | Absorption studies of a novel water-free hydrophilic absorbent for wound dressing application. | [88] |
5.2. Examples of Impact and Interactions
5.2.1. Responsive Dressings
5.2.2. Biomolecule-Loaded Dressings
6. Conclusions
- Developing more sensitive and specific analytical methods by resorting, for instance, to microfluidic-based approaches to improve the characterization of the content of wound exudate while reducing the amount of wound exudate required;
- Applying these methods to exudates obtained from large patient cohorts with the view of gaining a better understanding of the variability of wound exudate and its changes in composition as a function of the stage of the wound;
- Proposing enhanced approaches to better highlight the mutual interactions between the exudate and the wound care therapies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef]
- Agale, S.V. Chronic Leg Ulcers: Epidemiology, Aetiopathogenesis, and Management. Ulcers 2013, 2013, 413604. [Google Scholar] [CrossRef]
- Lal, B.K. Venous ulcers of the lower extremity: Definition, epidemiology, and economic and social burdens. Semin. Vasc. Surg. 2015, 28, 3–5. [Google Scholar] [CrossRef]
- Rani Raju, N.; Silina, E.; Stupin, V.; Manturova, N.; Chidambaram, S.B.; Achar, R.R. Multifunctional and Smart Wound Dressings—A Review on Recent Research Advancements in Skin Regenerative Medicine. Pharmaceutics 2022, 14, 1574. [Google Scholar] [CrossRef]
- Cooner, M. Biomaterial Interactions with Compromised Surfaces; Aston University: Birmingham, UK, 2014; Available online: https://research.aston.ac.uk/files/34643039/Cooner_Manpreet_K._2015_Redacted.pdf (accessed on 12 May 2023).
- Fernandez, M.L.; Upton, Z.; Edwards, H.; Finlayson, K.; Shooter, G.K. Elevated uric acid correlates with wound severity. Int. Wound J. 2012, 9, 139–149. [Google Scholar] [CrossRef]
- Fan, X.; Yang, L.; Wang, T.; Sun, T.; Lu, S. pH-responsive cellulose-based dual drug-loaded hydrogel for wound dressing. Eur. Polym. J. 2019, 121, 109290. [Google Scholar] [CrossRef]
- Walker, M.; Parsons, D. The biological fate of silver ions following the use of silver-containing wound care products—A review. Int. Wound J. 2014, 11, 496–504. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Khalil, R.A. Mechanisms of lower extremity vein dysfunction in chronic venous disease and implications in management of varicose veins. Vessel. Plus. 2021, 5, 36. [Google Scholar] [CrossRef]
- Mannello, F.; Ligi, D.; Canale, M.; Raffetto, J.D. Omics profiles in chronic venous ulcer wound fluid: Innovative applications for translational medicine. Expert Rev. Mol. Diagn. 2014, 14, 737–762. [Google Scholar] [CrossRef]
- Whittam, A.J.; Maan, Z.N.; Duscher, D.; Wong, V.W.; Barrera, J.A.; Januszyk, M.; Gurtner, G.C. Challenges and Opportunities in Drug Delivery for Wound Healing. Adv. Wound Care 2016, 5, 79–88. [Google Scholar] [CrossRef]
- Maeseneer, M.G.D.; Kakkos, S.K.; Aherne, T.; Baekgaard, N.; Black, S.; Blomgren, L.; Giannoukas, A.; Gohel, M.; de Graaf, R.; Hamel-Desnos, C.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 184–267. [Google Scholar]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; De Maeseneer, M.; et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J. Clin. Med. 2021, 10, 29. [Google Scholar] [CrossRef]
- Lyons, O.T.A.; Saha, P.; Smith, A. Redox dysregulation in the pathogenesis of chronic venous ulceration. Free Radic. Biol. Med. 2020, 149, 23–29. [Google Scholar] [CrossRef]
- Eberhardt, R.T.; Raffetto, J.D. Chronic Venous Insufficiency. Circulation 2014, 130, 333–346. [Google Scholar] [CrossRef]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef]
