Three-Dimensional Transesophageal Echocardiography in Percutaneous Catheter-Based Cardiac Interventions
Abstract
1. Introduction
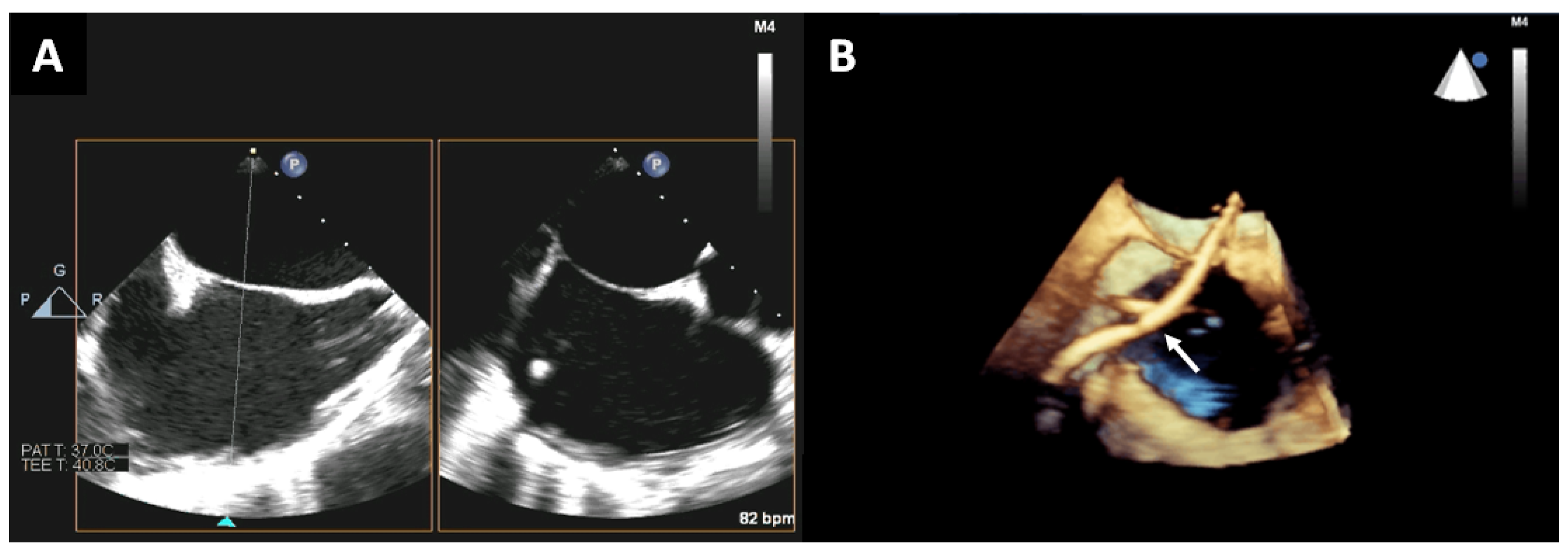
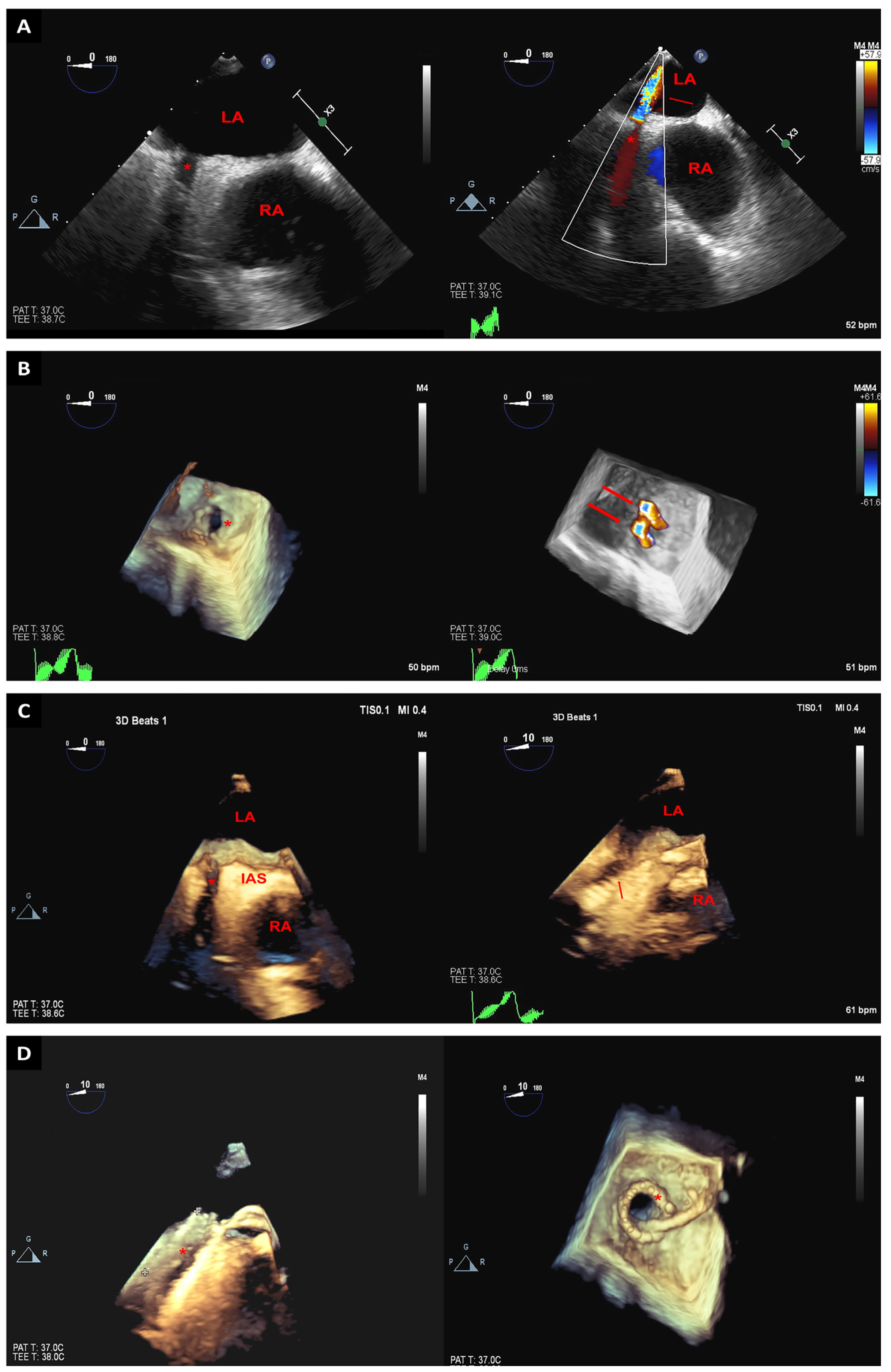
2. Trans-Septal Puncture
3. Cardiac Valve Interventions
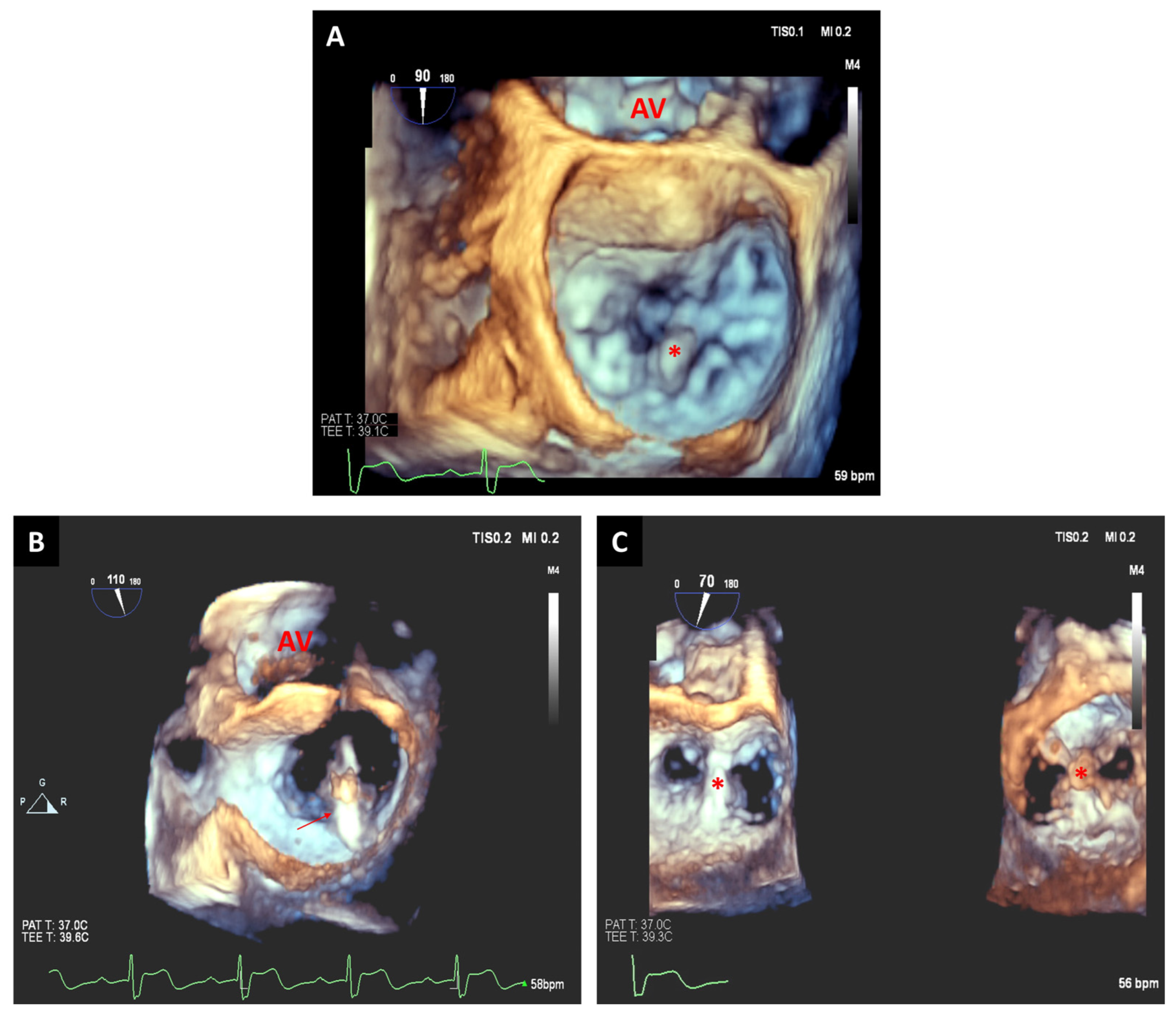
3.1. Transcatheter Aortic Valve Replacement
3.2. Mitral Valve Interventions
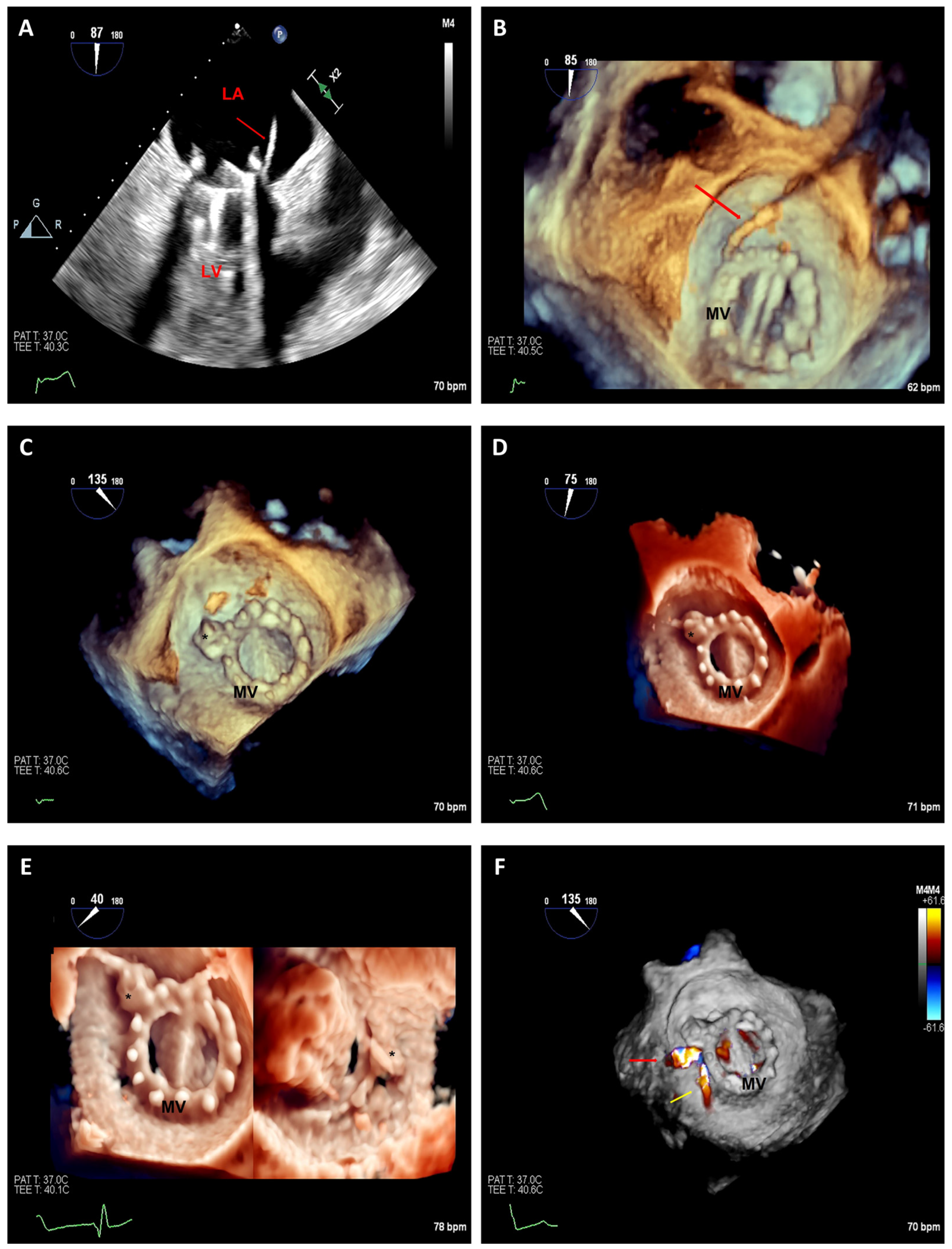
3.2.1. Edge-to-Edge Mitral Valve Repair
3.2.2. Transcatheter Mitral Valve Replacement
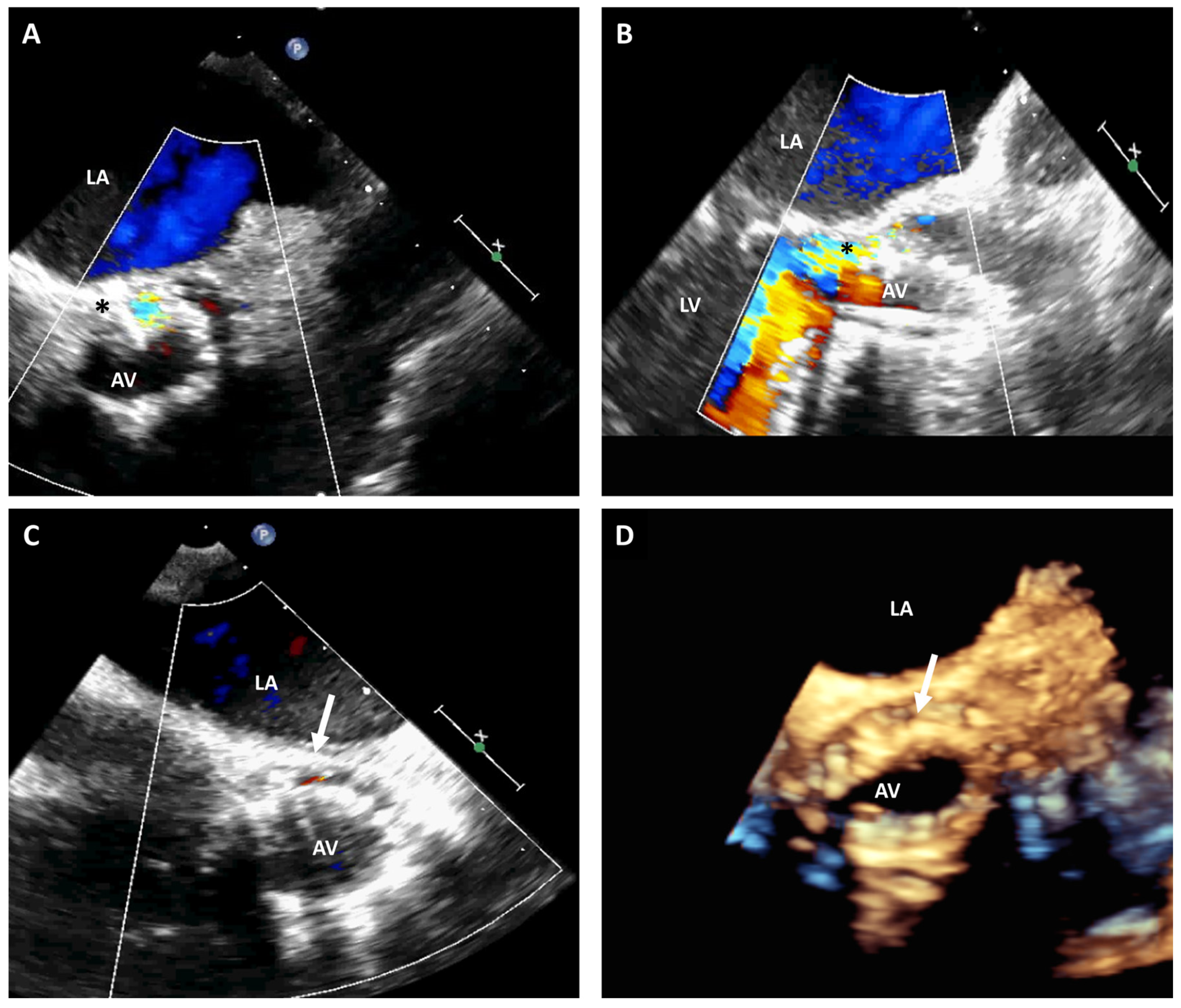
3.3. Paravalvular Leak Closure
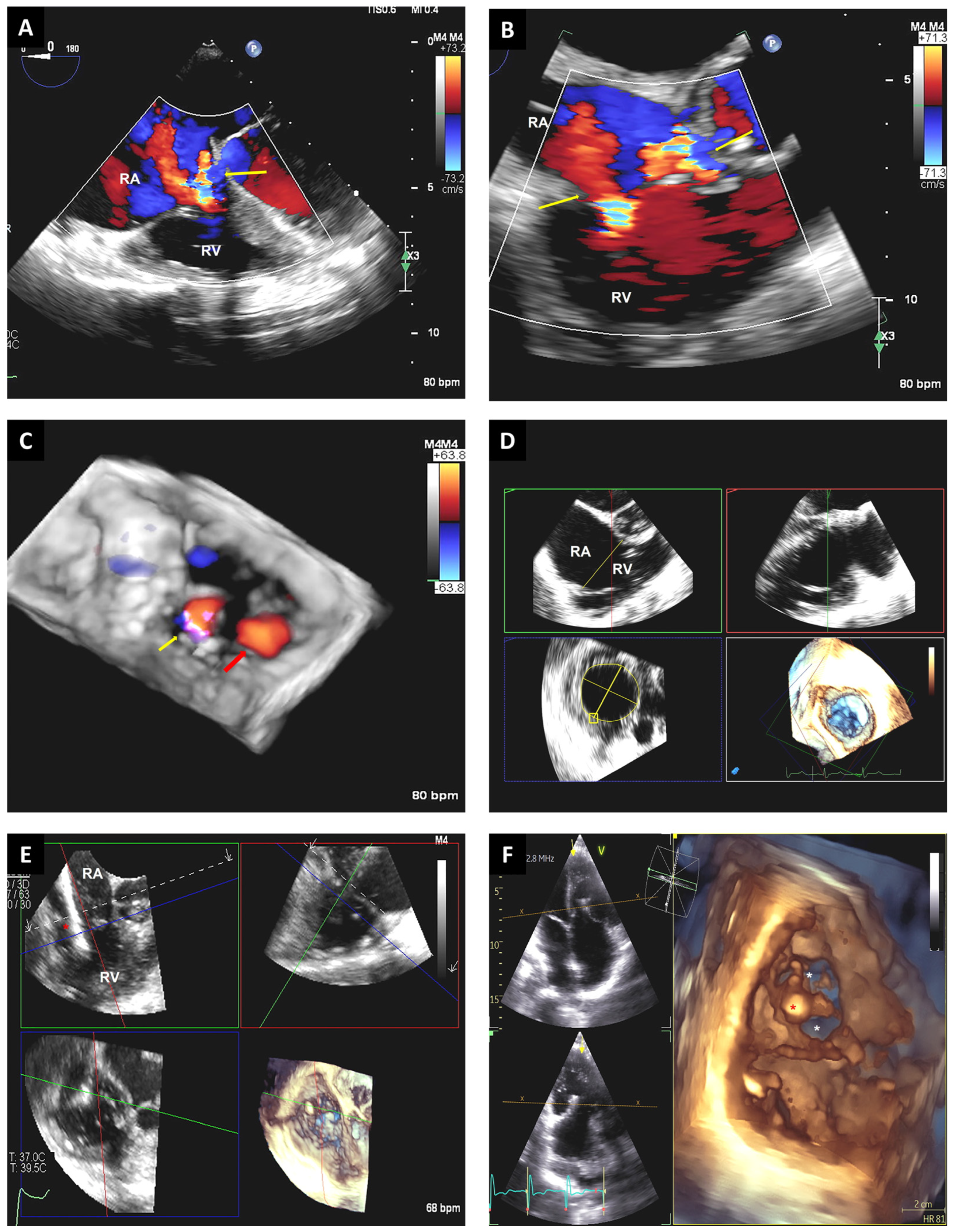
3.4. Tricuspid Valve Interventions
3.5. Left Atrial Appendage Closure
3.6. Pulmonary Veins Interventions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agricola, E.; Meucci, F.; Ancona, F.; Pardo Sanz, A.; Zamorano, J.L. Echocardiographic guidance in transcatheter structural cardiac interventions. EuroIntervention 2022, 17, 1205–1226. [Google Scholar] [CrossRef] [PubMed]
- Ro, R.; Bamira, D.; Bernard, S.; Vainrib, A.; Ibrahim, H.; Staniloae, C.; Williams, M.R.; Saric, M. Transesophageal Echocardiographic Screening for Structural Heart Interventions. Curr. Cardiol. Rep. 2023, 25, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Faletra, F.F.; Pedrazzini, G.; Pasotti, E.; Muzzarelli, S.; Dequarti, M.C.; Murzilli, R.; Schlossbauer, S.A.; Slater, I.P.; Moccetti, T. 3D TEE during catheter-based interventions. JACC Cardiovasc. Imaging 2014, 7, 292–308. [Google Scholar] [CrossRef]
- Hahn, R.T.; Abraham, T.; Adams, M.S.; Bruce, C.J.; Glas, K.E.; Lang, R.M.; Reeves, S.T.; Shanewise, J.S.; Siu, S.C.; Stewart, W.; et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: Recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J. Am. Soc. Echocardiogr. 2013, 26, 921–964. [Google Scholar] [CrossRef]
- Perk, G.; Lang, R.M.; Garcia-Fernandez, M.A.; Lodato, J.; Sugeng, L.; Lopez, J.; Knight, B.P.; Messika-Zeitoun, D.; Shah, S.; Slater, J.; et al. Use of real time three-dimensional transesophageal echocardiography in intracardiac catheter based interventions. J. Am. Soc. Echocardiogr. 2009, 22, 865–882. [Google Scholar] [CrossRef] [PubMed]
- Bruckheimer, E.; Rotschild, C.; Dagan, T.; Amir, G.; Kaufman, A.; Gelman, S.; Birk, E. Computer-generated real-time digital holography: First time use in clinical medical imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 845–849. [Google Scholar] [CrossRef]
- Hasnie, A.A.; Parcha, V.; Hawi, R.; Trump, M.; Shetty, N.S.; Ahmed, M.I.; Booker, O.J.; Arora, P.; Arora, G. Complications Associated with Transesophageal Echocardiography in Transcatheter Structural Cardiac Interventions. J. Am. Soc. Echocardiogr. 2023, 36, 381–390. [Google Scholar] [CrossRef]
- Freitas-Ferraz, A.B.; Bernier, M.; Vaillancourt, R.; Ugalde, P.A.; Nicodeme, F.; Paradis, J.M.; Champagne, J.; O’Hara, G.; Junquera, L.; Del Val, D.; et al. Safety of Transesophageal Echocardiography to Guide Structural Cardiac Interventions. J. Am. Coll. Cardiol. 2020, 75, 3164–3173. [Google Scholar] [CrossRef]
- Simard, T.; El Sabbagh, A.; Lane, C.; Killu, A.M.; Alkhouli, M.; Pollak, P.M.; Thaden, J.J.; Eleid, M.F.; Friedman, P.A.; Rihal, C.S. Anatomic Approach to Transseptal Puncture for Structural Heart Interventions. JACC Cardiovasc. Interv. 2021, 14, 1509–1522. [Google Scholar] [CrossRef]
- Sharma, T.; Krishnan, A.M.; Lahoud, R.; Polomsky, M.; Dauerman, H.L. National Trends in TAVR and SAVR for Patients with Severe Isolated Aortic Stenosis. J. Am. Coll. Cardiol. 2022, 80, 2054–2056. [Google Scholar] [CrossRef]