- World Union of Wound Healing Societies (WUWHS). Principles of Best Practice: Wound Exudate and the Role of Dressings; MEP Ltd.: London, UK, 2007. [Google Scholar]
- Spear, M. Wound Exudate—The Good, the Bad, and the Ugly. Plast. Aesthetic Nurs. 2012, 32, 77–79. [Google Scholar] [CrossRef]
- Cutting, K.F. Wound exudate: Composition and functions. Br. J. Community Nurs. 2003, 8 (Suppl. S3), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Hienne, S.; Cuny, J.F.; Callanquin, J.; Faure, P.; Labrude, P. Les Pansements des Plaies; Les Guides des Pharmathèmes; Pharmathèmes Edition—Communication Santé: Paris, France, 2008; 160p. [Google Scholar]
- Broszczak, D.A.; Sydes, E.R.; Wallace, D.; Parker, T.J. Molecular Aspects of Wound Healing and the Rise of Venous Leg Ulceration: Omics Approaches to Enhance Knowledge and Aid Diagnostic Discovery. Clin. Biochem. Rev. 2017, 38, 35–55. [Google Scholar]
- de Oliveira Gonzalez, A.C.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Kalan, L.; Schultz, G.; Malone, M.; Bjarnsholt, T.; Townsend, E.; Cheong, J.Z.A.; Gibson, A.; Radzieta, M.; Fritz, B.; Ousey, K.; et al. Slough: Composition, Analysis and Effect on Healing; Wounds International: London, UK, 2023; Available online: https://woundsinternational.com/consensus-documents/slough-composition-analysis-and-effect-on-healing/ (accessed on 18 May 2023).
- Gethin, G.; Ivory, J.D.; Sezgin, D.; Muller, H.; O’Connor, G.; Vellinga, A. What is the “normal” wound bed temperature? A scoping review and new hypothesis. Wound Repair. Regen. 2021, 29, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Dini, V.; Salvo, P.; Janowska, A.; Di Francesco, F.; Barbini, A.; Romanelli, M. Correlation Between Wound Temperature Obtained with an Infrared Camera and Clinical Wound Bed Score in Venous Leg Ulcers. Wounds Compend. Clin. Res. Pract. 2015, 27, 274–278. [Google Scholar]
- Siah, C.J.R.; Childs, C.; Chia, C.K.; Cheng, K.F.K. An observational study of temperature and thermal images of surgical wounds for detecting delayed wound healing within four days after surgery. J. Clin. Nurs. 2019, 28, 2285–2295. [Google Scholar] [CrossRef]
- Wallace, L.A.; Gwynne, L.; Jenkins, T. Challenges and opportunities of pH in chronic wounds. Ther. Deliv. 2019, 10, 719–735. [Google Scholar] [CrossRef]
- Shukla, V.K.; Shukla, D.; Tiwary, S.; Agrawal, S.; Rastogi, A. Evaluation of pH Measurement as a Method of Wound Assessment. J. Wound Care 2013. Available online: https://www.magonlinelibrary.com/doi/epdf/10.12968/jowc.2007.16.7.27062 (accessed on 15 May 2022). [CrossRef]
- Rabello Sergio, F.; Caldas Santos, N.; Andrade Silveira, I.; Barreto Pires, B.M.F.; Cavalcanti Valente, G.L.; Arneiro Teixeira, L.; Teixeira, F.L.; de Paula, G.R.; de Oliveira, B.G. Does pH Influence the Bacterial Profile of Chronic Lesions? An Analysis of Venous Ulcer Samples. Adv. Skin. Wound Care. 2022, 35, 30–36. [Google Scholar] [CrossRef]
- Senkowsky, J.; Li, S.; Nair, A.; Pal, S.; Hu, W.; Tang, L. A wound alkalinity measurement to predict non-healing wound outcomes. J. Wound Care. 2022, 31, 987–995. [Google Scholar] [CrossRef]
- Tarricone, A.; De La Mata, K.; Chen, S.; Krishnan, P.; Landau, S.; Soave, R. Relationship Between pH Shifts and Rate of Healing in Chronic Nonhealing Venous Stasis Lower-Extremity Wounds. J. Foot Ankle Surg. 2020, 59, 748–752. [Google Scholar] [CrossRef]
- Strohal, R.; Mittlböck, M.; Hämmerle, G. The Management of Critically Colonized and Locally Infected Leg Ulcers with an Acid-Oxidizing Solution: A Pilot Study. Adv. Skin. Wound Care. 2018, 31, 163–171. [Google Scholar] [CrossRef]
- Kuo, S.H.; Shen, C.J.; Shen, C.F.; Cheng, C.M. Role of pH Value in Clinically Relevant Diagnosis. Diagnostics 2020, 10, 107. [Google Scholar] [CrossRef]
- Lr, B.; Cn, M.; Rj, S.; Amb, M. The pH of wounds during healing and infection: A descriptive literature review. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2017, 25, 63–69. [Google Scholar]
- Serra, R.; Grande, R.; Buffone, G.; Molinari, V.; Perri, P.; Perri, A.; Amato, B.; Colosimo, M.; de Franciscis, S. Extracellular matrix assessment of infected chronic venous leg ulcers: Role of metalloproteinases and inflammatory cytokines. Int. Wound J. 2014, 13, 53–58. [Google Scholar] [CrossRef]
- Kadam, S.; Madhusoodhanan, V.; Dhekane, R.; Bhide, D.; Ugale, R.; Tikhole, U.; Kaushik, K.S. Milieu matters: An in vitro wound milieu to recapitulate key features of, and probe new insights into, mixed-species bacterial biofilms. Biofilm 2021, 3, 100047. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Herrick, S.E.; Ireland, G.W.; Simon, D.; McCollum, C.N.; Ferguson, M.W. Venous ulcer fibroblasts compared with normal fibroblasts show differences in collagen but not fibronectin production under both normal and hypoxic conditions. J. Invest. Dermatol. 1996, 106, 187–193. [Google Scholar] [CrossRef]
- Wysocki, A.B.; Grinnell, F. Fibronectin profiles in normal and chronic wound fluid. Lab. Investig. J. Tech. Methods Pathol. 1990, 63, 825–831. [Google Scholar]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. The Role of the Extracellular Matrix Components in Cutaneous Wound Healing. BioMed Res. Int. 2014, 2014, e747584. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care. 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.J.; Burnand, K.G.; Abisi, S.; TeKoppele, J.M.; van Els, B.; Smith, A. Effect of collagen turnover and matrix metalloproteinase activity on healing of venous leg ulcers. Br. J. Surg. 2008, 95, 319–325. [Google Scholar] [CrossRef]
- Trengove, N.J.; Langton, S.R.; Stacey, M.C. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair. Regen. 1996, 4, 234–239. [Google Scholar] [CrossRef] [PubMed]
- McQuilling, J.P.; Carter, M.J.; Fulton, J.A.; Patel, K.; Doner, B.; Serena, T.E.; Mowry, K.C. A prospective clinical trial evaluating changes in the wound microenvironment in patients with chronic venous leg ulcers treated with a hypothermically stored amniotic membrane. Int. Wound J. 2022, 19, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Ligi, D.; Mosti, G.; Croce, L.; Raffetto, J.D.; Mannello, F. Chronic venous disease—Part II: Proteolytic biomarkers in wound healing. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2016, 1862, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, K.; Pech, C.M.; Stacey, M.C.; Wallace, H.J. Induction of MMP-1, MMP-3 and TIMP-1 in normal dermal fibroblasts by chronic venous leg ulcer wound fluid*. Int. Wound J. 2008, 5, 79–86. [Google Scholar] [CrossRef]
- Amato, B.; Coretti, G.; Compagna, R.; Amato, M.; Buffone, G.; Gigliotti, D.; Grande, R.; Serra, R.; de Franciscis, S. Role of matrix metalloproteinases in non-healing venous ulcers. Int. Wound J. 2015, 12, 641–645. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Bikowska, B.; Niderla-Bielińska, J.; Jóźwiak, J.; Kamiński, A.; Skopiński, P.; Grzela, T. Potential role of metalloproteinase inhibitors from radiation-sterilized amnion dressings in the healing of venous leg ulcers. Mol. Med. Rep. 2012, 6, 723–728. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, Inflammation and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, M.H.; Phillips, S.A.; Stacey, M.C. Growth factors for treating chronic venous leg ulcers: A systematic review and meta-analysis. Wound Repair. Regen. 2022, 30, 117–125. [Google Scholar] [CrossRef]
- Cho, H.J.; Yoo, J. Rho activation is required for transforming growth factor-β-induced epithelial-mesenchymal transition in lens epithelial cells. Cell Biol. Int. 2007, 31, 1225–1230. [Google Scholar] [CrossRef]