- Naqvi, T.Z. Echocardiography in transcatheter aortic (Core) Valve implantation: Part 2—Transesophageal echoardiography. Echocardiography 2018, 35, 1020–1041. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, C.; Monaghan, M. 3D transesophageal echocardiography in TAVR. Echocardiography 2020, 37, 1654–1664. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.Q.; Hameed, I.; Salemi, A.; Rahouma, M.; Khan, F.M.; Wijeysundera, H.C.; Angiolillo, D.J.; Shore-Lesserson, L.; Biondi-Zoccai, G.; Girardi, L.N.; et al. Three-Dimensional Echocardiography for Transcatheter Aortic Valve Replacement Sizing: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e013463. [Google Scholar] [CrossRef] [PubMed]
- Hana, D.; Miller, T.; Skaff, P.; Seetharam, K.; Suleiman, S.; Raybuck, B.; Kawsara, A.; Wei, L.; Roberts, H.; Cook, C.; et al. 3D Transesophageal Echocardiography for Guiding Transcatheter Aortic Valve Replacement without Prior Cardiac Computed Tomography in Patients with Renal Dysfunction. Cardiovasc. Revasc. Med. 2022, 41, 63–68. [Google Scholar] [CrossRef]
- Hahn, R.T.; Little, S.H.; Monaghan, M.J.; Kodali, S.K.; Williams, M.; Leon, M.B.; Gillam, L.D. Recommendations for comprehensive intraprocedural echocardiographic imaging during TAVR. JACC Cardiovasc. Imaging 2015, 8, 261–287. [Google Scholar] [CrossRef]
- Kim, M.S.; Bracken, J.; Nijhof, N.; Salcedo, E.E.; Quaife, R.A.; Messenger, J.C.; Carroll, J.D. Integrated 3D Echo-X-ray navigation to predict optimal angiographic deployment projections for TAVR. JACC Cardiovasc. Imaging 2014, 7, 847–848. [Google Scholar] [CrossRef]
- Onishi, T.; Sengoku, K.; Ichibori, Y.; Mizote, I.; Maeda, K.; Kuratani, T.; Sawa, Y.; Sakata, Y. The role of echocardiography in transcatheter aortic valve implantation. Cardiovasc. Diagn. Ther. 2018, 8, 3–17. [Google Scholar] [CrossRef]
- Goncalves, A.; Almeria, C.; Marcos-Alberca, P.; Feltes, G.; Hernandez-Antolin, R.; Rodriguez, E.; Cardoso, J.C.; Macaya, C.; Zamorano, J.L. Three-dimensional echocardiography in paravalvular aortic regurgitation assessment after transcatheter aortic valve implantation. J. Am. Soc. Echocardiogr. 2012, 25, 47–55. [Google Scholar] [CrossRef]
- Shibayama, K.; Mihara, H.; Jilaihawi, H.; Berdejo, J.; Harada, K.; Itabashi, Y.; Siegel, R.; Makkar, R.R.; Shiota, T. 3D Assessment of Features Associated with Transvalvular Aortic Regurgitation after TAVR: A Real-Time 3D TEE Study. JACC Cardiovasc. Imaging 2016, 9, 114–123. [Google Scholar] [CrossRef]
- Shiota, T. Role of modern 3D echocardiography in valvular heart disease. Korean J. Intern. Med. 2014, 29, 685–702. [Google Scholar] [CrossRef]
- Shibayama, K.; Harada, K.; Berdejo, J.; Mihara, H.; Tanaka, J.; Gurudevan, S.V.; Siegel, R.; Jilaihawi, H.; Makkar, R.R.; Shiota, T. Effect of transcatheter aortic valve replacement on the mitral valve apparatus and mitral regurgitation: Real-time three-dimensional transesophageal echocardiography study. Circ. Cardiovasc. Imaging 2014, 7, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Langer, N.B.; Hamid, N.B.; Nazif, T.M.; Khalique, O.K.; Vahl, T.P.; White, J.; Terre, J.; Hastings, R.; Leung, D.; Hahn, R.T.; et al. Injuries to the Aorta, Aortic Annulus, and Left Ventricle during Transcatheter Aortic Valve Replacement: Management and Outcomes. Circ. Cardiovasc. Interv. 2017, 10, e004735. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Rodriguez Diaz, C.A.; Trinh, M.A. Use of real-time three-dimensional transesophageal echocardiography in type A aortic dissections: Advantages of 3D TEE illustrated in three cases. Ann. Card. Anaesth. 2015, 18, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Labrousse, L.; Dijos, M.; Leroux, L.; Oses, P.; Seguy, B.; Markof, M.; Lafitte, S. Guidance of the MitraClip((R)) procedure by 2D and 3D imaging. Arch. Cardiovasc. Dis. 2018, 111, 432–440. [Google Scholar] [CrossRef]