- Lauer, G.; Sollberg, S.; Cole, M.; Krieg, T.; Eming, S.A.; Flamme, I.; Stürzebecher, J.; Mann, K. Expression and Proteolysis of Vascular Endothelial Growth Factor is Increased in Chronic Wounds. J. Investig. Dermatol. 2000, 115, 12–18. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Stechmiller, J.; Weaver, M.; Gibson, D.J.; Horgas, A.; Kelly, D.L.; Lyon, D.E. The association of wound factors and symptoms of fatigue and pain with wound healing in chronic venous leg ulcers. Int. Wound J. 2023, 20, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Stacey, M.C.; Phillips, S.A.; Farrokhyar, F.; Swaine, J.M. Evaluation of wound fluid biomarkers to determine healing in adults with venous leg ulcers: A prospective study. Wound Repair. Regen. 2019, 27, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Singh, K.; Sindrilaru, A.; Crisan, D.; Scharffetter-Kochanek, K. Iron and iron-dependent reactive oxygen species in the regulation of macrophages and fibroblasts in non-healing chronic wounds. Free Radic. Biol. Med. 2019, 133, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Tong, X.; You, S.; Mao, R.; Cai, E.; Pan, W.; Zhang, C.; Hu, R.; Shen, J. Mild Hyperthermia-Assisted ROS Scavenging Hydrogels Achieve Diabetic Wound Healing. ACS Macro Lett. 2022, 11, 861–867. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and Wounds: An Overview of the Evidence. Adv. Wound Care. 2015, 4, 373–381. [Google Scholar] [CrossRef]
- Grinnell, F.; Ho, C.H.; Wysocki, A. Degradation of fibronectin and vitronectin in chronic wound fluid: Analysis by cell blotting, immunoblotting, and cell adhesion assays. J. Investig. Dermatol. 1992, 98, 410–416. [Google Scholar] [CrossRef]
- Schmidtchen, A. Chronic Ulcers: A Method for Sampling and Analysis of Wound Fluid. Acta Derm. Venereol. 1999, 79, 291–295. [Google Scholar] [CrossRef]
- James, T.J.; Hughes, M.A.; Cherry, G.W.; Taylor, R.P. Simple biochemical markers to assess chronic wounds. Wound Repair. Regen. 2000, 8, 264–269. [Google Scholar] [CrossRef]
- Raffetto, J.D. Pathophysiology of wound healing and alterations in venous leg ulcers-review. Phlebol. J. Venous Dis. 2016, 31 (Suppl. S1), 56–62. [Google Scholar] [CrossRef]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of Wound Healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, AU, Australia, 2011. Available online: http://www.ncbi.nlm.nih.gov/books/NBK534261/ (accessed on 9 March 2023).
- Thomsen, T.R.; Aasholm, M.S.; Rudkjøbing, V.B.; Saunders, A.M.; Bjarnsholt, T.; Givskov, M.; Kirketerp-Møller, K.; Nielsen, P.H. The bacteriology of chronic venous leg ulcer examined by culture-independent molecular methods. Wound Repair. Regen. 2010, 18, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Parsons, D.; Bowler, P.G.; Myles, V.; Jones, S. Silver Antimicrobial Dressings in Wound Management: A Comparison of Antibacterial, Physical, and Chemical Characteristics. Wounds-A Compend. Clin. Res. Pract. 2005, 17, 11. [Google Scholar]
- Sopata, M.; Kucharzewski, M.; Tomaszewska, E. Antiseptic with modern wound dressings in the treatment of venous leg ulcers: Clinical and microbiological aspects. J. Wound Care. 2016, 25, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic Hydrogels with Robust Inherent Antibacterial Properties for Promoting Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces. 2022, 14, 11144–11155. [Google Scholar] [CrossRef] [PubMed]
- Canchy, L.; Kerob, D.; Demessant, A.; Amici, J.M. Wound healing and microbiome, an unexpected relationship. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 7–15. [Google Scholar] [CrossRef]