- Altiok, E.; Hamada, S.; Brehmer, K.; Kuhr, K.; Reith, S.; Becker, M.; Schroder, J.; Almalla, M.; Lehmacher, W.; Marx, N.; et al. Analysis of procedural effects of percutaneous edge-to-edge mitral valve repair by 2D and 3D echocardiography. Circ. Cardiovasc. Imaging 2012, 5, 748–755. [Google Scholar] [CrossRef]
- Hirasawa, K.; Izumo, M. Role of 3D Transesophageal Echocardiography for Transcatheter Mitral Valve Repair—A Mini Review. Front. Cardiovasc. Med. 2022, 9, 815304. [Google Scholar] [CrossRef]
- Qamruddin, S.; Naqvi, T.Z. Advances in 3D echocardiography for mitral valve. Expert. Rev. Cardiovasc. Ther. 2011, 9, 1431–1443. [Google Scholar] [CrossRef]
- Altiok, E.; Becker, M.; Hamada, S.; Grabskaya, E.; Reith, S.; Marx, N.; Hoffmann, R. Real-time 3D TEE allows optimized guidance of percutaneous edge-to-edge repair of the mitral valve. JACC Cardiovasc. Imaging 2010, 3, 1196–1198. [Google Scholar] [CrossRef]
- Avenatti, E.; Mackensen, G.B.; El-Tallawi, K.C.; Reisman, M.; Gruye, L.; Barker, C.M.; Little, S.H. Diagnostic Value of 3-Dimensional Vena Contracta Area for the Quantification of Residual Mitral Regurgitation After MitraClip Procedure. JACC Cardiovasc. Interv. 2019, 12, 582–591. [Google Scholar] [CrossRef]
- Natarajan, N.; Patel, P.; Bartel, T.; Kapadia, S.; Navia, J.; Stewart, W.; Tuzcu, E.M.; Schoenhagen, P. Peri-procedural imaging for transcatheter mitral valve replacement. Cardiovasc. Diagn. Ther. 2016, 6, 144–159. [Google Scholar] [CrossRef]
- Harloff, M.T.; Chowdhury, M.; Hirji, S.A.; Percy, E.D.; Yazdchi, F.; Shim, H.; Malarczyk, A.A.; Sobieszczyk, P.S.; Sabe, A.A.; Shah, P.B.; et al. A step-by-step guide to transseptal valve-in-valve transcatheter mitral valve replacement. Ann. Cardiothorac. Surg. 2021, 10, 113–121. [Google Scholar] [CrossRef]