- International Wound Infection Institute (IWII). Wound Infection in Clinical Practice; Wounds International: London, UK, 2022. [Google Scholar]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Wu, Y.K.; Cheng, N.C.; Cheng, C.M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Kamerling, J.P.; Boons, G.J. Comprehensive Glycoscience: From Chemistry to Systems Biology; Elsevier: Amsterdam, The Netherlands, 2007; 818p. [Google Scholar]
- Witten, J.; Samad, T.; Ribbeck, K. Molecular Characterization of Mucus Binding. Biomacromolecules 2019, 20, 1505–1513. Available online: https://pubs.acs.org/doi/pdf/10.1021/acs.biomac.8b01467 (accessed on 6 September 2022). [CrossRef]
- Thomas, S.; Fear, M.; Humphreys, J.; Disley, L.; Waring, M.J. The Effect of Dressings on the Production of Exudate from Venous Leg Ulcers. Wounds Compend. Clin. Res. Pract. 1996, 8, 145–150. [Google Scholar]
- Lutz, J.B.; Zehrer, C.L.; Solfest, S.E.; Walters, S.A. A new in vivo test method to compare wound dressing fluid handling characteristics and wear time. Ostomy Wound Manag. 2011, 57, 28–36. [Google Scholar]
- dos Santos, S.d.L.V.; Martins, M.A.; do Prado, M.A.; Soriano, J.V.; Bachion, M.M. Are there clinical signs and symptoms of infection to indicate the presence of multidrug-resistant bacteria in venous ulcers? J. Vasc. Nurs. 2017, 35, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Règlement (UE) 2017/745 du Parlement Européen et du Conseil du 5 Avril 2017 Relatif Aux Dispositifs Médicaux, Modifiant la Directive 2001/83/CE, le Règlement (CE) n° 178/2002 et le Règlement (CE) n° 1223/2009 et Abrogeant les Directives du Conseil 90/385/CEE et 93/42/CEE (Texte Présentant de L’intérêt Pour l’EEE.). Available online: http://data.europa.eu/eli/reg/2017/745/oj/fra (accessed on 5 April 2023).
- Test Methods for Primary Wound Dressings—Part 1: Aspects of Absorbency. EN 13726-1:2002; iTeh Standards: Etobicoke, ON, Canada, 2002. Available online: https://standards.iteh.ai/catalog/standards/cen/3eb17b79-e68a-4ea2-a2a8-dd6d98145e73/en-13726-1-2002 (accessed on 2 June 2023).
- Forss, J.R. Does exudate viscosity affect its rate of absorption into wound dressings? J. Wound Care 2022, 31, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, S.; Delbono, M.; Stevenson, R.; Stephens, S.I.; Cullen, B. The Silver Release Profile of Antimicrobial Wound Dressings: Standardizing In Vitro Evaluations. Undefined. 1992. Available online: https://www.semanticscholar.org/paper/The-silver-release-profile-of-antimicrobial-wound-Lindsay-Delbono/5a4a58d296a5819b66be390d24259a9928a275f7 (accessed on 13 July 2022).
- Akiyode, O.; Boateng, J. Composite Biopolymer-Based Wafer Dressings Loaded with Microbial Biosurfactants for Potential Application in Chronic Wounds. Polymers 2018, 10, 918. [Google Scholar] [CrossRef]
- Momoh, F.U.; Boateng, J.S.; Richardson, S.C.; Chowdhry, B.Z.; Mitchell, J.C. Development and functional characterization of alginate dressing as potential protein delivery system for wound healing. Int. J. Biol. Macromol. 2015, 81, 137–150. [Google Scholar] [CrossRef]
- Pawar, H.V.; Boateng, J.S.; Ayensu, I.; Tetteh, J. Multifunctional Medicated Lyophilised Wafer Dressing for Effective Chronic Wound Healing. J. Pharm. Sci. 2014, 103, 1720–1733. [Google Scholar] [CrossRef]
- Boateng, J.S.; Pawar, H.V.; Tetteh, J. Polyox and carrageenan based composite film dressing containing anti-microbial and anti-inflammatory drugs for effective wound healing. Int. J. Pharm. 2013, 441, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Hobson, W.D.; Jones, P.D.; Duque, P.P. Wasserfreier, Hydrophiler Absorbierender Wundverband. DE 60109487T2, 8 September 2005. Available online: https://patents.google.com/patent/DE60109487T2/en?oq=Hobson+%22artificial+wound+fluid%22 (accessed on 11 May 2023).
- Kaminski, A. Determination of Silver Release from Wound Care Products. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2013. [Google Scholar]
- McColl, D.; Cartlidge, B.; Connolly, P. Real-time monitoring of moisture levels in wound dressings in vitro: An experimental study. Int. J. Surg. 2007, 5, 316–322. [Google Scholar] [CrossRef]
- Khabbaz, B.; Solouk, A.; Mirzadeh, H. Polyvinyl alcohol/soy protein isolate nanofibrous patch for wound-healing applications. Prog. Biomater. 2019, 8, 185–196. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef]
- Dumetz, A.C.; Chockla, A.M.; Kaler, E.W.; Lenhoff, A.M. Effects of pH on protein–protein interactions and implications for protein phase behavior. Biochim. Biophys. Acta BBA-Proteins Proteom. 2008, 1784, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Rui, J.Z.; Peng, H.H.; Guan, Y.X.; Yao, S.J. Preparation of ROS-responsive drug-loaded hydrogels applied in wound dressings using supercritical solvent impregnation. J. Supercrit. Fluids. 2022, 188, 105682. [Google Scholar] [CrossRef]
- Catanzano, O.; Boateng, J. Local Delivery of Growth Factors Using Wound Dressings. In Therapeutic Dressings and Wound Healing Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 291–314. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119433316.ch13 (accessed on 16 June 2023).

| Clinical classification |
| C0—No visible or palpable signs of venous disease |
| C1—Telangiectasias or reticular veins |
| C2—Varicose veins |
| C2r—Recurrent varicose veins |
| C3—Edema |
| C4—Changes in skin and subcutaneous tissue secondary to CVD |
| C4a—Pigmentation or eczema |
| C4b—Lipodermatosclerosis or atrophie blanche |
| C4c—Corona phlebectatica |
| C5—Healed |
| C6—Active venous ulcers |
| C6r—Recurrent active venous ulcer |
| Etiologic classification |
| Ep—Primary |
| Es—Secondary |
| Esi—Secondary-intravenous |
| Ese—Secondary-extravenous |
| Ec—Congenital |
| En—No cause identified |
| Anatomic classification |
| As—Superficial |
| Ad—Deep |
| Ap—Perforator |
| An—No venous anatomic location identified |
| Pathophysiologic classification * |
| Pr—Reflux |
| Po—Obstruction |
| Pr,o—Reflux and obstruction |
| Pn—No pathophysiology identifiable |
| Serous | Clear or slightly yellow fluid is typically seen in the early stages of wound healing. |
| Sanguineous | Red and contains red blood cells, indicating damage to blood vessels. |
| Serosanguineous | Consisted of a mixture of serous and sanguineous exudate. |
| Purulent | Thick and opaque with a yellow or green colour, indicating the presence of infection. |
| Unwounded Skin | Healing Wound | Chronic Wounds | Highly Infected Wounds |
|---|---|---|---|
| 4.5–6.0 [34] | 6.5–8.5 [35] | 8.64–9.86 [33] |
| MMP/TIMP | Non-Healing VLU (pg/mL) | Healing VLU (or Acute Wounds for Some Studies) (pg/mL) | References |
|---|---|---|---|
| MMP-1 | 79,460 ± 26,370 | 142,800 ± 26,370 | [47] |
| 100,000 ± 10,000 | 60,000 (acute graft) | [48] | |
| 500,000 | ~150,000 | [49] | |
| MMP-2 | 943,900 ± 119,600 | 414,700 ± 65,300 | [47] |
| 251,000 ± 101,000 | 178,000 ± 84,000 | [50] | |
| MMP-3 | 11,000 ± 3 | 8000 ± 4000 (acute graft) | [48] |
| 623.6 ± 165.8 | 3072 ± 1076 | [47] | |
| MMP-8 | 6,000,000 | 400,000 | [49] |
| MMP-9 | 483,100 ± 68,190 | 173,900 ± 47,060 | [47] |