- Garcia-Fernandez, M.A.; Cortes, M.; Garcia-Robles, J.A.; Gomez de Diego, J.J.; Perez-David, E.; Garcia, E. Utility of real-time three-dimensional transesophageal echocardiography in evaluating the success of percutaneous transcatheter closure of mitral paravalvular leaks. J. Am. Soc. Echocardiogr. 2010, 23, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Zendjebil, S.; d’Angelo, L.; Doguet, F.; Dumont, N.; Benamer, H.; Fourchy, D.; Djebbar, M.; Garot, J.; Vaillant, R.; Garot, P. Computed Tomography/Fluoroscopy Fusion and 3D Transesophageal Echocardiography-Guided Percutaneous Paravalvular Leak Closure. JACC Case Rep. 2023, 5, 101690. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.K.; Porras, D.; Maschietto, N.; Marx, G.R. Three-dimensional transesophageal echocardiography-guided transcatheter closure of multiple mitral paravalvular leaks demonstrating real time avoidance of device-induced valve malfunction. Echocardiography 2019, 36, 1418–1420. [Google Scholar] [CrossRef] [PubMed]
- Beneduce, A.; Ancona, M.B.; Russo, F.; Ferri, L.A.; Bellini, B.; Vella, C.; Romano, V.; Ancona, F.; Agricola, E.; Montorfano, M. Transcatheter Mitral Paravalvular Leak Closure Using Arteriovenous Rail Across Aortic Bileaflet Mechanical Prosthesis: Multimodality Imaging Approach. Circ. Cardiovasc. Imaging 2023, 16, e014267. [Google Scholar] [CrossRef]
- Biner, S.; Kar, S.; Siegel, R.J.; Rafique, A.; Shiota, T. Value of color Doppler three-dimensional transesophageal echocardiography in the percutaneous closure of mitral prosthesis paravalvular leak. Am. J. Cardiol. 2010, 105, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Y.; Zhai, M.; Jin, P.; Li, L.; Ma, Y.; Yang, J. Transcatheter Closure of a Paravalvular Leak Guided by Transesophageal Echocardiography and Three-Dimensional Printing. Front. Cardiovasc. Med. 2022, 9, 750896. [Google Scholar] [CrossRef]