| 293,000 ± 96,000 | 219,000 ± 113,000 | [50] | |
| MMP-10 | 4158 (2274, 6042) | 6910 (5234, 8585) | [46] |
| MMP-12 | 67,550 ± 12,350 | 22,780 ± 7478 | [47] |
| MMP-13 | 3093 ± 930.3 | 10,290 ± 3775 | [47] |
| TIMP-1 | 2500 | 29,000 ± 2000 (acute surgical wound) | [48] |
| TIMP-4 | 2.7 (0.6, 6.1) | 41.2 (19.8, 63.0) | [46] |
| Cytokines | Non-Healing VLU (pg/mL) | Healing VLU (pg/mL) |
|---|---|---|
| IL-1a | 4610 (3194, 6027) | 2355 (1782, 2928) |
| IL-1ra | 7328 (6232, 8423) | 5250 (4388, 6113) |
| IL-2 | 4.6 (2.2, 7.1) | 2.4 (1.7, 3.1) |
| IL-3 | 0.58 (0.27, 0.89) | 0.3 (0.09, 0.50) |
| IL-6 | 3550.5 (3258.4–3842.7) | 3962.7 (3702–4223.3) |
| IL-9 | 1.9 (1.4, 2.3) | 0.93 (0.66, 1.2) |
| Biochemical Component | Healing Exudate | Non-Healing Exudate |
|---|---|---|
| Serum components, i.e., Na+, K+, Cl− | Present | Present |
| Bicarbonate | Present | Lower than AWF and serum |
| Glucose | Lower than serum values | Lower than AWF and serum |
| Total protein | 44 g/L (Lower than serum values of 73 g/L) | 30 g/L Lower than AWF and serum |
| Albumin | 25 g/L (Lower than serum values of 73 g/L) | 17 g/L Lower than AWF and serum |
| C-reactive protein | Same as serum values | Raised levels—denotes inflammatory phase |
| Cytokines | Present | Increased levels (in particular IL-6 and TNF-a) |
| Matrix metalloproteinases | Present | Increased levels (in particular MMP-2 and MMP-9) |
| Tissue inhibitors of metalloproteinases | Present | Absent or low levels |
| Growth factors | Present | Degraded or completely absent |
| Vitronectin and Fibronectin | Present in their usual forms | Degraded into lower molecular-weight proteins |
| Free radicals | Low levels | Raised levels |
| Biochemical Component | Healing Exudate | Non-Healing Exudate |
|---|---|---|
| Bicarbonate (mmol/L) | 19 (16–22) | 17.5 (14–20) |
| Glucose (mmol/L) | 2 (1.1–5.9) | 1.2 (0.6–3.7) |
| Total protein (g/L) | 41 (36–51) | 34 (26–46) |
| Albumin (g/L) | 23 (18–28) | 19 (14–24) |
| C-reactive protein (mg/L) | 5 (2.5–21) | 13 (5–25) |
| Gamma globulin (g/L) | 6 (4.4–9.0) | 4.5 (3.9–6.6) |
| Cholesterol (mmol/L) | 1.8 (1.3–3.2) | 1.6 (1.2–3.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, G.A.; Secretan, P.-H.; Tortolano, L.; Charvet, L.; Yagoubi, N. Evolution of the Chronic Venous Leg Ulcer Microenvironment and Its Impact on Medical Devices and Wound Care Therapies. J. Clin. Med. 2023, 12, 5605. https://doi.org/10.3390/jcm12175605
Coelho GA, Secretan P-H, Tortolano L, Charvet L, Yagoubi N. Evolution of the Chronic Venous Leg Ulcer Microenvironment and Its Impact on Medical Devices and Wound Care Therapies. Journal of Clinical Medicine. 2023; 12(17):5605. https://doi.org/10.3390/jcm12175605
Chicago/Turabian StyleCoelho, Gisele Abreu, Philippe-Henri Secretan, Lionel Tortolano, Loïc Charvet, and Najet Yagoubi. 2023. "Evolution of the Chronic Venous Leg Ulcer Microenvironment and Its Impact on Medical Devices and Wound Care Therapies" Journal of Clinical Medicine 12, no. 17: 5605. https://doi.org/10.3390/jcm12175605
APA StyleCoelho, G. A., Secretan, P.-H., Tortolano, L., Charvet, L., & Yagoubi, N. (2023). Evolution of the Chronic Venous Leg Ulcer Microenvironment and Its Impact on Medical Devices and Wound Care Therapies. Journal of Clinical Medicine, 12(17), 5605. https://doi.org/10.3390/jcm12175605