- Matli, K.; Mahdi, A.; Zibara, V.; Costanian, C.; Ghanem, G. Transcatheter tricuspid valve intervention techniques and procedural steps for the treatment of tricuspid regurgitation: A review of the literature. Open Heart 2022, 9, e002030. [Google Scholar] [CrossRef]
- Hahn, R.T. State-of-the-Art Review of Echocardiographic Imaging in the Evaluation and Treatment of Functional Tricuspid Regurgitation. Circ. Cardiovasc. Imaging 2016, 9, e005332. [Google Scholar] [CrossRef]
- Muraru, D.; Hahn, R.T.; Soliman, O.I.; Faletra, F.F.; Basso, C.; Badano, L.P. 3-Dimensional Echocardiography in Imaging the Tricuspid Valve. JACC Cardiovasc. Imaging 2019, 12, 500–515. [Google Scholar] [CrossRef]
- Dahou, A.; Ong, G.; Hamid, N.; Avenatti, E.; Yao, J.; Hahn, R.T. Quantifying Tricuspid Regurgitation Severity: A Comparison of Proximal Isovelocity Surface Area and Novel Quantitative Doppler Methods. JACC Cardiovasc. Imaging 2019, 12, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Mediratta, A.; Addetia, K.; Yamat, M.; Moss, J.D.; Nayak, H.M.; Burke, M.C.; Weinert, L.; Maffessanti, F.; Jeevanandam, V.; Mor-Avi, V.; et al. 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc. Imaging 2014, 7, 337–347. [Google Scholar] [CrossRef]
- Naqvi, T.Z.; Rafie, R.; Ghalichi, M. Real-time 3D TEE for the diagnosis of right-sided endocarditis in patients with prosthetic devices. JACC Cardiovasc. Imaging 2010, 3, 325–327. [Google Scholar] [CrossRef][Green Version]
- Belzile, D.H.M.; Lang, R.M.; Tsang, W. Pre-Procedural Interventional TEE: Tricuspid Valve. 2023. Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2022/07/29/17/19/Pre-Procedural-Interventional-TEE-Tricuspid-Valve (accessed on 1 May 2023).
- Qamruddin, S.; Shinbane, J.; Shriki, J.; Naqvi, T.Z. Left atrial appendage: Structure, function, imaging modalities and therapeutic options. Expert. Rev. Cardiovasc. Ther. 2010, 8, 65–75. [Google Scholar] [CrossRef]
- Shah, S.J.; Bardo, D.M.; Sugeng, L.; Weinert, L.; Lodato, J.A.; Knight, B.P.; Lopez, J.J.; Lang, R.M. Real-time three-dimensional transesophageal echocardiography of the left atrial appendage: Initial experience in the clinical setting. J. Am. Soc. Echocardiogr. 2008, 21, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, G.; Faletra, F.F.; Regoli, F.; Pasotti, E.; Pedrazzini, G.; Moccetti, T.; Auricchio, A. Evaluation of the left atrial appendage with real-time 3-dimensional transesophageal echocardiography: Implications for catheter-based left atrial appendage closure. Circ. Cardiovasc. Imaging 2011, 4, 514–523. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Z.; Han, Z.; Zeng, L.; Wang, C. Role of real time-three dimensional transesophageal echocardiography in left atrial appendage closure with LACBES((R)) devices. Exp. Ther. Med. 2019, 17, 1456–1462. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, H.; Zhang, L.; Deng, Q.; Chen, J.; Hu, B.; Wang, Y.; Guo, R. Roles of real-time three-dimensional transesophageal echocardiography in peri-operation of transcatheter left atrial appendage closure. Medicine 2017, 96, e5637. [Google Scholar] [CrossRef] [PubMed]
- Pazos-Lopez, P.; Garcia-Rodriguez, C.; Guitian-Gonzalez, A.; Paredes-Galan, E.; Alvarez-Moure, M.A.; Rodriguez-Alvarez, M.; Baz-Alonso, J.A.; Teijeira-Fernandez, E.; Calvo-Iglesias, F.E.; Iniguez-Romo, A. Pulmonary vein stenosis: Etiology, diagnosis and management. World J. Cardiol. 2016, 8, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.S.; Todoran, T.M.; Kinlay, S.; Sobieszczyk, P.S.; Shook, D.; Gross, W.L.; Eisenhauer, A.C. Use of real-time 3D transesophageal echocardiography in percutaneous intervention of a flush-occluded pulmonary vein. Circ. Cardiovasc. Interv. 2010, 3, 394–395. [Google Scholar] [CrossRef]
- Faletra, F.F.; Nucifora, G.; Regoli, F.; Ho, S.Y.; Moccetti, T.; Auricchio, A. Anatomy of pulmonary veins by real-time 3D TEE: Implications for catheter-based pulmonary vein ablation. JACC Cardiovasc. Imaging 2012, 5, 456–462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heyns, M.D.; Schroder, J.N.; Jaquis, R.D.; Mackensen, G.B. Three-dimensional transesophageal echocardiography enhances multimodal imaging of a successful repair of a case with scimitar syndrome. Circ. Cardiovasc. Imaging 2012, 5, 164–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Basman, C.; Parmar, Y.J.; Kronzon, I. Intracardiac Echocardiography for Structural Heart and Electrophysiological Interventions. Curr. Cardiol. Rep. 2017, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Karagodin, I.; Addetia, K.; Singh, A.; Dow, A.; Rivera, L.; DeCara, J.M.; Soulat-Dufour, L.; Yamat, M.; Kruse, E.; Shah, A.P.; et al. Improved Delineation of Cardiac Pathology Using a Novel Three-Dimensional Echocardiographic Tissue Transparency Tool. J. Am. Soc. Echocardiogr. 2020, 33, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Genovese, D.; Addetia, K.; Kebed, K.; Kruse, E.; Yamat, M.; Narang, A.; Patel, A.R.; Badano, L.P.; Muraru, D.; Goncalves, A.; et al. First Clinical Experience with 3-Dimensional Echocardiographic Transillumination Rendering. JACC Cardiovasc. Imaging 2019, 12, 1868–1871. [Google Scholar] [CrossRef]
- Mantegazza, V.; Gripari, P.; Tamborini, G.; Muratori, M.; Fusini, L.; Ghulam Ali, S.; Garlasche, A.; Pepi, M. 3D echocardiography in mitral valve prolapse. Front. Cardiovasc. Med. 2022, 9, 1050476. [Google Scholar] [CrossRef]
- Castro-Verdes, M.; Yuan, X.; Mitsis, A.; Li, W.; Nienaber, C.A. Transesophageal Ultrasound Guidance for Endovascular Interventions on the Aorta. Aorta 2022, 10, 3–12. [Google Scholar] [CrossRef]
- Agricola, E.; Ingallina, G.; Ancona, F.; Biondi, F.; Margonato, D.; Barki, M.; Tavernese, A.; Belli, M.; Stella, S. Evolution of interventional imaging in structural heart disease. Eur. Heart J. Suppl. 2023, 25 (Suppl. SC), C189–C199. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, J.M.; Barry, T.; Arsanjani, R.; Ayoub, C.; Naqvi, T.Z. Three-Dimensional Transesophageal Echocardiography in Percutaneous Catheter-Based Cardiac Interventions. J. Clin. Med. 2023, 12, 5664. https://doi.org/10.3390/jcm12175664
Farina JM, Barry T, Arsanjani R, Ayoub C, Naqvi TZ. Three-Dimensional Transesophageal Echocardiography in Percutaneous Catheter-Based Cardiac Interventions. Journal of Clinical Medicine. 2023; 12(17):5664. https://doi.org/10.3390/jcm12175664
Chicago/Turabian StyleFarina, Juan M., Timothy Barry, Reza Arsanjani, Chadi Ayoub, and Tasneem Z. Naqvi. 2023. "Three-Dimensional Transesophageal Echocardiography in Percutaneous Catheter-Based Cardiac Interventions" Journal of Clinical Medicine 12, no. 17: 5664. https://doi.org/10.3390/jcm12175664
APA StyleFarina, J. M., Barry, T., Arsanjani, R., Ayoub, C., & Naqvi, T. Z. (2023). Three-Dimensional Transesophageal Echocardiography in Percutaneous Catheter-Based Cardiac Interventions. Journal of Clinical Medicine, 12(17), 5664. https://doi.org/10.3390/jcm12175664





